Lessons Learned in Scalp Reconstruction and Tailoring Free Tissue Transfer in the Elderly: A Case Series and Literature Review
Abstract
:Methods
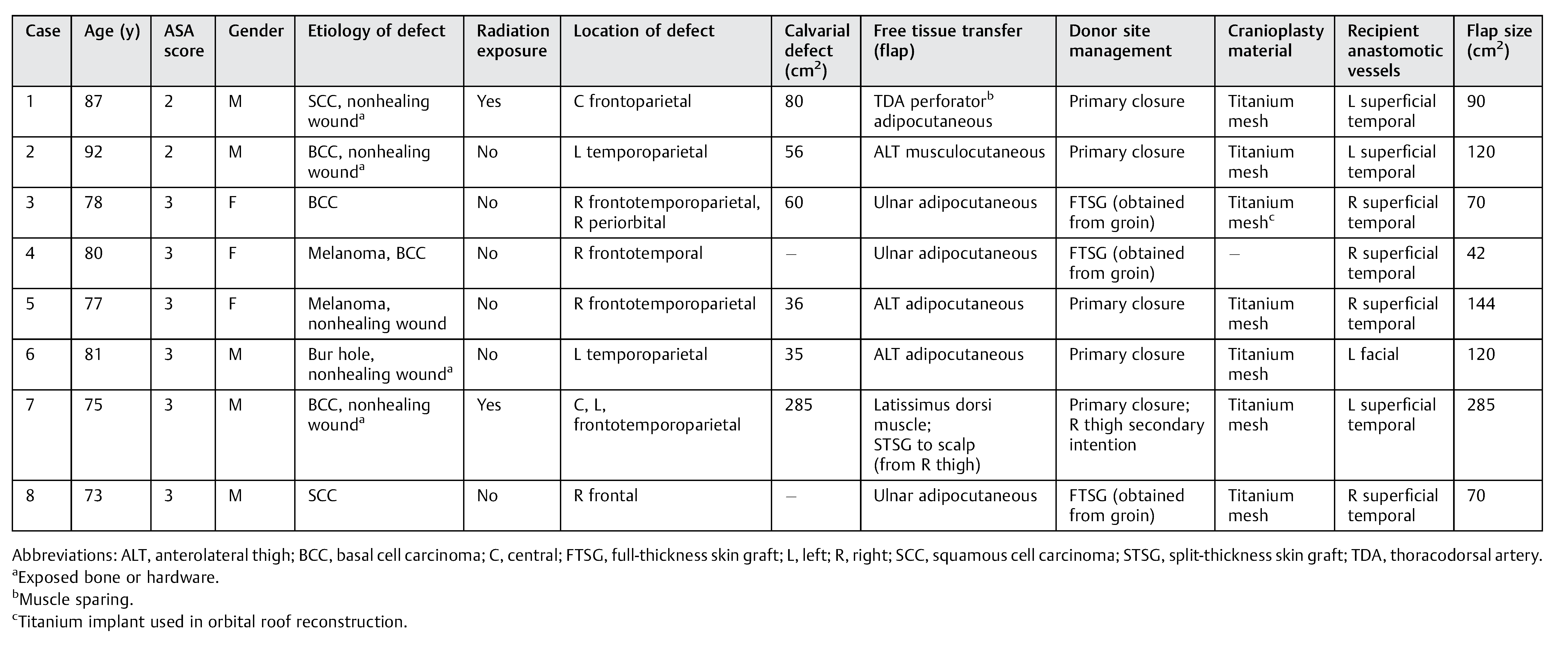
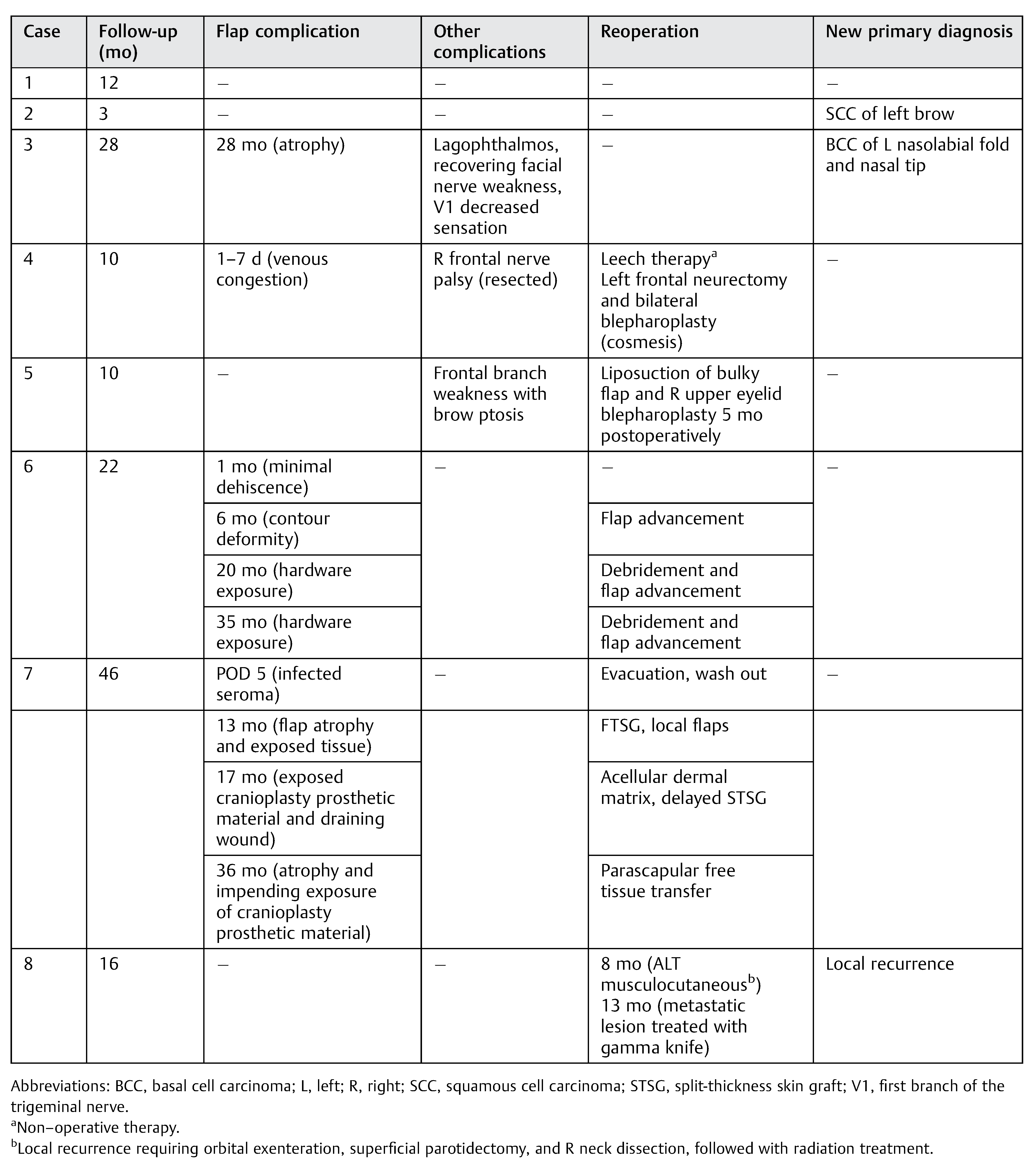
Results
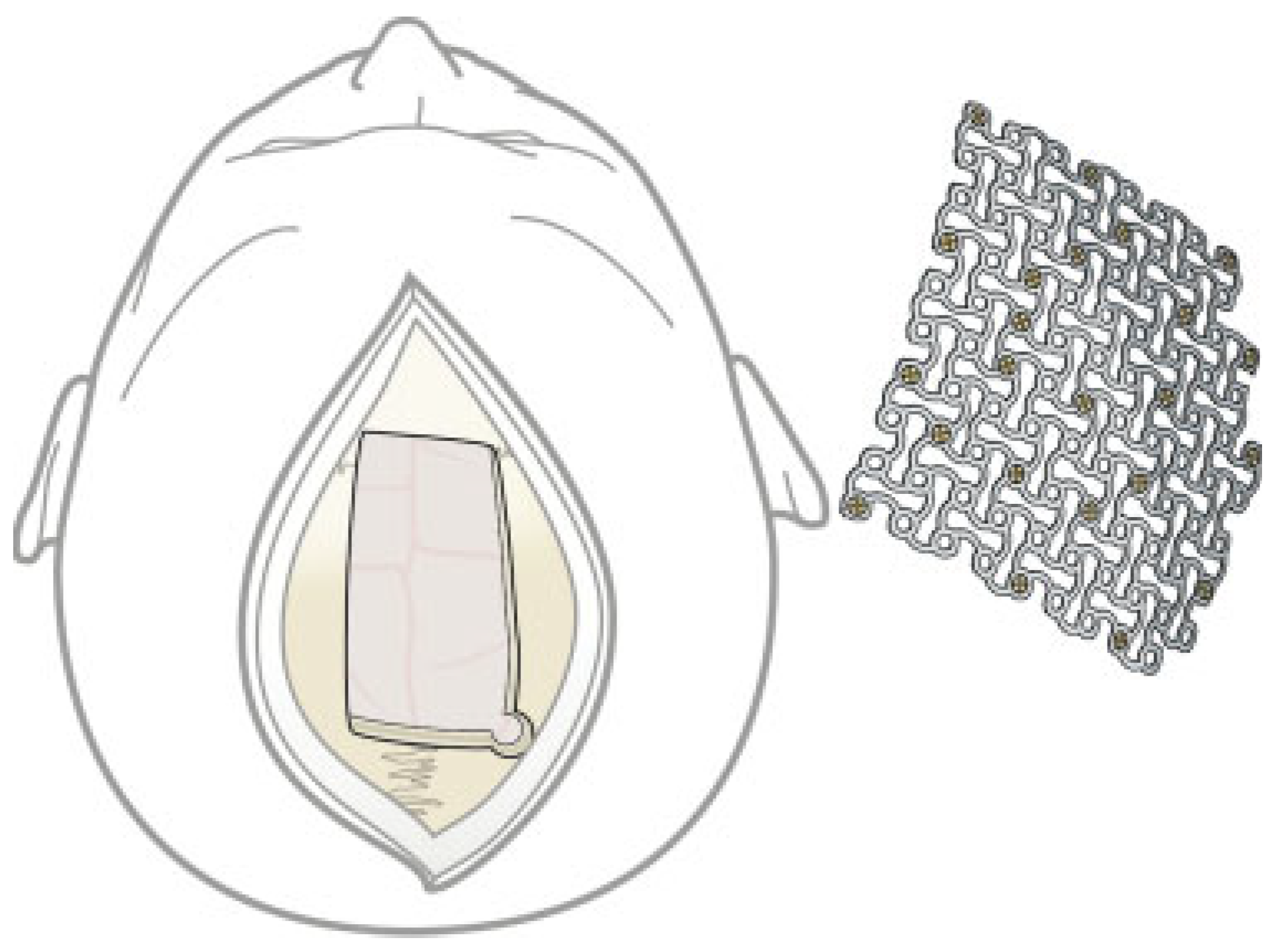
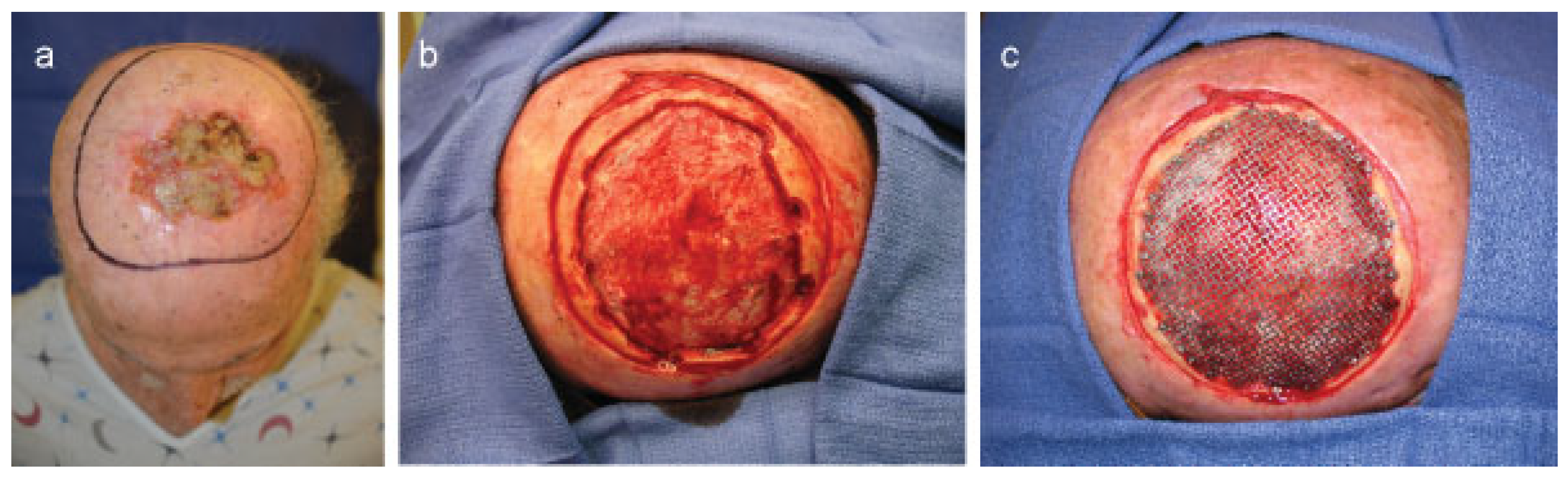
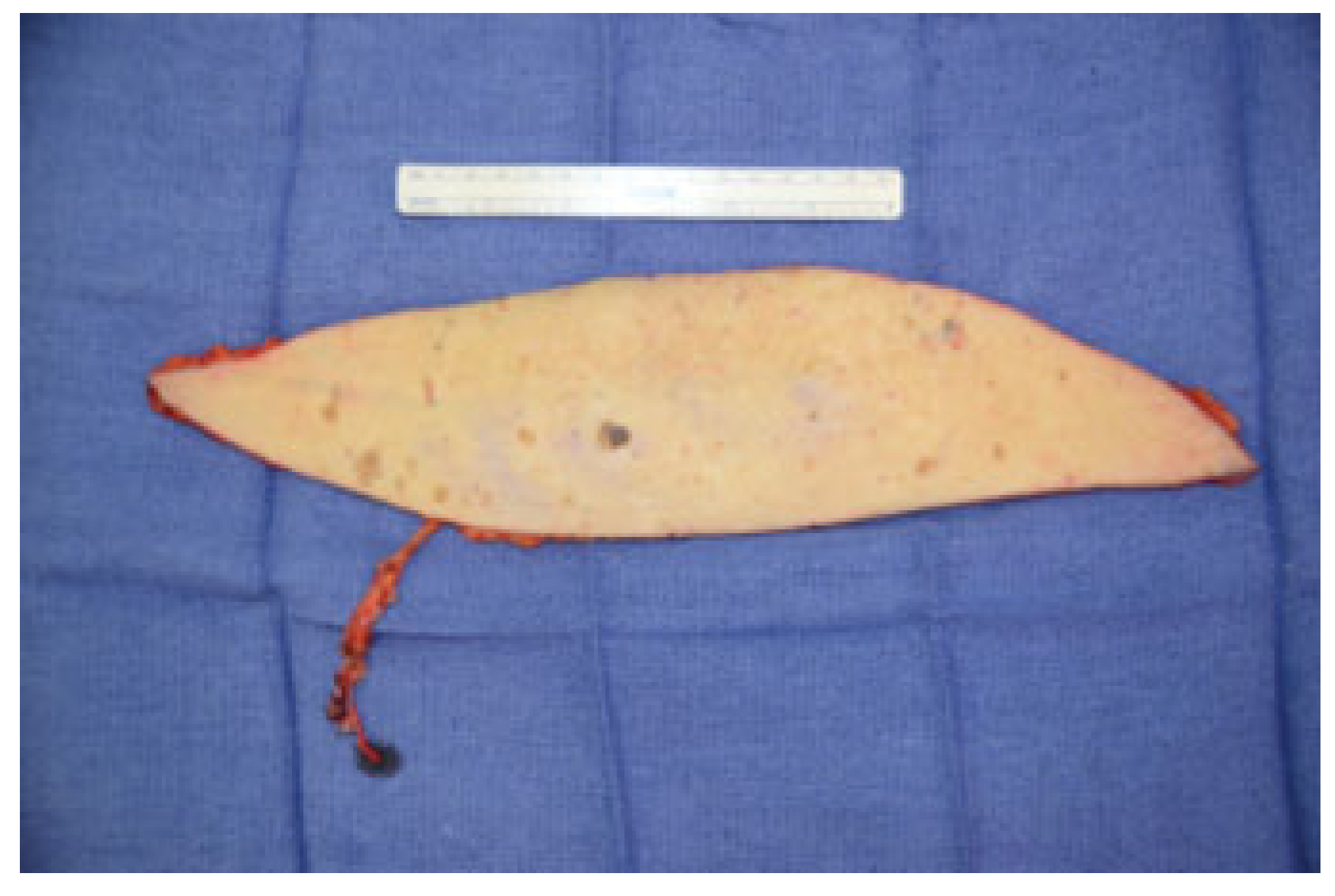
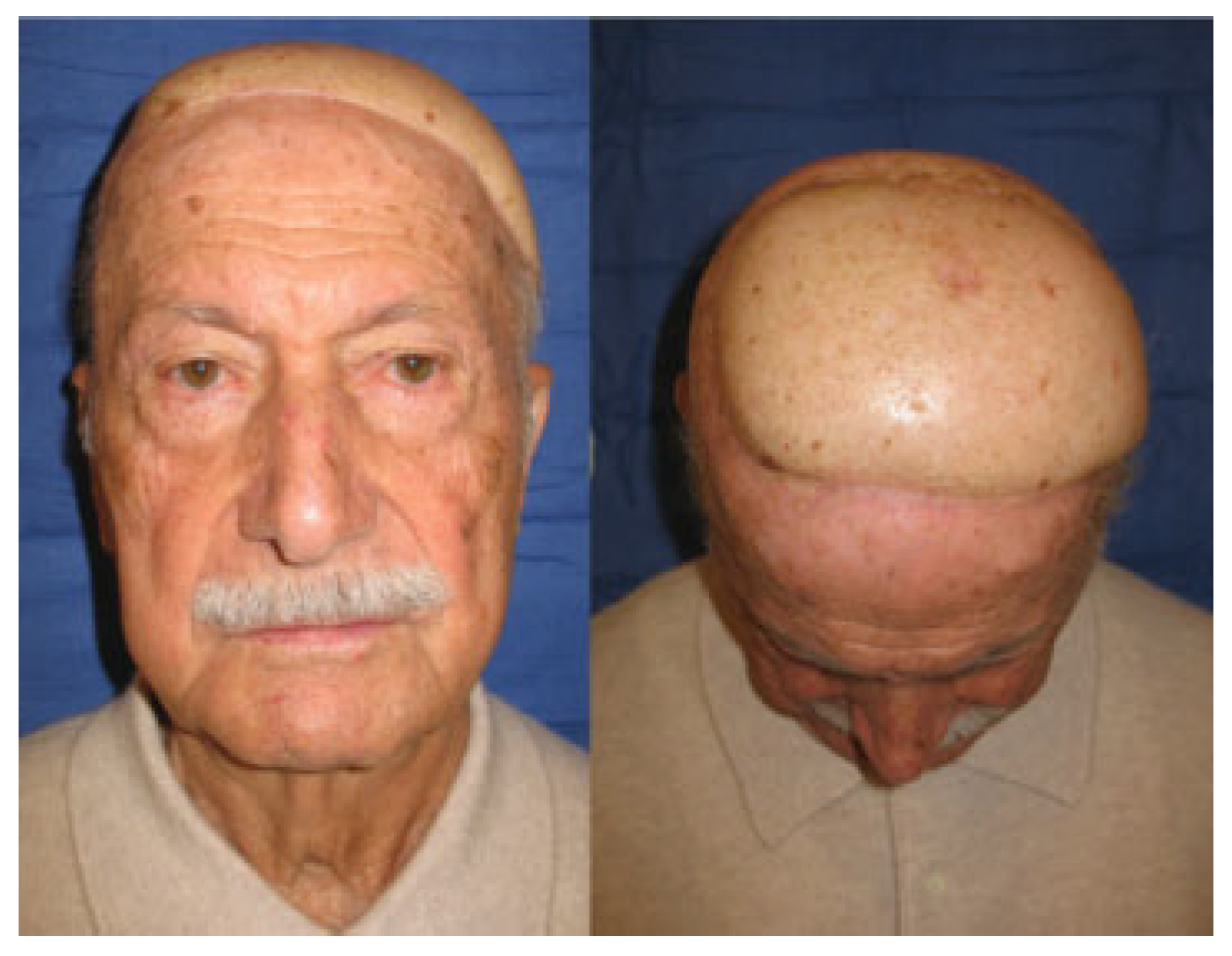
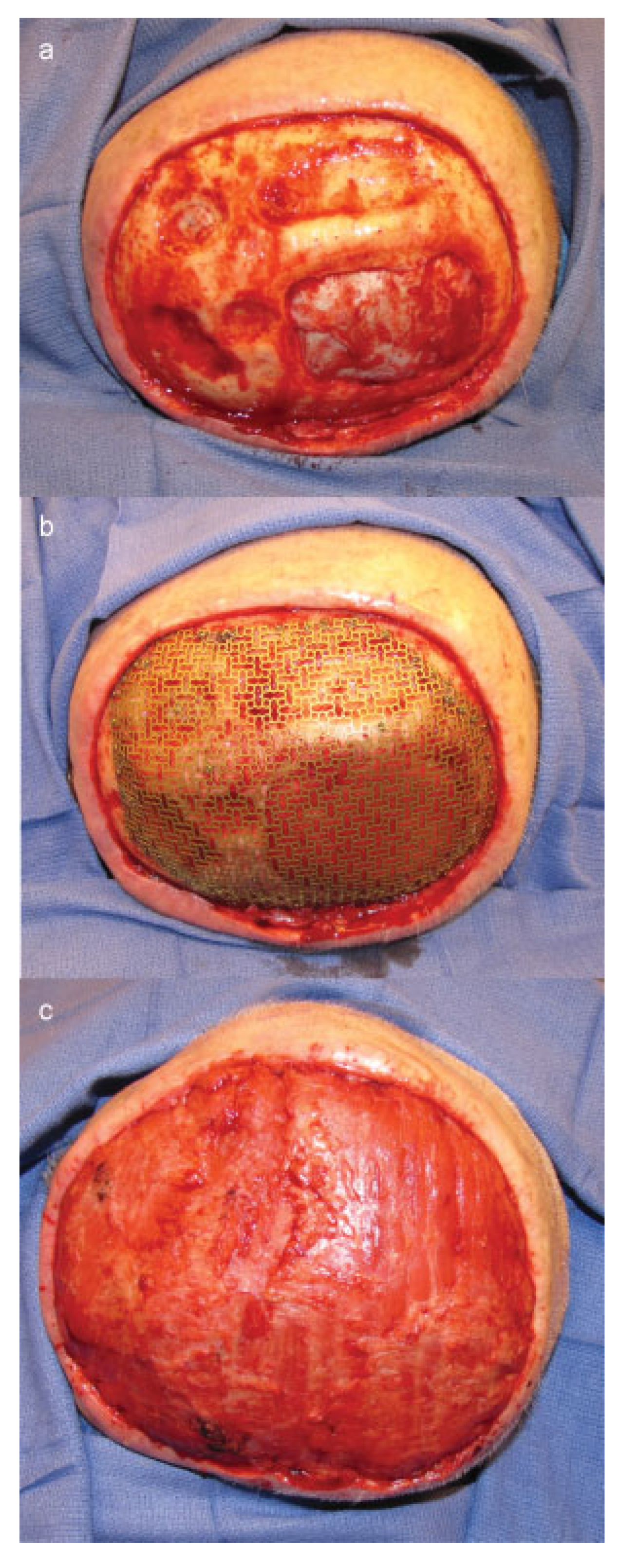
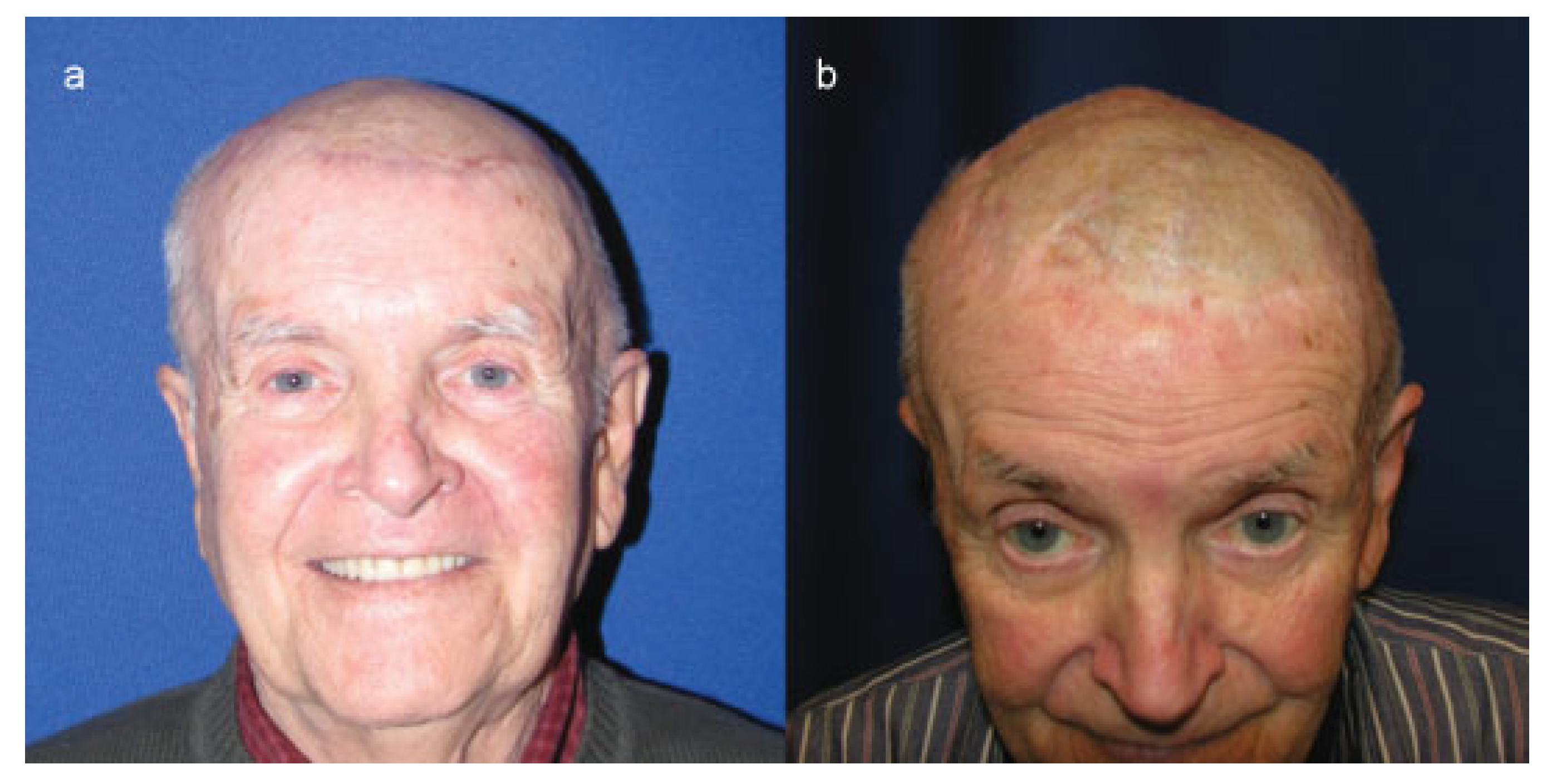
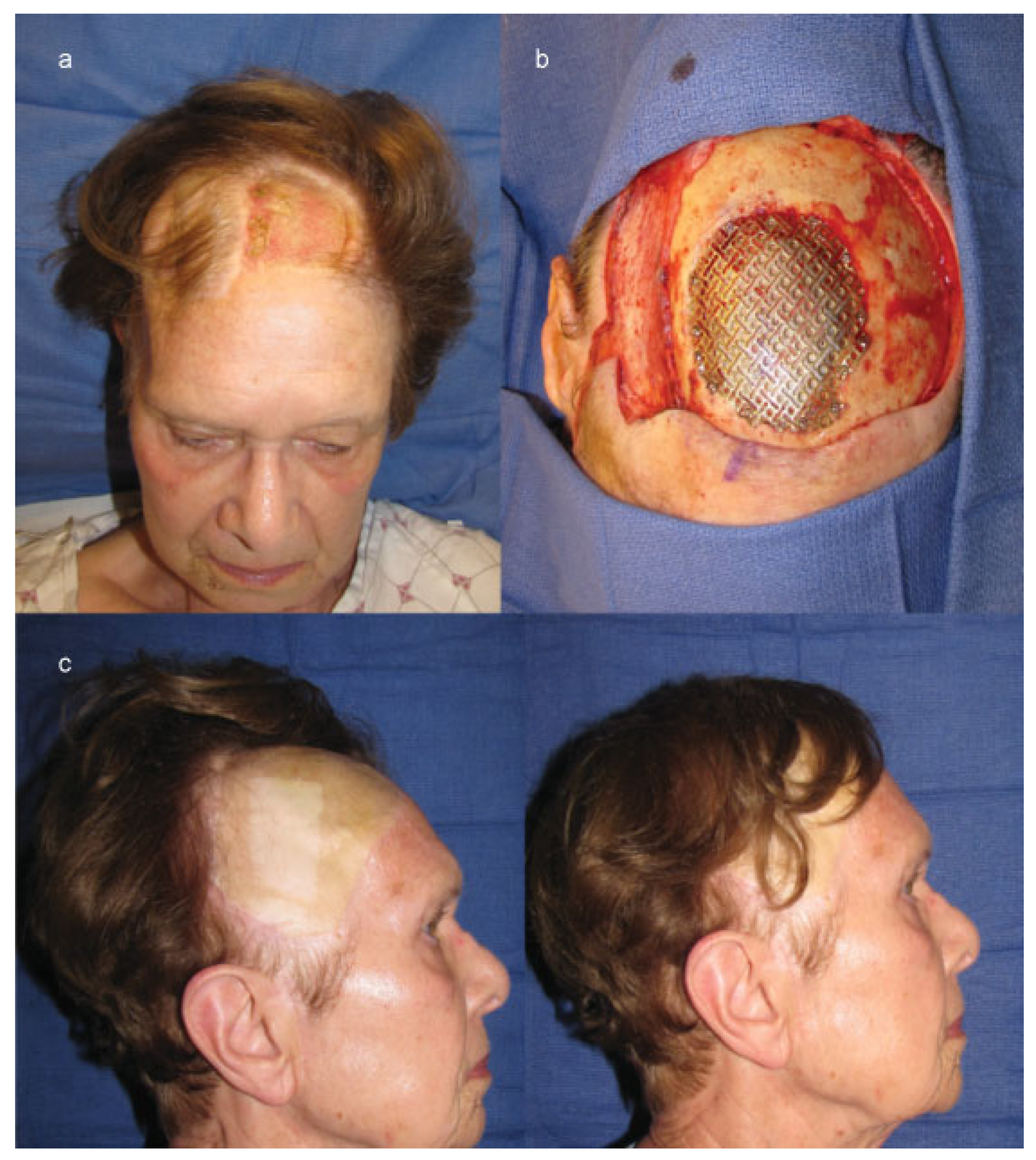
Cases
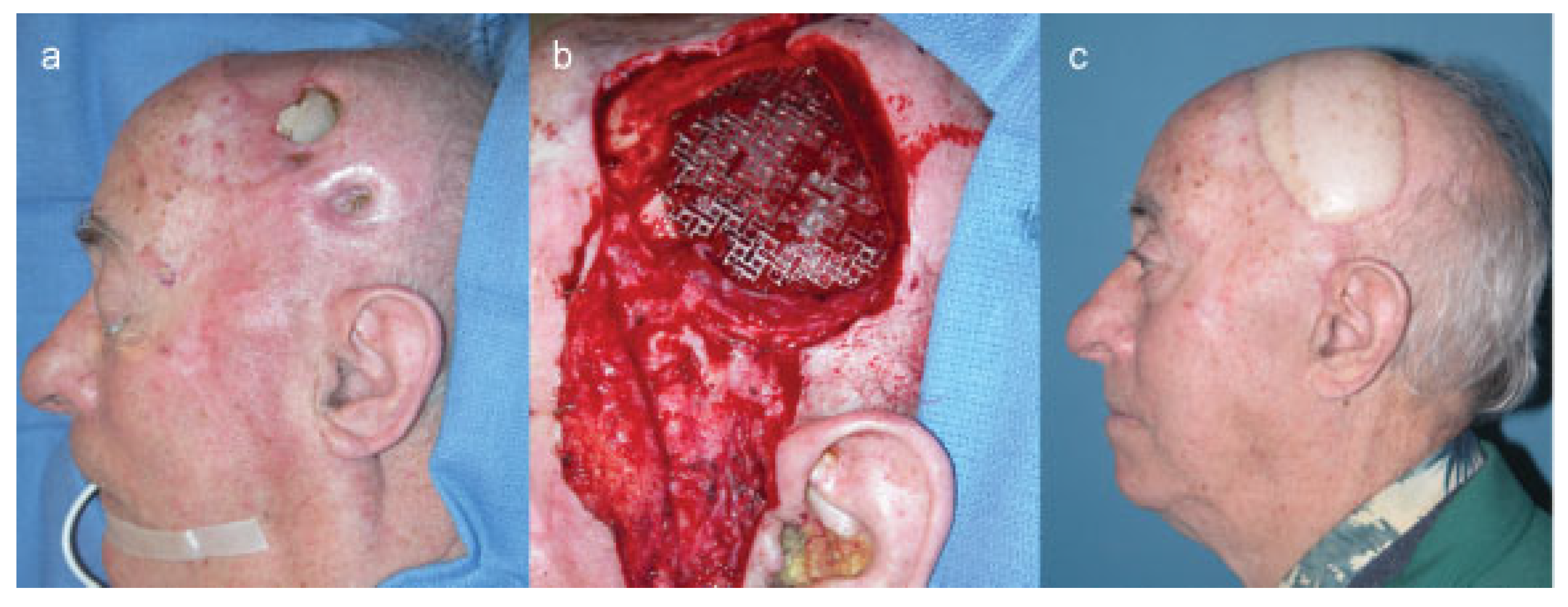
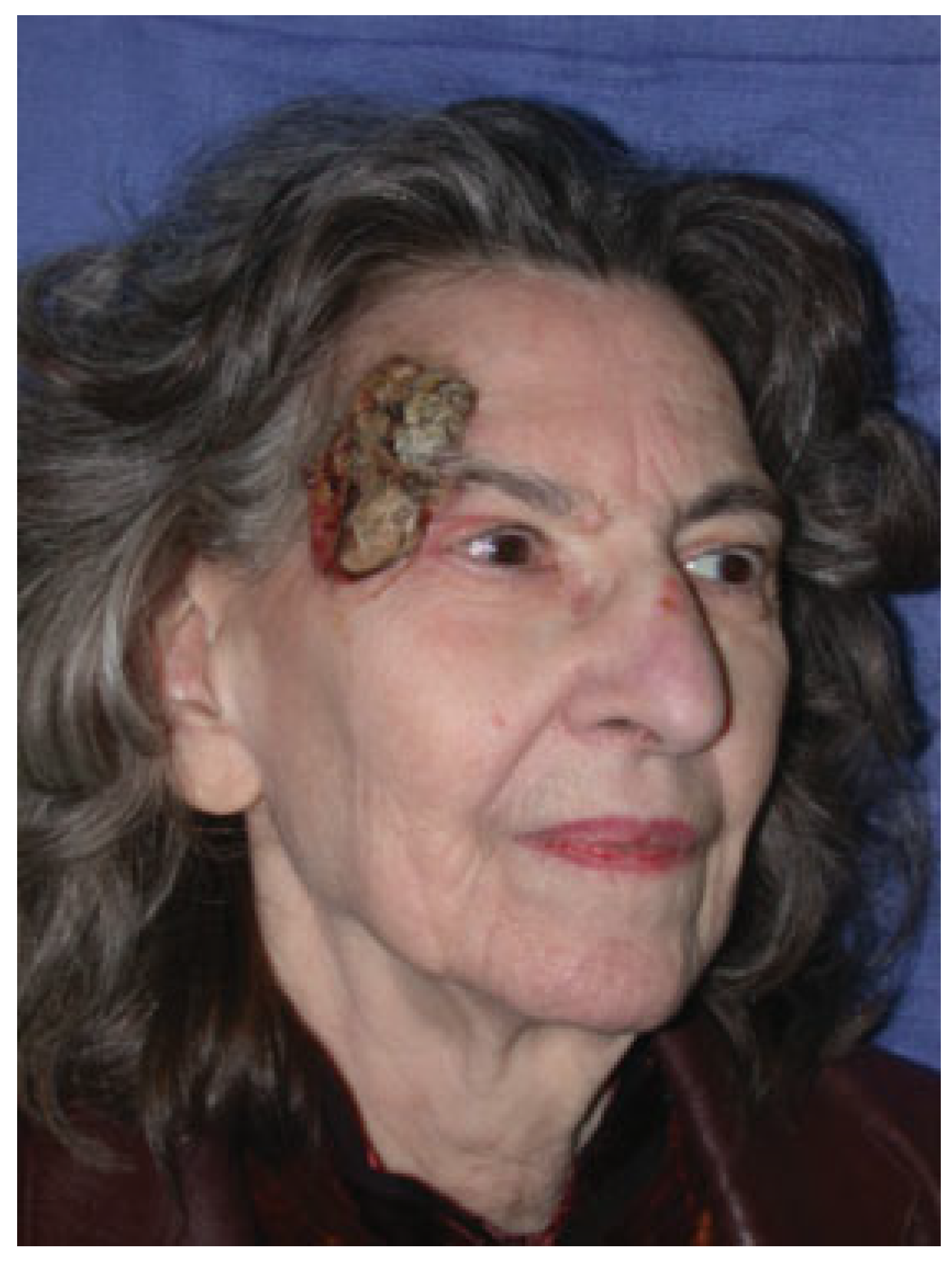
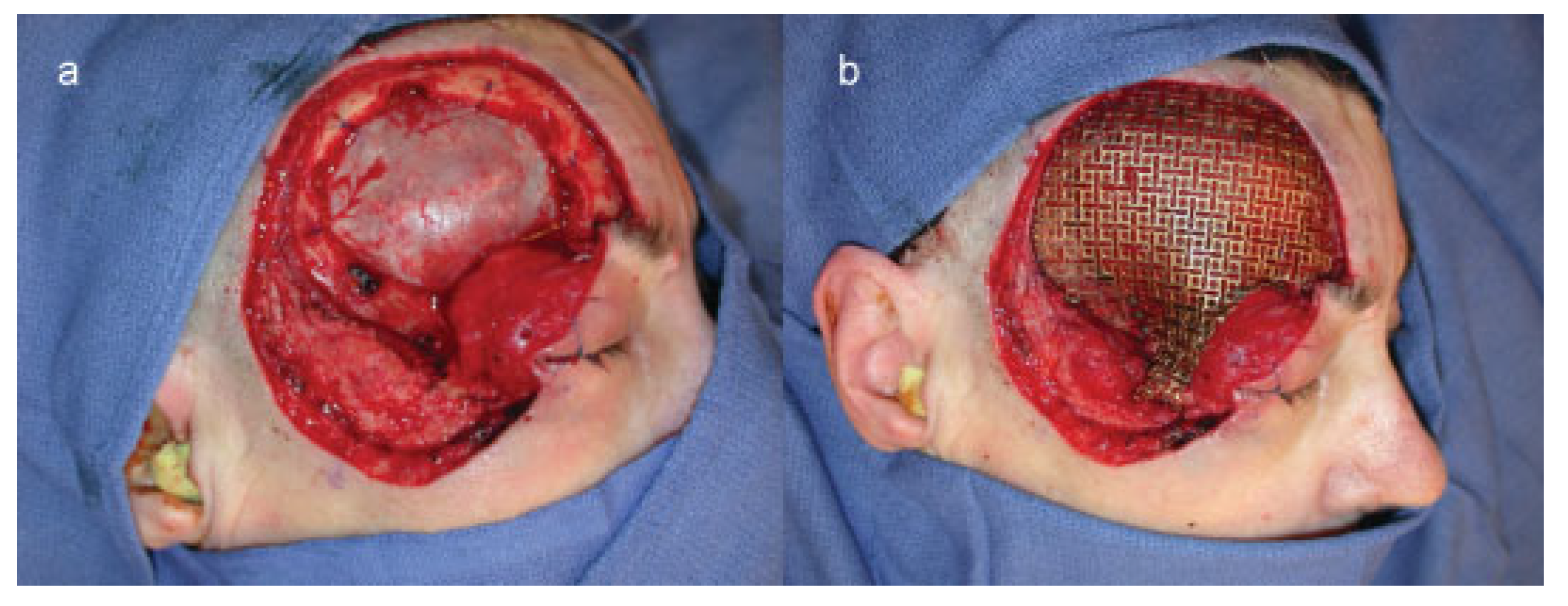
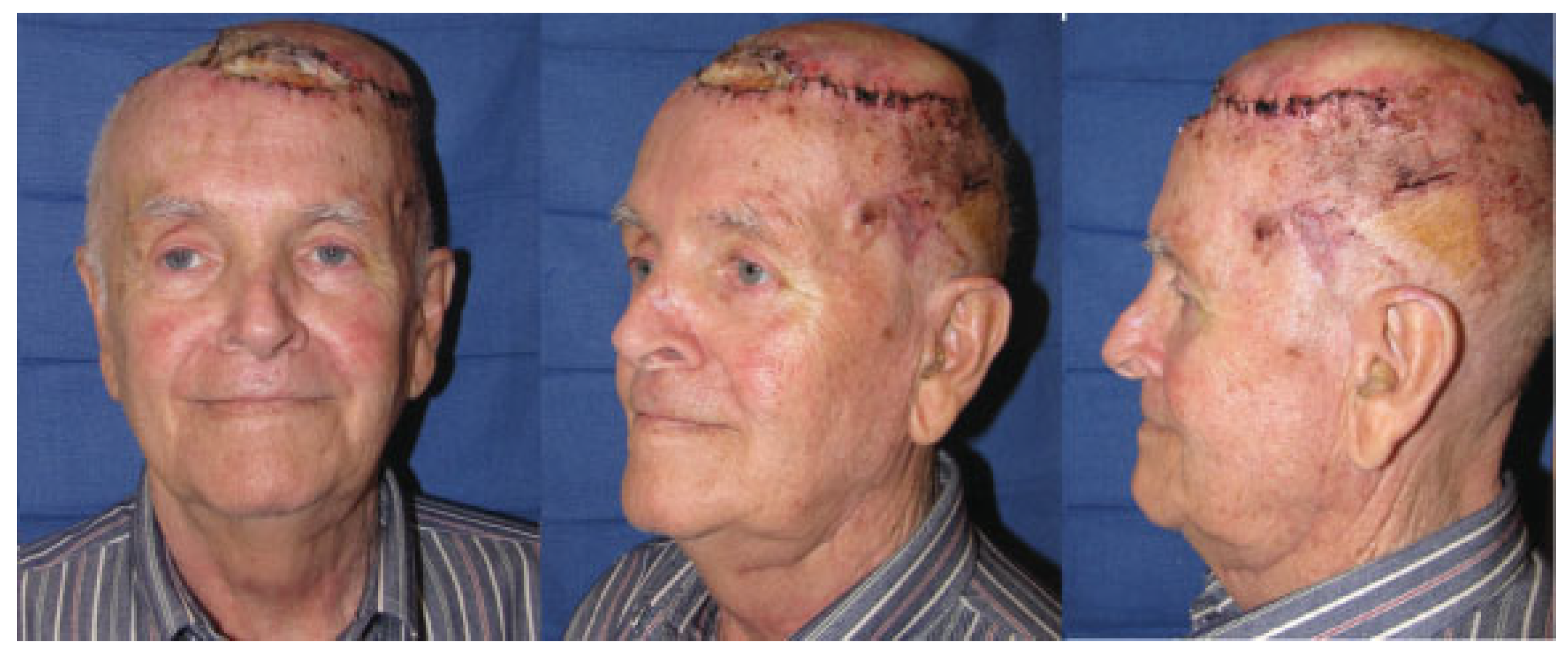
Case 1
Case 2
Case 3
Discussion
Conclusion
References
- Furnas, H.; Lineaweaver, W.C.; Alpert, B.S.; Buncke, H.J. Scalp reconstruction by microvascular free tissue transfer. Ann Plast Surg 1990, 24, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.; Zhou, S.; Jiang, K.; et al. Microsurgical replantation of the avulsed scalp: Report of 20 cases. Plast Reconstr Surg 1996, 97, 1099–1106, discussion 1107–1108. [Google Scholar] [PubMed]
- García del Campo, J.A.; García de Marcos, J.A.; del Castillo Pardo de Vera, J.L.; García de Marcos, M.J. Local flap reconstruction of large scalp defects. Med Oral Patol Oral Cir Bucal 2008, 13, E666–E670. [Google Scholar]
- Lesavoy, M.A.; Dubrow, T.J.; Schwartz, R.J.; Wackym, P.A.; Eisenhauer, D.M.; McGuire, M. Management of large scalp defects with local pedicle flaps. Plast Reconstr Surg 1993, 91, 783–790. [Google Scholar] [CrossRef]
- Newman, M.I.; Hanasono, M.M.; Disa, J.J.; Cordeiro, P.G.; Mehrara, B.J. Scalp reconstruction: A 15-year experience. Ann Plast Surg 2004, 52, 501–506, discussion 506. [Google Scholar] [CrossRef] [PubMed]
- Labow, B.I.; Rosen, H.; Pap, S.A.; Upton, J. Microsurgical reconstruction: A more conservative method of managing large scalp defects? J Reconstr Microsurg 2009, 25, 465–474. [Google Scholar] [CrossRef]
- Wang, H.T.; Erdmann, D.; Olbrich, K.C.; Friedman, A.H.; Levin, L.S.; Zenn, M.R. Free flap reconstruction of the scalp and calvaria of major neurosurgical resections in cancer patients: Lessons learned closing large, difficult wounds of the dura and skull. Plast Reconstr Surg 2007, 119, 865–872. [Google Scholar] [CrossRef]
- Mehrara, B.J.; Disa, J.J.; Pusic, A. Scalp reconstruction. J Surg Oncol 2006, 94, 504–508. [Google Scholar] [CrossRef]
- Werner, C.A. The Older Population: 2010 Census Briefs. C2010BR-09; US Department of Commerce Economics and Statistics Administration: Washington, DC, USA, 2011. [Google Scholar]
- Dhiwakar, M.; Khan, N.A.; McClymont, L.G. Surgery for head and neck skin tumors in the elderly. Head Neck 2007, 29, 851–856. [Google Scholar] [CrossRef]
- Carey, J.N.; Watt, A.J.; Ho, O.; Zeidler, K.; Lee, G.K. Free flap scalp reconstruction in a 91-year-old patient under local-regional anesthesia: Case report and review of the literature. J Reconstr Microsurg 2012, 28, 189–193. [Google Scholar] [CrossRef]
- Shestak, K.C.; Jones, N.F. Microsurgical free-tissue transfer in the elderly patient. Plast Reconstr Surg 1991, 88, 259–263. [Google Scholar] [PubMed]
- Shestak, K.C.; Jones, N.F.; Wu, W.; Johnson, J.T.; Myers, E.N. Effect of advanced age and medical disease on the outcome of microvascular reconstruction for head and neck defects. Head Neck 1992, 14, 14–18. [Google Scholar] [PubMed]
- Howard, M.A.; Cordeiro, P.G.; Disa, J.; et al. Free tissue transfer in the elderly: Incidence of perioperative complications following microsurgical reconstruction of 197 septuagenarians and octogenarians. Plast Reconstr Surg 2005, 116, 1659–1668, discussion 1669–1671. [Google Scholar]
- Kim, H.K.; Park, B.; Bae, T.H.; Kim, W.S. Comparative study of the postoperative complications of microvascular surgery in elderly and young patients. J Reconstr Microsurg 2011, 27, 219–224. [Google Scholar]
- Shonka, D.C., Jr.; Potash, A.E.; Jameson, M.J.; Funk, G.F. Successful reconstruction of scalp and skull defects: Lessons learned from a large series. Laryngoscope 2011, 121, 2305–2312. [Google Scholar] [PubMed]
- Ozkan, O.; Ozgentas, H.E.; Islamoglu, K.; Boztug, N.; Bigat, Z.; Dikici, M.B. Experiences with microsurgical tissue transfers in elderly patients. Microsurgery 2005, 25, 390–395. [Google Scholar]
- Ozkan, O.; Coskunfirat, O.K.; Ozgentas, H.E.; Derin, A. Rationale for reconstruction of large scalp defects using the anterolateral thigh flap: Structural and aesthetic outcomes. J Reconstr Microsurg 2005, 21, 539–545. [Google Scholar]
- Chao, A.H.; Yu, P.; Skoracki, R.J.; Demonte, F.; Hanasono, M.M. Microsurgical reconstruction of composite scalp and calvarial defects in patients with cancer: A 10-year experience. Head Neck 2012, 34, 1759–1764. [Google Scholar]
- Shaari, C.M.; Buchbinder, D.; Costantino, P.D.; Lawson, W.; Biller, H.F.; Urken, M.L. Complications of microvascular head and neck surgery in the elderly. Arch Otolaryngol Head Neck Surg 1998, 124, 407–411. [Google Scholar]
- Jones, N.F.; Jarrahy, R.; Song, J.I.; Kaufman, M.R.; Markowitz, B. Postoperative medical complications—Not microsurgical complications—Negatively influence the morbidity, mortality, and true costs after microsurgical reconstruction for head and neck cancer. Plast Reconstr Surg 2007, 119, 2053–2060. [Google Scholar]
- Derks, W.; de Leeuw, R.J.; Hordijk, G.J. Elderly patients with head and neck cancer: The influence of comorbidity on choice of therapy, complication rate, and survival. Curr Opin Otolaryngol Head Neck Surg 2005, 13, 92–96. [Google Scholar]
- Chick, L.R.; Walton, R.L.; Reus, W.; Colen, L.; Sasmor, M. Free flaps in the elderly. Plast Reconstr Surg 1992, 90, 87–94. [Google Scholar] [PubMed]
- Coskunfirat, O.K.; Chen, H.C.; Spanio, S.; Tang, Y.B. The safety of microvascular free tissue transfer in the elderly population. Plast Reconstr Surg 2005, 115, 771–775. [Google Scholar] [PubMed]
- Chang, K.P.; Lai, C.H.; Chang, C.H.; Lin, C.L.; Lai, C.S.; Lin, S.D. Free flap options for reconstruction of complicated scalp and calvarial defects: Report of a series of cases and literature review. Microsurgery 2010, 30, 13–18. [Google Scholar] [PubMed]
- Schuurmans, H.; Steverink, N.; Lindenberg, S.; Frieswijk, N.; Slaets, J.P. Old or frail: What tells us more? J Gerontol A Biol Sci Med Sci 2004, 59, M962–M965. [Google Scholar]
- Yoshioka, N.; Haraoka, G.; Muraoka, M.; Tominaga, S. Single stage reconstruction of scalp and skull using free muscle flap and titanium mesh in patients with epidural infection. J Craniomaxillofac Surg 1996, 24, 118–121. [Google Scholar]
- van der Schroeff, M.P.; Derks, W.; Hordijk, G.J.; de Leeuw, R.J. The effect of age on survival and quality of life in elderly head and neck cancer patients: A long-term prospective study. Eur Arch Otorhinolaryngol 2007, 264, 415–422. [Google Scholar]
- Derks, W.; De Leeuw, J.R.; Hordijk, G.J.; Winnubst, J.A. Elderly patients with head and neck cancer: Short-term effects of surgical treatment on quality of life. Clin Otolaryngol Allied Sci 2003, 28, 399–405. [Google Scholar]
- Herrera, F.; Buntic, R.; Brooks, D.; Buncke, G.; Antony, A.K. Microvascular approach to scalp replantation and reconstruction: A thirty-six year experience. Microsurgery 2012, 32, 591–597. [Google Scholar] [CrossRef]
- Lee, S.H.; Mun, G.H. Transverse thoracodorsal artery perforator flaps: Experience with 31 free flaps. J Plast Reconstr Aesthet Surg 2008, 61, 372–379. [Google Scholar] [CrossRef]
- Isenberg, J.S.; Costigan, W. Microvascular transplantation in the salvage of lower extremity trauma in the elderly. Ann Plast Surg 1996, 36, 273–275. [Google Scholar]
- Wester, J.L.; Lindau, R.H.; Wax, M.K. Efficacy of free flap reconstruction of the head and neck in patients 90 years and older. JAMA Otolaryngol Head Neck Surg 2013, 139, 49–53. [Google Scholar]
- Chang, E.I.; Vaca, L.; DaLio, A.L.; Festekjian, J.H.; Crisera, C.A. Assessment of advanced age as a risk factor in microvascular breast reconstruction. Ann Plast Surg 2011, 67, 255–259. [Google Scholar]
- Barzin, A.; Hernandez-Boussard, T.; Lee, G.K.; Curtin, C. Adverse events following digital replantation in the elderly. J Hand Surg Am 2011, 36, 870–874. [Google Scholar] [PubMed]
- McLean, D.H.; Buncke, H.J., Jr. Autotransplant of omentum to a large scalp defect, with microsurgical revascularization. Plast Reconstr Surg 1972, 49, 268–274. [Google Scholar] [PubMed]
- Lynch, J.R.; Hansen, J.E.; Chaffoo, R.; Seyfer, A.E. The lower trapezius musculocutaneous flap revisited: Versatile coverage for complicated wounds to the posterior cervical and occipital regions based on the deep branch of the transverse cervical artery. Plast Reconstr Surg 2002, 109, 444–450. [Google Scholar]
- Potparić, Z.; Starović, B. Reconstruction of extensive defects of the cranium using free-tissue transfer. Head Neck 1993, 15, 97–104. [Google Scholar] [PubMed]
- De Haro, F.; Giraldo, F. Bipedicled fronto-occipital flap for reconstruction of postoncologic defects of the lateral scalp. Plast Reconstr Surg 2001, 107, 506–510. [Google Scholar]
- Borah, G.L.; Hidalgo, D.A.; Wey, P.D. Reconstruction of extensive scalp defects with rectus free flaps. Ann Plast Surg 1995, 34, 281–285, discussion 285–287. [Google Scholar]
- Kaplan, H.; Wexler, M.R.; Fiensod, M. Free groin flap for the reconstruction of the scalp following tumor resection. Ann Plast Surg 1979, 2, 445–447. [Google Scholar]
- Sweeny, L.; Eby, B.; Magnuson, J.S.; Carroll, W.R.; Rosenthal, E.L. Reconstruction of scalp defects with the radial forearm free flap. Head Neck Oncol 2012, 4, 21. [Google Scholar] [CrossRef]
- Bo, B.; Qun, Y.; Zheming, P.; Haitao, X.; Tianyi, L. Reconstruction scalp defects after malignant tumor resection with anterolateral thigh flaps. J Craniofac Surg 2011, 22, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Kobienia, B.J.; Migliori, M.; Schubert, W. Preexpanded radial forearm free flap to the scalp. Ann Plast Surg 1996, 37, 629–632. [Google Scholar] [CrossRef]
- Santamaria, E.; Granados, M.; Barrera-Franco, J.L. Radial forearm free tissue transfer for head and neck reconstruction: Versatility and reliability of a single donor site. Microsurgery 2000, 20, 195–201. [Google Scholar] [CrossRef]
- Pennington, D.G.; Stern, H.S.; Lee, K.K. Free-flap reconstruction of large defects of the scalp and calvarium. Plast Reconstr Surg 1989, 83, 655–661. [Google Scholar] [CrossRef]
- Lackey, P.L.; Sargent, L.A.; Wong, L.; Brzezienski, M.; Kennedy, J.W. Giant basal cell carcinoma surgical management and reconstructive challenges. Ann Plast Surg 2007, 58, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hierner, R.; van Loon, J.; Goffin, J.; van Calenbergh, F. Free latissimus dorsi flap transfer for subtotal scalp and cranium defect reconstruction: Report of 7 cases. Microsurgery 2007, 27, 425–428. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, D.A.; Teng, M.S.; Mendez, E.; Futran, N.D. Microvascular free tissue transfer in the reconstruction of scalp and lateral temporal bone defects. Craniomaxillofac Trauma Reconstr 2011, 4, 179–188. [Google Scholar] [CrossRef]
- Calikapan, G.T.; Yildirim, S.; Aköz, T. One-stage reconstruction of large scalp defects: Anterolateral thigh flap. Microsurgery 2006, 26, 155–159. [Google Scholar] [CrossRef]
- Kwee, M.M.; Rozen, W.M.; Ting, J.W.; Mirkazemi, M.; Leong, J.; Baillieu, C. Total scalp reconstruction with bilateral anterolateral thigh flaps. Microsurgery 2012, 32, 393–396. [Google Scholar] [CrossRef]
- Trignano, E.; Ciudad, P.; Fallico, N.; Chen, H.C. Nodular cutaneous amyloidosis of the scalp reconstructed with a free anterolateral thigh flap: A case report. J Oral Maxillofac Surg 2012, 70, e481–e483. [Google Scholar] [CrossRef] [PubMed]
- St-Hilaire, H.; Mithani, S.K.; Taylor, J.; Simmons, O.P.; Singh, N.; Rodriguez, E.D. Restoring the failed cranioplasty: Nonanatomical titanium mesh with perforator flap. Plast Reconstr Surg 2009, 123, 1813–1817. [Google Scholar] [CrossRef]
- Lutz, B.S.; Wei, F.C.; Chen, H.C.; Lin, C.H.; Wei, C.Y. Reconstruction of scalp defects with free flaps in 30 cases. Br J Plast Surg 1998, 51, 186–190. [Google Scholar] [CrossRef]
- Manders, E.K.; Schenden, M.J.; Furrey, J.A.; Hetzler, P.T.; Davis, T.S.; Graham, W.P., III. Soft-tissue expansion: Concepts and complications. Plast Reconstr Surg 1984, 74, 493–507. [Google Scholar] [CrossRef]
- Corradino, B.; Di Lorenzo, S.; Leto Barone, A.A.; Maresi, E.; Moschella, F. Reconstruction of full thickness scalp defects after tumour excision in elderly patients: Our experience with Integra dermal regeneration template. J Plast Reconstr Aesthet Surg 2010, 63, e245–e247. [Google Scholar] [CrossRef]
- Momoh, A.O.; Lypka, M.A.; Echo, A.; Rizvi, M.; Klebuc, M.; Friedman, J.D. Reconstruction of full-thickness calvarial defect: A role for artificial dermis. Ann Plast Surg 2009, 62, 656–659. [Google Scholar] [CrossRef]
- Mureau, M.A.; Posch, N.A.; Meeuwis, C.A.; Hofer, S.O. Anterolateral thigh flap reconstruction of large external facial skin defects: A followup study on functional and aesthetic recipientand donor-site outcome. Plast Reconstr Surg 2005, 115, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Zheng, P.S.; Dong, G. Morphology of aged skin. Clin Geriatr Med 1989, 5, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mun, G.H.; Lim, S.Y.; Hyon, W.S.; Bang, S.I.; Oh, K.S. A novel reconstruction of 2 distinct defects: Concomitant use of a thoracodorsal artery perforator flap and its corresponding muscle flap. Ann Plast Surg 2005, 55, 676–678. [Google Scholar] [CrossRef]
- Ayhan, S.; Tuncer, S.; Demir, Y.; Kandal, S. Thoracodorsal artery perforator flap: A versatile alternative for various soft tissue defects. J Reconstr Microsurg 2008, 24, 285–293. [Google Scholar] [CrossRef]
- van Driel, A.A.; Mureau, M.A.; Goldstein, D.P.; et al. Aesthetic and oncologic outcome after microsurgical reconstruction of complex scalp and forehead defects after malignant tumor resection: An algorithm for treatment. Plast Reconstr Surg 2010, 126, 460–470. [Google Scholar] [PubMed]
- Kruse-Lösler, B.; Presser, D.; Meyer, U.; Schul, C.; Luger, T.; Joos, U. Reconstruction of large defects on the scalp and forehead as an interdisciplinary challenge: Experience in the management of 39 cases. Eur J Surg Oncol 2006, 32, 1006–1014. [Google Scholar] [PubMed]
- Afifi, A.; Djohan, R.S.; Hammert, W.; Papay, F.A.; Barnett, A.E.; Zins, J.E. Lessons learned reconstructing complex scalp defects using free flaps and a cranioplasty in one stage. J Craniofac Surg 2010, 21, 1205–1209. [Google Scholar] [PubMed]
- Lutz, B.S. Aesthetic and functional advantages of the anterolateral thigh flap in reconstruction of tumor-related scalp defects. Microsurgery 2002, 22, 258–264. [Google Scholar]

 |
 |
© 2014 by the author. The Author(s) 2014.
Share and Cite
Sosin, M.; Chaudhry, A.; Cruz, C.D.L.; Bojovic, B.; Manson, P.N.; Rodriguez, E.D. Lessons Learned in Scalp Reconstruction and Tailoring Free Tissue Transfer in the Elderly: A Case Series and Literature Review. Craniomaxillofac. Trauma Reconstr. 2015, 8, 179-189. https://doi.org/10.1055/s-0034-1393725
Sosin M, Chaudhry A, Cruz CDL, Bojovic B, Manson PN, Rodriguez ED. Lessons Learned in Scalp Reconstruction and Tailoring Free Tissue Transfer in the Elderly: A Case Series and Literature Review. Craniomaxillofacial Trauma & Reconstruction. 2015; 8(3):179-189. https://doi.org/10.1055/s-0034-1393725
Chicago/Turabian StyleSosin, Michael, Arif Chaudhry, Carla De La Cruz, Branko Bojovic, Paul N. Manson, and Eduardo D. Rodriguez. 2015. "Lessons Learned in Scalp Reconstruction and Tailoring Free Tissue Transfer in the Elderly: A Case Series and Literature Review" Craniomaxillofacial Trauma & Reconstruction 8, no. 3: 179-189. https://doi.org/10.1055/s-0034-1393725
APA StyleSosin, M., Chaudhry, A., Cruz, C. D. L., Bojovic, B., Manson, P. N., & Rodriguez, E. D. (2015). Lessons Learned in Scalp Reconstruction and Tailoring Free Tissue Transfer in the Elderly: A Case Series and Literature Review. Craniomaxillofacial Trauma & Reconstruction, 8(3), 179-189. https://doi.org/10.1055/s-0034-1393725



