Optimal Duration of Antibiotic Therapy for Space Infections in the Maxillofacial Region: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Review Protocol
2.2. Research Question
2.3. Information Sources and Search Strategies
2.4. Eligibility Criteria
- Population: Individuals diagnosed with maxillofacial space infections of odontogenic origin.
- Exposure: Administration of oral or parenteral antibiotics as part of the treatment for odontogenic and maxillofacial space infections.
- Outcome: Clinical outcomes associated with antibiotic therapy, specifically the effectiveness and optimal duration of antibiotic regimens used as adjuvant treatment following surgical intervention.
2.5. Study Selection and Data Extraction
2.6. Quality Assessment
3. Results
3.1. Selection of Studies
3.2. Study Characteristics
3.3. Antibiotic Protocols Utilized
- Beta-lactams (used in eight studies): amoxicillin (±clavulanic acid), cephalexin (±CV), cefuroxime, and phenoxymethylpenicillin.
- Nitroimidazoles: metronidazole (used in five studies).
- Macrolides: azithromycin and erythromycin (used in two studies).
- Lincosamides: clindamycin (used in three studies).
- Fluoroquinolones: moxifloxacin (used in one study).
- Aminoglycosides: amikacin (used in one study).
- Oral: used in seven studies.
- Parenteral (IV): reported in two studies (Keswani et al., Bali et al.).
- Mixed: some protocols shifted from IV to oral based on clinical improvement.
3.4. Clinical Outcomes of Antibiotics for OI
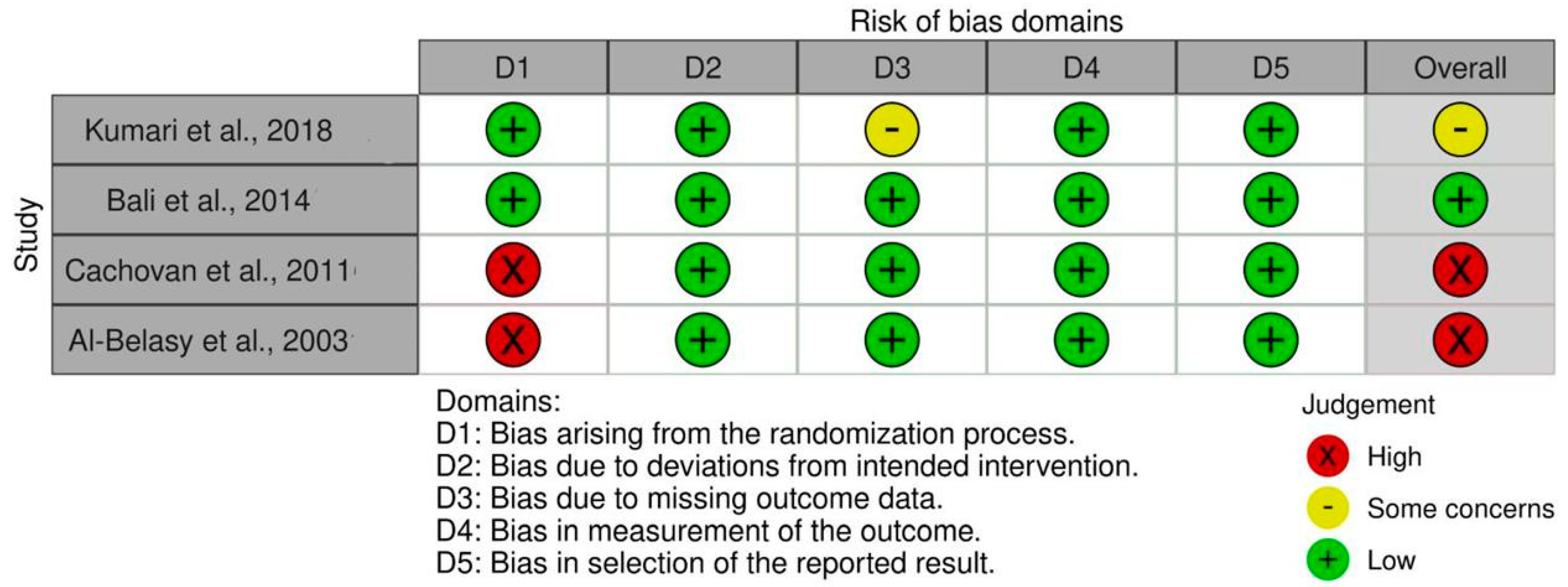
3.5. Assessment of the Quality of the Examined Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kudiyirickal, M.G.; Hollinshead, F. Clinical profile of orofacial infections: An experience from two primary care dental practices. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e533–e537. [Google Scholar] [CrossRef] [PubMed]
- Nadig, K.; Taylor, N.G. Management of odontogenic infection at a district general hospital. Br. Dent. J. 2018, 224, 962–966. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.; Mathur, A.; Kohli, M.; Siddiqui, S.R. In vitro evaluation of microbiological flora of orofacial infections. J. Maxillofac. Oral Surg. 2009, 8, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Igoumenakis, D.; Gkinis, G.; Kostakis, G.; Mezitis, M.; Rallis, G. Severe odontogenic infections: Causes of spread and their management. Surg. Infect. (Larchmt) 2014, 15, 64–68. [Google Scholar] [CrossRef]
- Bascones Martínez, A.; Aguirre Urízar, J.M.; Bermejo Fenoll, A.; Blanco Carrión, A.; Gay-Escoda, C.; González-Moles, M.A.; Gutiérrez Pérez, J.L.; Jiménez Soriano, Y.; Liébana Ureña, J.; López Marcos, J.F.; et al. Consensus statement on antimicrobial treatment of odontogenic bacterial infections. Med. Oral Patol. Oral Cir. Bucal 2004, 9, 369–376. [Google Scholar]
- Koyuncuoglu, C.Z.; Aydin, M.; Kirmizi, N.I.; Aydin, V.; Aksoy, M.; Isli, F.; Akici, A. Rational use of medicine in dentistry: Do dentists prescribe antibiotics in appropriate indications? Eur. J. Clin. Pharmacol. 2017, 73, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.R.; Chagas, O.L., Jr.; Velasques, B.D.; Bobrowski, Â.N.; Correa, M.B.; Torriani, M.A. The Use of Antibiotics in Odontogenic Infections: What Is the Best Choice? A Systematic Review. J. Oral Maxillofac. Surg. 2017, 75, 2606.e1–2606.e11. [Google Scholar] [CrossRef]
- Halling, F.; Neff, A.; Heymann, P.; Ziebart, T. Trends in antibiotic prescribing by dental practitioners in Germany. J. Craniomaxillofac. Surg. 2017, 45, 1854–1859. [Google Scholar] [CrossRef]
- Rodríguez Sánchez, F.; Arteagoitia, I.; Teughels, W.; Rodríguez Andrés, C.; Quirynen, M. Antibiotic dosage prescribed in oral implant surgery: A meta-analysis of cross-sectional surveys. PLoS ONE 2020, 15, e0236981. [Google Scholar] [CrossRef]
- Poveda Roda, R.; Bagan, J.V.; Sanchis Bielsa, J.M.; Carbonell Pastor, E. Antibiotic use in dental practice. A review. Med. Oral Patol. Oral Cir. Bucal 2007, 12, E186–E192. [Google Scholar]
- Ahmadi, H.; Ebrahimi, A.; Ahmadi, F. Antibiotic Therapy in Dentistry. Int. J. Dent. 2021, 2021, 6667624. [Google Scholar] [CrossRef] [PubMed]
- Bassiony, M.; Yang, J.; Abdel-Monem, T.M.; Elmogy, S.; Elnagdy, M. Exploration of ultrasonography in assessment of fascial space spread of odontogenic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 861–869. [Google Scholar] [CrossRef]
- Rega, A.J.; Aziz, S.R.; Ziccardi, V.B. Microbiology and antibiotic sensitivities of head and neck space infections of odontogenic origin. J. Oral Maxillofac. Surg. 2006, 64, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Stefanopoulos, P.K.; Kolokotronis, A.E. The clinical significance of anaerobic bacteria in acute orofacial odontogenic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 398–408. [Google Scholar] [CrossRef]
- Flynn, T.R.; Shanti, R.M.; Levi, M.H.; Adamo, A.K.; Kraut, R.A.; Trieger, N. Severe odontogenic infections, part 1: Prospective report. J. Oral Maxillofac. Surg. 2006, 64, 1093–1103. [Google Scholar] [CrossRef]
- Ribeiro, E.D.; Santana, I.H.G.; Viana, M.R.M.; Fan, S.; Mohamed, A.; Dias, J.C.P.; Forte, A.G.; Pereira Júnior, J.M.; Ferreira, A.J.; Sant’Ana, E. Optimal treatment time with systemic antimicrobial therapy in odontogenic infections affecting the jaws: A systematic review. BMC Oral Health 2025, 25, 253. [Google Scholar] [CrossRef]
- Page, M.J.; Mckenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Savović, J.; Page, M.; Elbers, R.; Sterne, J. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Version 64 (Updated August 2023); Cochrane: London, UK, 2023; Available online: www.training.cochrane.org/handbook (accessed on 13 March 2025).
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Banerjee, K.; Kakkar, A.; Shamsi, K.A.; Bansal, D.; Mathur, P.; Potode, N.M.; Pagariya, P.; Azher, S.P.; Chaudhari, A.; Mandal, R.; et al. Effectiveness of Oral Cephalexin-Clavulanic Acid, Cefuroxime, and Amoxicillin-Clavulanic Acid in the Management of Dental Infections: A Real-World, Retrospective, Electronic Medical Record-Based Study in India. Drugs Real. World Outcomes 2024, 11, 53–68. [Google Scholar] [CrossRef]
- Keswani, E.S.; Venkateshwar, G. Odontogenic Maxillofacial Space Infections: A 5-Year Retrospective Review in Navi Mumbai. J. Maxillofac. Oral Surg. 2019, 18, 345–353. [Google Scholar] [CrossRef]
- Kumari, S.; Mohanty, S.; Sharma, P.; Dabas, J.; Kohli, S.; Diana, C. Is the routine practice of antibiotic prescription and microbial culture and antibiotic sensitivity testing justified in primary maxillofacial space infection patients? A prospective, randomized clinical study. J. Craniomaxillofac. Surg. 2018, 46, 446–452. [Google Scholar] [CrossRef]
- Bali, R.; Sharma, P.; Gaba, S. Use of metronidazole as part of an empirical antibiotic regimen after incision and drainage of infections of the odontogenic spaces. Br. J. Oral Maxillofac. Surg. 2015, 53, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Cachovan, G.; Böger, R.H.; Giersdorf, I.; Hallier, O.; Streichert, T.; Haddad, M.; Platzer, U.; Schön, G.; Wegscheider, K.; Sobottka, I. Comparative efficacy and safety of moxifloxacin and clindamycin in the treatment of odontogenic abscesses and inflammatory infiltrates: A phase II, double-blind, randomized trial. Antimicrob. Agents Chemother. 2011, 55, 1142–1147. [Google Scholar] [CrossRef]
- Ellison, S.J. An outcome audit of three-day antimicrobial prescribing for the acute dentoalveolar abscess. Br. Dent. J. 2011, 211, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Matijević, S.; Lazić, Z.; Kuljić-Kapulica, N.; Nonković, Z. Empirical antimicrobial therapy of acute dentoalveolar abscess. Vojnosanit. Pregl. 2009, 66, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, T.; Absi, E.G.; Williams, D.W.; Lewis, M.A. An outcome audit of the treatment of acute dentoalveolar infection: Impact of penicillin resistance. Br. Dent. J. 2005, 198, 759–763, discussion 754; quiz 778. [Google Scholar] [CrossRef]
- Al-Belasy, F.A.; Hairam, A.R. The efficacy of azithromycin in the treatment of acute infraorbital space infection. J. Oral Maxillofac. Surg. 2003, 61, 310–316. [Google Scholar] [CrossRef]
- Lang, P.M.; Jacinto, R.C.; Dal Pizzol, T.S.; Ferreira, M.B.; Montagner, F. Resistance profiles to antimicrobial agents in bacteria isolated from acute endodontic infections: Systematic review and meta-analysis. Int. J. Antimicrob. Agents 2016, 48, 467–474. [Google Scholar] [CrossRef]
- Shilnikova, I.I.; Dmitrieva, N.V. Evaluation of antibiotic susceptibility of Bacteroides, Prevotella and Fusobacterium species isolated from patients of the N. N. Blokhin Cancer Research Center, Moscow, Russia. Anaerobe 2015, 31, 15–18. [Google Scholar] [CrossRef]
- Bancescu, G.; Didilescu, A.; Bancescu, A.; Bari, M. Antibiotic susceptibility of 33 Prevotella strains isolated from Romanian patients with abscesses in head and neck spaces. Anaerobe 2015, 35 Pt A, 41–44. [Google Scholar] [CrossRef]
- Veloo, A.C.M.; Tokman, H.B.; Jean-Pierre, H.; Dumont, Y.; Jeverica, S.; Lienhard, R.; Novak, A.; Rodloff, A.; Rotimi, V.; Wybo, I.; et al. Antimicrobial susceptibility profiles of anaerobic bacteria, isolated from human clinical specimens, within different European and surrounding countries. A joint ESGAI study. Anaerobe 2020, 61, 102111. [Google Scholar] [CrossRef] [PubMed]
- Liau, I.; Han, J.; Bayetto, K.; May, B.; Goss, A.; Sambrook, P.; Cheng, A. Antibiotic resistance in severe odontogenic infections of the South Australian population: A 9-year retrospective audit. Aust. Dent. J. 2018, 63, 187–192. [Google Scholar] [CrossRef]
- Teoh, L.; Cheung, M.C.; Dashper, S.; James, R.; McCullough, M.J. Oral Antibiotic for Empirical Management of Acute Dentoalveolar Infections-A Systematic Review. Antibiotics 2021, 10, 240. [Google Scholar] [CrossRef] [PubMed]
- Bahl, R.; Sandhu, S.; Singh, K.; Sahai, N.; Gupta, M. Odontogenic infections: Microbiology and management. Contemp. Clin. Dent. 2014, 5, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Sato, F.R.; Hajala, F.A.; Freire Filho, F.W.; Moreira, R.W.; de Moraes, M. Eight-year retrospective study of odontogenic origin infections in a postgraduation program on oral and maxillofacial surgery. J. Oral Maxillofac. Surg. 2009, 67, 1092–1097. [Google Scholar] [CrossRef]
- Heimdahl, A.; von Konow, L.; Satoh, T.; Nord, C.E. Clinical appearance of orofacial infections of odontogenic origin in relation to microbiological findings. J. Clin. Microbiol. 1985, 22, 299–302. [Google Scholar] [CrossRef]
- Durkin, M.J.; Hsueh, K.; Sallah, Y.H.; Feng, Q.; Jafarzadeh, S.R.; Munshi, K.D.; Lockhart, P.B.; Thornhill, M.H.; Henderson, R.R.; Fraser, V.J. An evaluation of dental antibiotic prescribing practices in the United States. J. Am. Dent. Assoc. 2017, 148, 878–886.e1. [Google Scholar] [CrossRef]
- Gross, A.E.; Hanna, D.; Rowan, S.A.; Bleasdale, S.C.; Suda, K.J. Successful Implementation of an Antibiotic Stewardship Program in an Academic Dental Practice. Open Forum Infect. Dis. 2019, 6, ofz067. [Google Scholar] [CrossRef]
- Suda, K.J.; Henschel, H.; Patel, U.; Fitzpatrick, M.A.; Evans, C.T. Use of Antibiotic Prophylaxis for Tooth Extractions, Dental Implants, and Periodontal Surgical Procedures. Open Forum Infect. Dis. 2017, 5, ofx250. [Google Scholar] [CrossRef]
- Löffler, C.; Böhmer, F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care-A systematic review. PLoS ONE 2017, 12, e0188061. [Google Scholar] [CrossRef]
- Lockhart, P.B.; Tampi, M.P.; Abt, E.; Aminoshariae, A.; Durkin, M.J.; Fouad, A.F.; Gopal, P.; Hatten, B.W.; Kennedy, E.; Lang, M.S.; et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J. Am. Dent. Assoc. 2019, 150, 906–921.e12. [Google Scholar] [CrossRef] [PubMed]
- Llewelyn, M.J.; Fitzpatrick, J.M.; Darwin, E.; Tonkin-Crine, S.; Gorton, C.; Paul, J.; Peto, T.E.A.; Yardley, L.; Hopkins, S.; Walker, A.S. The antibiotic course has had its day. BMJ 2017, 358, j3418. [Google Scholar] [CrossRef]
- Natarajan, B.; Balakrishnan, G.; Thangavelu, K. Comparison of efficacy of amoxicillin versus ciprofloxacin in postsurgical management of transalveolar extraction. J Pharm Bioallied Sci. 2017, 9 (Suppl. 1), S187–S190. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohanty, R.; Awasthi, N.; Hosmani, S.B.; Sankaranarayanan, A.I.; Oberoi, N.H.; Singh, P.K.; Singh, N.; Patel, D. Comparing the efficacy of postoperative antibiotic regimens in the treatment of maxillofacial fractures: A prospective study. J. Contemp. Dent. Pract. 2023, 24, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Luaces-Rey, R.; Arenaz-Búa, J.; Lopez-Cedrun-Cembranos, J.L.; Martínez-Roca, C.; Pértega-Díaz, S.; Sironvalle-Soliva, S. Efficacy and safety comparison of two amoxicillin administration schedules after third molar removal. A randomized, double-blind and controlled clinical trial. Med. Oral Patol. Oral Cir. Bucal. 2010, 15, e633–e638. [Google Scholar] [CrossRef] [PubMed]
- Arteagoitia, I.; Diez, A.; Barbier, L.; Santamaría, G.; Santamaría, J. Efficacy of amoxicillin/clavulanic acid in preventing infectious and inflammatory complications following impacted mandibular third molar extraction. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, e11–e18. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Mehdi, S.Z.; Motiwala, Z.; Iqbal, H.U. Comparative analysis of treatment outcomes of amoxicillin and cephedrine in oral and dental infections. Pak. J. Med. Health Sci. 2021, 15, 2833–2836. [Google Scholar] [CrossRef]


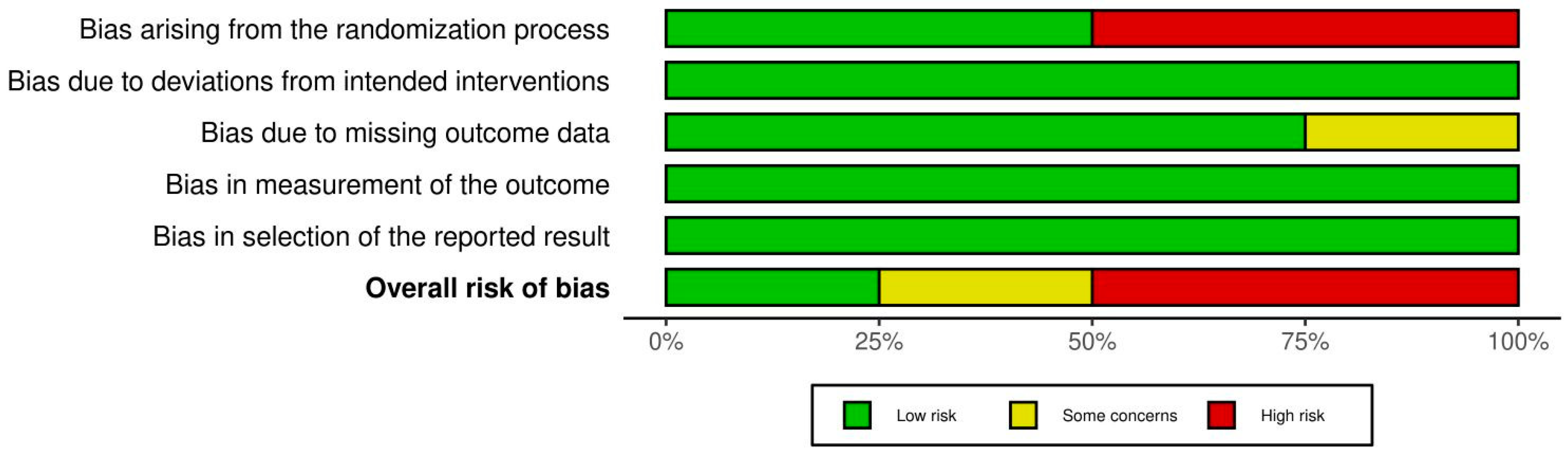
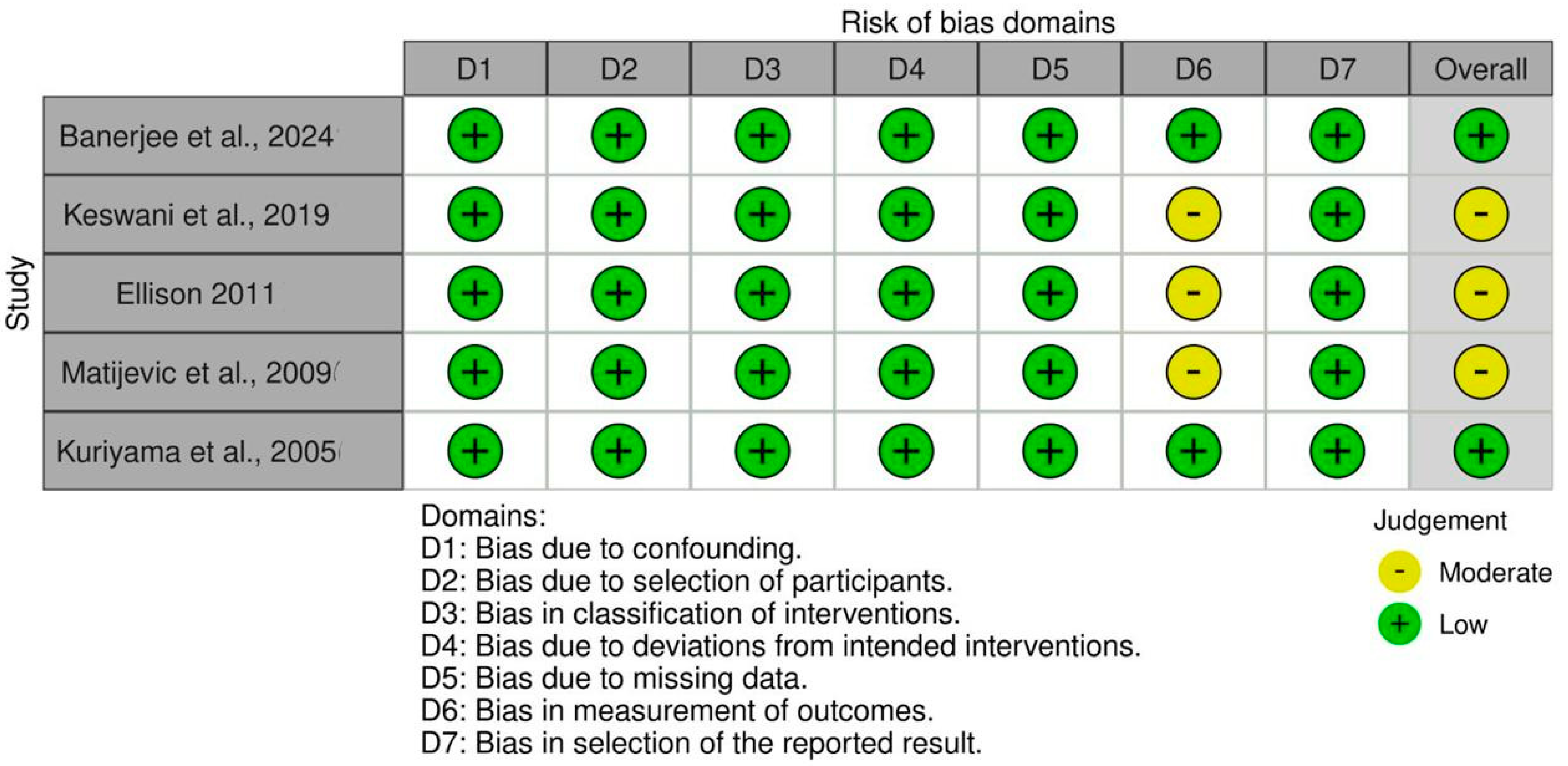
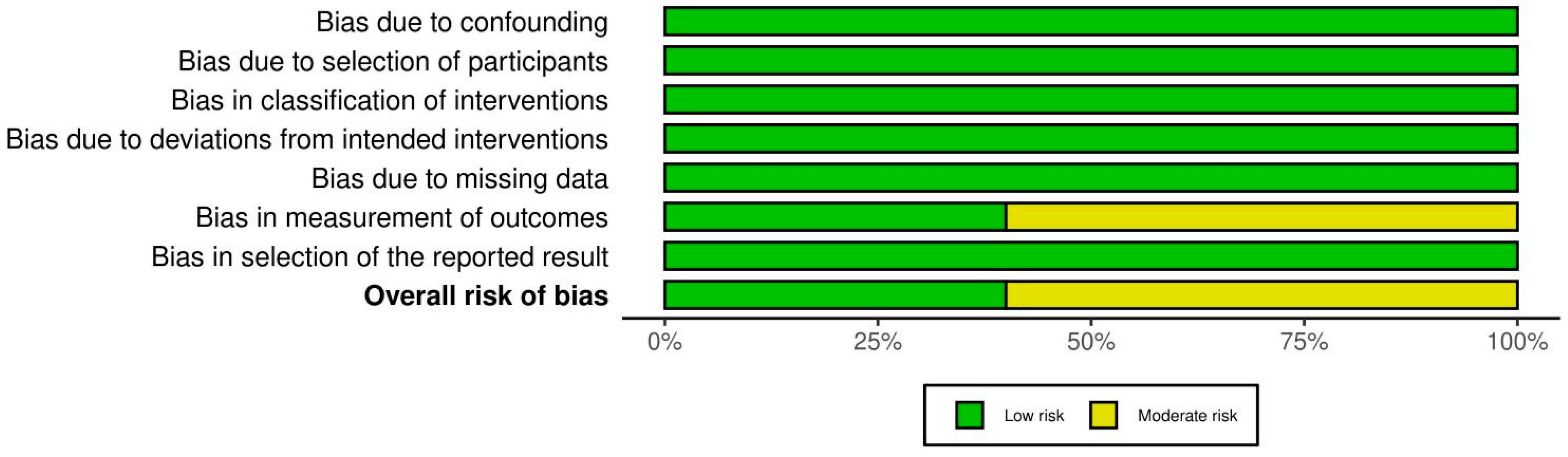
| Study | Study Design | Sample Size (Age Range/Mean) | Intervention Type | Antibiotics Used (Dosage, Route, Duration) |
|---|---|---|---|---|
| Banerjee et al., 2024 [20] | Retrospective, multicentric | 355 adults (mean 39 years) | Oral antibiotics | Cephalexin–CV (375–750 + 125 mg); co-amoxiclav 625 mg; cefuroxime 250–500 mg for ~5 days |
| Keswani et al., 2019 [21] | Retrospective | 315 (mean ~38 years) | Intraoral/extraoral I&D | IV amoxicillin–CV 1.2 g BD; metronidazole; amikacin |
| Kumari et al., 2018 [22] | RCT (Prospective) | 40 (10–50 years, mean 27 years) | Extraction + I&D | Amoxicillin–CV 625 mg + metronidazole 400 mg TID vs. no antibiotics |
| Bali et al., 2014 [23] | RCT (double-blind) | 60 (mean 33 years) | I&D | Amoxicillin–CV + metronidazole IV, 8-hourly |
| Cachovan et al., 2011 [24] | RCT (double-blind) | 31 (>18 years) | Surgical + extraction | Clindamycin 300 mg QID or moxifloxacin 400 mg OD for 5 days |
| Ellison, 2011 [25] | Retrospective | 188 (>18 years) | Drainage + extraction | Amoxicillin, metronidazole, clindamycin—all for 3 days |
| Matijevic et al., 2009 [26] | Prospective comparative | 90 | Extraction and/or I&D | Amoxicillin or cefalexin 500 mg QID for ~5 days |
| Kuriyama et al., 2005 [27] | Retrospective | 112 (17–81 years) | Drainage | Multiple regimens—2–3 days |
| Al-Belasy et al., 2003 [28] | RCT (prospective) | 60 (18–47 years) | Extraction ± I&D | Azithromycin 500 mg OD, erythromycin 250 mg QID, or none |
| Author-Year | Outcome Summary |
|---|---|
| Banerjee et al. [20] | Cephalexin–CV showed faster symptom resolution than co-amoxiclav and cefuroxime. |
| Keswani et al. [21] | Infections resolved within 72 h with IV antibiotics and early intervention. |
| Kumari et al. [22] | Similar recovery in both groups; 75% showed drainage cessation within 3 days. |
| Bali et al. [23] | No difference in resolution between antibiotic combinations; reassessment done after 48–72 h. |
| Cachovan et al. [24] | Moxifloxacin had better tolerability than clindamycin; similar improvement in both groups. |
| Ellison [25] | Three-day antibiotics effective post-drainage for systemic dentoalveolar abscess. |
| Matijevic et al. [26] | Symptom duration ~4.5–4.7 days with antibiotics; surgery-only group took ~6.2 days to resolve. |
| Kuriyama et al. [27] | All regimens effective by 72 h; penicillin resistance did not affect outcomes. |
| Al-Belasy et al. [28] | Azithromycin showed better swelling reduction; both antibiotics improved outcomes by day 3. |
| Study | Study Design | Sample Size | I&D Approach | Findings | Limitations |
|---|---|---|---|---|---|
| Banerjee et al. (2024) [20] | Retrospective | 355 patients | Not specified | Cephalexin–clavulanic acid showed faster symptom resolution than co-amoxiclav. | Retrospective design; no randomization. |
| Keswani et al. (2019) [21] | Retrospective | 315 patients | Extraoral | Extraoral I&D cases required longer antibiotic courses than intraoral cases. | No standardized protocol; lacks RCT design. |
| Kumari et al. (2018) [22] | RCT | 40 patients | Intraoral | No significant difference between I&D alone and I&D with antibiotics. | Small sample; lacks subgroup analysis. |
| Bali et al. (2014) [23] | RCT | 60 patients | Intraoral | Metronidazole offered no added benefit over amoxicillin-clavulanic acid alone. | No culture-based pathogen analysis. |
| Cachovan et al. (2011) [24] | RCT | 31 patients | Not specified | Moxifloxacin and clindamycin had similar outcomes; moxifloxacin better tolerated. | Small sample; no long-term follow-up. |
| Ellison (2011) [25] | Retrospective | 188 patients | Intraoral | Three-day antibiotic regimens were sufficient post-I&D for acute abscesses. | No comparison to longer regimens or alternatives. |
| Matijevic et al. (2009) [26] | Prospective comparative | 90 patients | Not specified | Cephalexin and amoxicillin had similar outcomes; cephalexin slightly superior. | No bacterial resistance analysis. |
| Kuriyama et al. (2005) [27] | Retrospective | 112 patients | Not specified | Penicillin resistance did not impact outcomes when drainage was performed. | No prospective tracking of resistance. |
| Al-Belasy et al. (2003) [28] | RCT | 60 patients | Extraoral | Azithromycin more effective than erythromycin in reducing pain and swelling. | Small sample; limited long-term outcome data. |
| Study ID | Included in Ribeiro et al. [16] | Included in Current Review | Reason for Inclusion/Exclusion |
|---|---|---|---|
| Natarajan et al., [44] | Yes | Yes | Overlapping study on transalveolar extraction |
| Bali et al., [23] | Yes | Yes | Metronidazole-based therapy after I&D |
| Matijević et al., [26] | Yes | Yes | Acute dentoalveolar abscesses |
| Mohanty et al., [45] | Yes | Yes | Maxillofacial fractures and antibiotic course |
| Luaces-Rey et al., [46] | Yes | No | Focused on prophylactic regimens, not space infections |
| Arteagoitia et al., [47] | Yes | No | No data on fascial space involvement |
| Salim et al., [48] | Yes | No | Observational study with limited data on space infections |
| Banerjee et al., [20] | No | Yes | New study on submandibular and buccal infections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhudaithi, A.S.; Almutairi, F.J.; Almansour, A.S.; Aljeadi, A.A.; Kolarkodi, S.H. Optimal Duration of Antibiotic Therapy for Space Infections in the Maxillofacial Region: A Systematic Review. Craniomaxillofac. Trauma Reconstr. 2025, 18, 31. https://doi.org/10.3390/cmtr18030031
Alhudaithi AS, Almutairi FJ, Almansour AS, Aljeadi AA, Kolarkodi SH. Optimal Duration of Antibiotic Therapy for Space Infections in the Maxillofacial Region: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction. 2025; 18(3):31. https://doi.org/10.3390/cmtr18030031
Chicago/Turabian StyleAlhudaithi, Abdullah Saleh, Faris Jaser Almutairi, Abdullah Saleh Almansour, Abdurrahman Abdurrazzaq Aljeadi, and Shaul Hameed Kolarkodi. 2025. "Optimal Duration of Antibiotic Therapy for Space Infections in the Maxillofacial Region: A Systematic Review" Craniomaxillofacial Trauma & Reconstruction 18, no. 3: 31. https://doi.org/10.3390/cmtr18030031
APA StyleAlhudaithi, A. S., Almutairi, F. J., Almansour, A. S., Aljeadi, A. A., & Kolarkodi, S. H. (2025). Optimal Duration of Antibiotic Therapy for Space Infections in the Maxillofacial Region: A Systematic Review. Craniomaxillofacial Trauma & Reconstruction, 18(3), 31. https://doi.org/10.3390/cmtr18030031