Impact of Freeze Storage on the Estimation of Phenotypic Antimicrobial Resistance Prevalence in Escherichia coli Collected from Faecal Samples from Healthy Humans and Chickens
Abstract
1. Introduction
2. Results
2.1. Counts of E. coli Colonies in Fresh and Stored Samples
2.2. Prevalence of Resistance over Time in Faecal Samples
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Sample Collection
4.3. Estimation of the Prevalence of Resistant E. coli
4.4. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- E Clinical Medicine. Antimicrobial resistance: A top ten global public health threat. EClinicalMedicine 2021, 41, 101221. [Google Scholar] [CrossRef] [PubMed]
- Nji, E.; Kazibwe, J.; Hambridge, T.; Joko, C.A.; Larbi, A.A.; Damptey, L.A.O.; Nkansa-Gyamfi, N.A.; Lundborg, C.S.; Lien, L.T.Q. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Cuong, N.V.; Thwaites, G.; Carrique-Mas, J. Antimicrobial Usage and Antimicrobial Resistance in Animal Production in Southeast Asia: A Review. Antibiotics 2016, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Poirel, L.; Madec, J.-Y.; Lupo, A.; Schink, A.-K.; Kieffer, N.; Nordmann, P.; Schwarz, S. Antimicrobial Resistance in Escherichia coli. Microbiol. Spectr. 2018, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Poole, T.; Callaway, T.; Norman, K.; Scott, H.; Loneragan, G.; Ison, S.; Beier, R.; Harhay, D.; Norby, B.; Nisbet, D. Transferability of antimicrobial resistance from multidrug-resistant Escherichia coli isolated from cattle in the USA to E. coli and Salmonella Newport recipients. J. Glob. Antimicrob. Resist. 2017, 11, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Yen, N.T.P.; Dung, N.T.T.; Nhan, N.T.M.; Phu, D.H.; Kiet, B.T.; Thwaites, G.; Geskus, R.B.; Baker, S.; Carrique-Mas, J.; et al. Antimicrobial resistance in commensal Escherichia coli from humans and chickens in the Mekong Delta of Vietnam is driven by antimicrobial usage and potential cross-species transmission. JAC-Antimicrob. Resist. 2022, 4, dlac054. [Google Scholar] [CrossRef] [PubMed]
- Purohit, M.R.; Chandran, S.; Shah, H.; Diwan, V.; Tamhankar, A.J.; Lundborg, C.S. Antibiotic Resistance in an Indian Rural Community: A ‘One-Health’ Observational Study on Commensal Coliform from Humans, Animals, and Water. Int. J. Environ. Res. Public Health 2017, 14, 386. [Google Scholar] [CrossRef] [PubMed]
- Nhung, N.T.; Cuong, N.V.; Campbell, J.; Hoa, N.T.; Bryant, J.E.; Truc, V.N.T.; Kiet, B.T.; Jombart, T.; Trung, N.V.; Hien, V.B.; et al. High Levels of Antimicrobial Resistance among Escherichia coli Isolates from Livestock Farms and Synanthropic Rats and Shrews in the Mekong Delta of Vietnam. Appl. Environ. Microbiol. 2015, 81, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Carrique-Mas, J.J.; Ngo, T.H.; Ho, H.M.; Ha, T.T.; Campbell, J.I.; Nguyen, T.N.; Hoang, N.N.; Pham, V.M.; Wagenaar, J.A.; et al. Prevalence and risk factors for carriage of antimicrobial-resistant Escherichia coli on household and small-scale chicken farms in the Mekong Delta of Vietnam. J. Antimicrob. Chemother. 2015, 70, 2144–2152. [Google Scholar] [CrossRef] [PubMed]
- Bassis, C.M.; Program, F.T.C.P.E.; Moore, N.M.; Lolans, K.; Seekatz, A.M.; Weinstein, R.A.; Young, V.B.; Hayden, M.K. Comparison of stool versus rectal swab samples and storage conditions on bacterial community profiles. BMC Microbiol. 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gavriliuc, S.; Stothart, M.R.; Henry, A.; Poissant, J. Long-term storage of feces at −80 °C versus −20 °C is negligible for 16S rRNA amplicon profiling of the equine bacterial microbiome. PeerJ 2021, 9, e10837. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Zhou, N.; Gordon, J.I.; Knight, R.; Fierer, N. Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol. Lett. 2010, 307, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Vogwill, T.; MacLean, R.C. The genetic basis of the fitness costs of antimicrobial resistance: A meta-analysis approach. Evol. Appl. 2015, 8, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Dorsaz, S.; Charretier, Y.; Girard, M.; Gaïa, N.; Leo, S.; Schrenzel, J.; Harbarth, S.; Huttner, B.; Lazarevic, V. Changes in Microbiota Profiles After Prolonged Frozen Storage of Stool Suspensions. Front. Cell. Infect. Microbiol. 2020, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Navajas-Porras, B.; Blasco, T.; Balzerani, F.; Lerma-Aguilera, A.; León, D.; Pastoriza, S.; Apaolaza, I.; Planes, F.; et al. Effect of Freezing on Gut Microbiota Composition and Functionality for In Vitro Fermentation Experiments. Nutrients 2021, 13, 2207. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, J.D.; Semenov, A.v.; Costa, R.; Trevors, J.T. Survival of Escherichia coli in the Environment: Fundamental and Public Health Aspects. ISME J. 2011, 5, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.; Schubert, H.; Puddy, E.F.; Sealey, J.E.; Gould, V.C.; Cogan, T.A.; Avison, M.B.; Reyher, K.K. Factors influencing the detection of antibacterial-resistant Escherichia coli in faecal samples from individual cattle. J. Appl. Microbiol. 2021, 132, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, C.S.; Kaas, R.S.; Aarestrup, F.M.; Pamp, S.J. Standard Sample Storage Conditions Have an Impact on Inferred Microbiome Composition and Antimicrobial Resistance Patterns. Microbiol. Spectr. 2021, 9, e01387-21. [Google Scholar] [CrossRef] [PubMed]
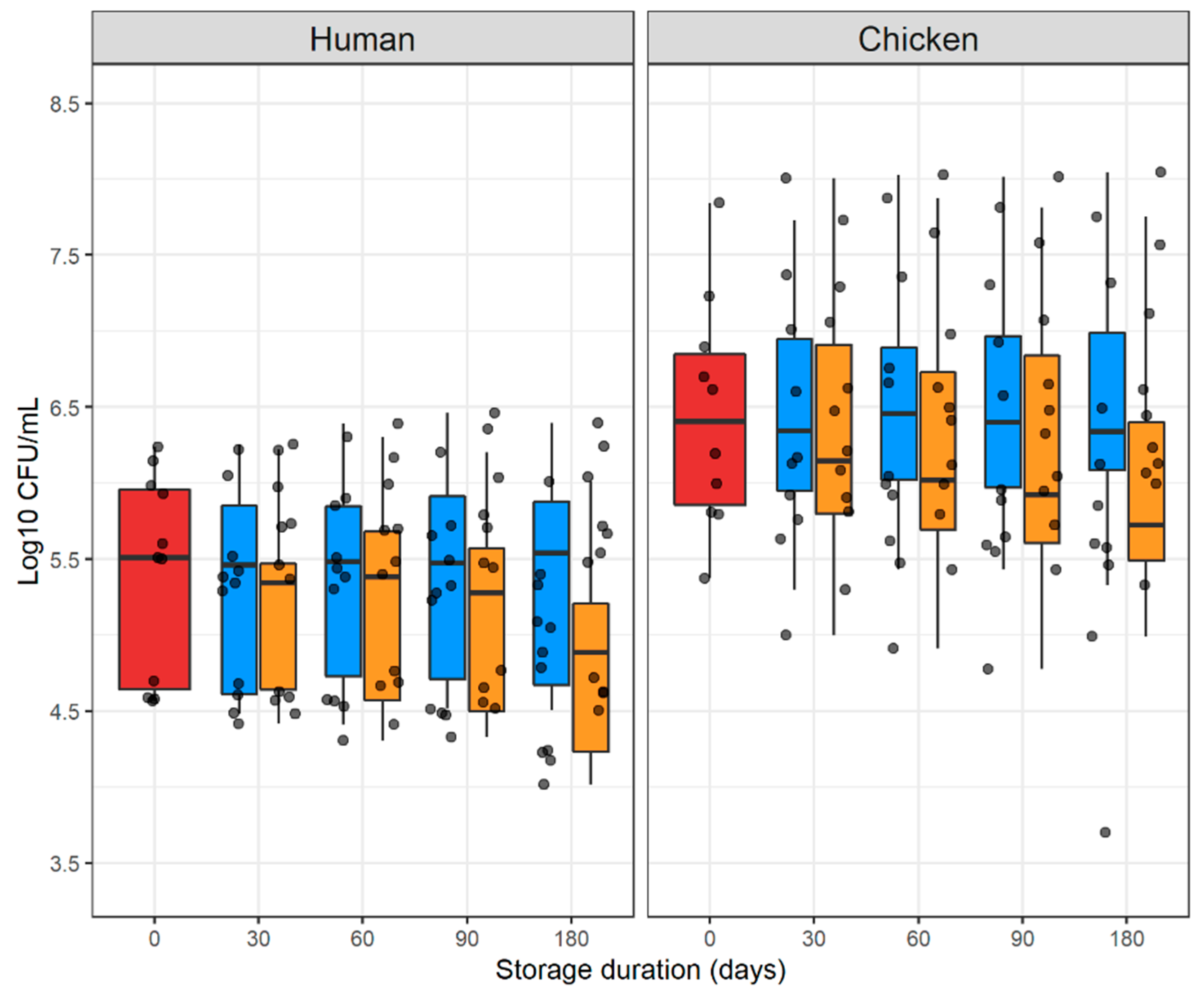
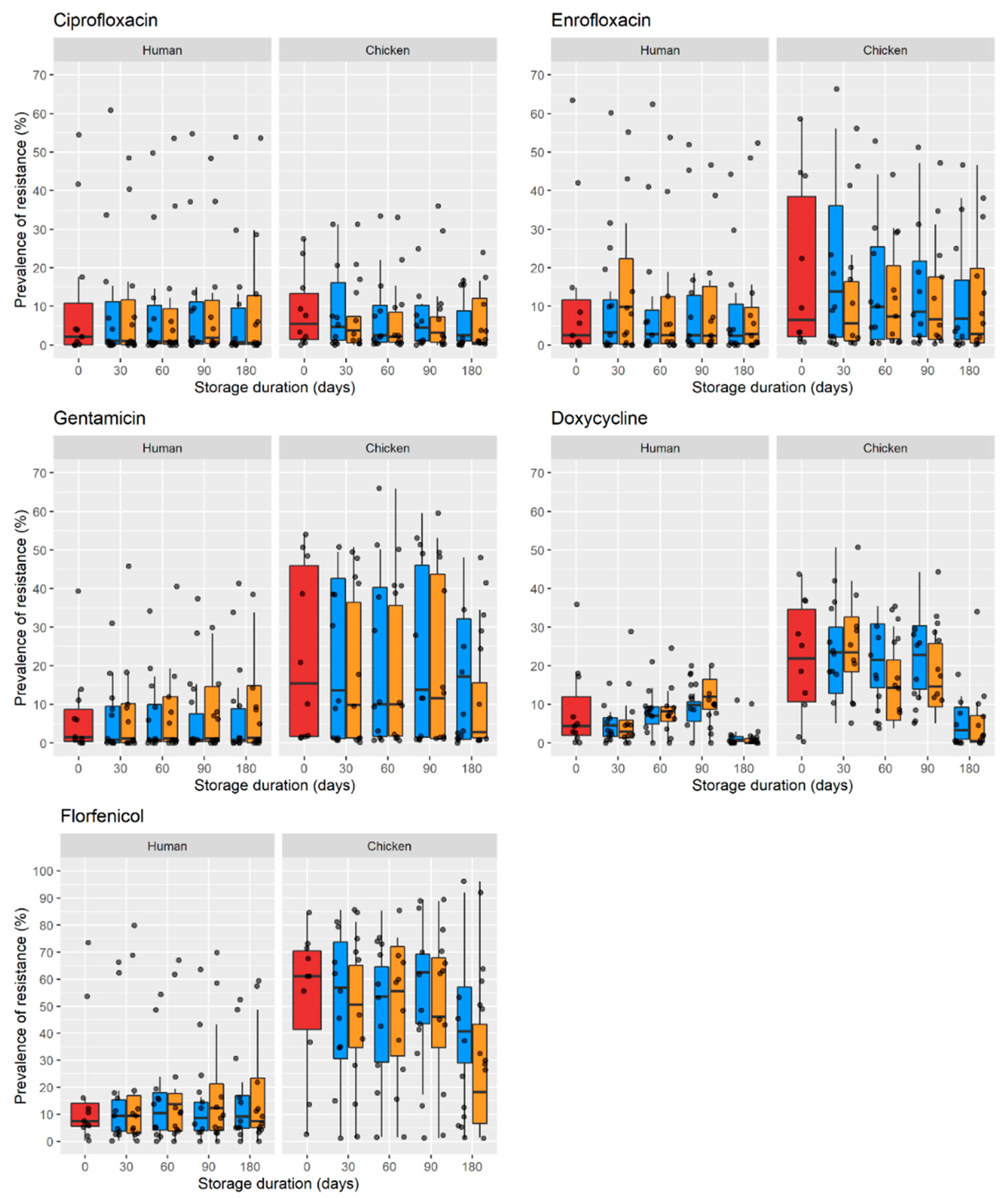
| Sample | Days | Human 1 | Chicken 2 |
|---|---|---|---|
| Fresh | 0 | 235,626 | 2,520,581 |
| −20 °C | 30 | 194,989 | 2,389,513 |
| 100 | 125,367 | 2,109,581 | |
| 300 | 35,490 | 1,477,704 | |
| −80 °C | 30 | 244,850 | 2,707,943 |
| 100 | 267,801 | 3,201,084 | |
| 300 | 345,933 | 5,162,854 |
| Host | Antimicrobial | Model-Predicted Prevalence of Resistance (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fresh Sample | Storage at −20 °C | Storage at −80 °C | ||||||
| 30 d | 100 d | 300 d | 30 d | 100 d | 300 d | |||
| Human | Ciprofloxacin | 0.054 | 0.053 | 0.051 | 0.046 | 0.053 | 0.049 | 0.040 |
| Enrofloxacin | 0.08 | 0.081 | 0.078 | 0.069 | 0.077 | 0.065 | 0.041 | |
| Doxycycline | 4.46 | 4.28 | 3.89 | 2.97 | 3.89 | 2.82 | 1.12 | |
| Gentamicin | 0.21 | 0.23 | 0.29 | 0.51 | 0.21 | 0.19 | 0.15 | |
| Florfenicol | 7.65 | 7.25 | 6.39 | 4.45 | 7.47 | 7.04 | 5.97 | |
| Chicken | Ciprofloxacin | 3.88 | 2.92 | 1.50 | 0.22 | 3.45 | 2.64 | 1.22 |
| Enrofloxacin | 6.99 | 5.96 | 4.11 | 1.42 | 6.65 | 5.90 | 4.19 | |
| Doxycycline | 20.60 | 17.10 | 11.08 | 3.21 | 18.60 | 14.66 | 7.43 | |
| Gentamicin | 10.03 | 8.32 | 5.38 | 1.55 | 9.27 | 7.72 | 4.57 | |
| Florfenicol | 41.48 | 35.35 | 24.34 | 8.38 | 39.37 | 34.85 | 24.61 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiet, B.T.; Nhung, N.T.; Yen, N.T.P.; Phu, D.H.; Dung, N.T.T.; Yen, L.K.; Thu, H.T.V.; Carrique-Mas, J.J. Impact of Freeze Storage on the Estimation of Phenotypic Antimicrobial Resistance Prevalence in Escherichia coli Collected from Faecal Samples from Healthy Humans and Chickens. Antibiotics 2022, 11, 1643. https://doi.org/10.3390/antibiotics11111643
Kiet BT, Nhung NT, Yen NTP, Phu DH, Dung NTT, Yen LK, Thu HTV, Carrique-Mas JJ. Impact of Freeze Storage on the Estimation of Phenotypic Antimicrobial Resistance Prevalence in Escherichia coli Collected from Faecal Samples from Healthy Humans and Chickens. Antibiotics. 2022; 11(11):1643. https://doi.org/10.3390/antibiotics11111643
Chicago/Turabian StyleKiet, Bach Tuan, Nguyen Thi Nhung, Nguyen Thi Phuong Yen, Doan Hoang Phu, Nguyen Thi Thuy Dung, Lam Kim Yen, Ho Thi Viet Thu, and Juan J. Carrique-Mas. 2022. "Impact of Freeze Storage on the Estimation of Phenotypic Antimicrobial Resistance Prevalence in Escherichia coli Collected from Faecal Samples from Healthy Humans and Chickens" Antibiotics 11, no. 11: 1643. https://doi.org/10.3390/antibiotics11111643
APA StyleKiet, B. T., Nhung, N. T., Yen, N. T. P., Phu, D. H., Dung, N. T. T., Yen, L. K., Thu, H. T. V., & Carrique-Mas, J. J. (2022). Impact of Freeze Storage on the Estimation of Phenotypic Antimicrobial Resistance Prevalence in Escherichia coli Collected from Faecal Samples from Healthy Humans and Chickens. Antibiotics, 11(11), 1643. https://doi.org/10.3390/antibiotics11111643








