Factors Influencing Mandibular Invasion, Lymph Node Metastasis and Extracapsular Spread in Squamous Cell Carcinoma of the Oral Cavity
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Setting
2.2. Participants
2.3. Evaluation and Outcome
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Interpretation
- Subcapsular Sinus Invasion: Tumor cells initially proliferate in the subcapsular sinus, progressively replacing the nodal architecture. Extracapsular spread (ECS) arises either through direct capsular penetration or via tumor emboli trapped in adjacent capsular and juxtacapsular lymphatics.
- Lymphatic Sinus Infiltration: Widespread tumor infiltration of the lymphatic sinuses occurs while sparing germinal centers and trabeculae. ECS may develop through mechanisms similar to those described above.
- Concurrent Intra- and Extranodal Proliferation: Tumor cells exhibit simultaneous and proportional growth both inside the lymph node and in surrounding tissues.
- Extranodal Embolic Growth: Tumor emboli proliferate without significant intranodal involvement, potentially leading to early ECS in the disease course.
4.2. Limitations
- Prior radiotherapy and secondary tumors exhibit different POI, which was not taken into consideration in our study.
- Several other factors like comorbidities, lifestyle, and patient physical status might impact survival. Further studies are necessary to evaluate the combined effects of each of these factors on survival.
- In our study, the pattern of invasion was determined histologically. Various imaging modalities have been shown to determine the pattern of invasion, comparison of which was not performed in our study.
- Smaller sample size and retrospective nature of the study.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarode, G.; Maniyar, N.; Sarode, S.C.; Jafer, M.; Patil, S.; Awan, K.H. Epidemiologic aspects of oral cancer. Dis. Mon. 2020, 66, 100988. [Google Scholar] [CrossRef]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef]
- Arora, A.; Husain, N.; Bansal, A.; Neyaz, A.; Jaiswal, R.; Jain, K.; Chaturvedi, A.; Anand, N.; Malhotra, K.; Shukla, S. Development of a New Outcome Prediction Model in Early-stage Squamous Cell Carcinoma of the Oral Cavity Based on Histopathologic Parameters With Multivariate Analysis: The Aditi-Nuzhat Lymph-node Prediction Score (ANLPS) System. Am. J. Surg. Pathol. 2017, 41, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Mermod, M.; Tolstonog, G.; Simon, C.; Monnier, Y. Extracapsular spread in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral. Oncol. 2016, 62, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.D.; Shetty, R.; Nair, D.; Mathur, Y.; Nair, S.; Deshmukh, A.; Thiagarajan, S.; Pantvaidya, G.; Lashkar, S.; Prabhash, K.; et al. Depth of invasion, size and number of metastatic nodes predicts extracapsular spread in early oral cancers with occult metastases. Oral. Oncol. 2018, 81, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Jakobsson, P.A.; Eneroth, C.M.; Killander, D.; Moberger, G.; Mårtensson, B. Histologic classification and grading of malignancy in carcinoma of the larynx. Acta Radiol. Ther. Phys. Biol. 1973, 12, 1–8. [Google Scholar] [CrossRef]
- Bryne, M.; Koppang, H.S.; Lilleng, R.; Kjaerheim, A. Malignancy grading of the deep invasive margins of oral squamous cell carcinomas has high prognostic value. J. Pathol. 1992, 166, 375–381. [Google Scholar] [CrossRef]
- Anneroth, G.; Batsakis, J.; Luna, M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand. J. Dent. Res. 1987, 95, 229–249. [Google Scholar] [CrossRef]
- Brandwein-Gensler, M.; Teixeira, M.S.; Lewis, C.M.; Lee, B.; Rolnitzky, L.; Hille, J.J.; Genden, E.; Urken, M.L.; Wang, B.Y. Oral squamous cell carcinoma: Histologic risk assessment, but not margin status, is strongly predictive of local disease-free and overall survival. Am. J. Surg. Pathol. 2005, 29, 167–178. [Google Scholar] [CrossRef]
- Müller, S.; Boy, S.C.; Day, T.A.; Magliocca, K.R.; Richardson, M.S.; Sloan, P.; Tilakaratne, W.M.; Zain, R.B.; Thompson, L.D.R. Data set for the reporting of oral cavity carcinomas: Explanations and recommendations of the guidelines from the International Collaboration of Cancer Reporting. Arch. Pathol. Lab. Med. 2019, 143, 439–446. [Google Scholar] [CrossRef]
- Xu, B.; Salama, A.M.; Valero, C.; Yuan, A.; Khimraj, A.; Saliba, M.; Zanoni, D.K.; Ganly, I.; Patel, S.G.; Katabi, N.; et al. The prognostic role of histologic grade, worst pattern of invasion, and tumor budding in early oral tongue squamous cell carcinoma: A comparative study. Virchows Arch. 2021, 479, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, J.; Men, Y.; Yang, W.; Mi, F.; Li, L. Does Medullary Versus Cortical Invasion of the Mandible Affect Prognosis in Patients With Oral Squamous Cell Carcinoma? J. Oral Maxillofac. Surg. 2017, 75, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.P.; Das, S.R.; Mathews, A.; Naik, B.R.; Chacko, E.; Pandey, M. Mandibular invasion in oral squamous cell carcinoma: Investigation by clinical examination and orthopantomogram. Int. J. Oral Maxillofac. Surg. 2004, 33, 454–457. [Google Scholar] [CrossRef]
- Tsue, T.T.; McCulloch, T.M.; Girod, D.A.; Couper, D.J.; Weymuller, E.A., Jr.; Glenn, M.G. Predictors of carcinomatous invasion of the mandible. Head Neck 1994, 16, 116–126. [Google Scholar] [CrossRef]
- Gou, L.; Yang, W.; Qiao, X.; Ye, L.; Yan, K.; Li, L.; Li, C. Marginal or segmental mandibulectomy: Treatment modality selection for oral cancer: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 1–10. [Google Scholar] [CrossRef]
- Rao, L.P.; Shukla, M.; Sharma, V.; Pandey, M. Mandibular conservation in oral cancer. Surg. Oncol. 2012, 21, 109–118. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Strobe Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Brown, J. Mechanisms of cancer invasion of the mandible. Curr. Opin. Otolaryngol. Head. Neck Surg. 2003, 11, 96–102. [Google Scholar] [CrossRef]
- Carter, R.L.; Tsao, S.W.; Burman, J.F.; Pittam, M.R.; Clifford, P.; Shaw, H.J. Patterns and mechanisms of bone invasion by squamous carcinomas of the head and neck. Am. J. Surg. 1983, 146, 451–455. [Google Scholar] [CrossRef]
- Brown, J.S.; Browne, R.M. Factors influencing the patterns of invasion of the mandible by oral squamous cell carcinoma. Int. J. Oral. Maxillofac. Surg. 1995, 24, 417–426. [Google Scholar] [CrossRef]
- Brown, J.S.; Lowe, D.; Kalavrezos, N.; D’Souza, J.; Magennis, P.; Woolgar, J. Patterns of invasion and routes of tumor entry into the mandible by oral squamous cell carcinoma. Head Neck 2002, 24, 370–383. [Google Scholar] [CrossRef]
- Fives, C.; Nae, A.; Roche, P.; O’Leary, G.; Fitzgerald, B.; Feeley, L.; Sheahan, P. Impact of mandibular invasion on prognosis in oral squamous cell carcinoma four centimeters or less in size. Laryngoscope 2017, 127, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, T.; Tayaar, A.S.; Acharya, S.; Bhushan, J.; Muddapur, M.V. Pattern of invasion as a factor in determining lymph node metastasis in oral squamous cell carcinoma. J. Cancer Res. Ther. 2018, 14, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Bansal, V.; Malik, V.; Bhagat, R.; Punia, R.S.; Handa, U.; Gupta, A.; Dass, A. Tumor Budding and Worse Pattern of Invasion Can Predict Nodal Metastasis in Oral Cancers and Associated with Poor Survival in Early-Stage Tumors. Ear Nose Throat J. 2019, 98, E112–E119. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, L.; Stenlund, H.; Laurell, G.; Nylander, K. The importance of stromal inflammation in squamous cell carcinoma of the tongue. J. Oral Pathol. Med. 2012, 41, 379–383. [Google Scholar] [CrossRef]
- Kane, S.V.; Gupta, M.; Kakade, A.C.; D’ Cruz, A. Depth of invasion is the most significant histological predictor of subclinical cervical lymph node metastasis in early squamous carcinomas of the oral cavity. Eur. J. Surg. Oncol. 2006, 32, 795–803. [Google Scholar] [CrossRef]
- Lewis, J.S., Jr.; Carpenter, D.H.; Thorstad, W.L.; Zhang, Q.; Haughey, B.H. Extracapsular extension is a poor predictor of disease recurrence in surgically treated oropharyngeal squamous cell carcinoma. Mod. Pathol. 2011, 24, 1413–1420. [Google Scholar] [CrossRef]
- Puri, S.K.; Fan, C.Y.; Hanna, E. Significance of extracapsular lymph node metastases in patients with head and neck squamous cell carcinoma. Curr. Opin. Otolaryngol. Head. Neck Surg. 2003, 11, 119–123. [Google Scholar] [CrossRef]
- Tokerc, C. Some observations on the deposition of metastatic carcinoma within cervical lymph nodes. Cancer 1963, 16, 364–374. [Google Scholar] [CrossRef]


| Characteristics | Frequency | ||
|---|---|---|---|
| No. of Patients | Percentage | ||
| Mean Age M:F ratio | 54.78 ± 6.4 years 1:0.18 | ||
| Site of primary tumor | Buccal mucosa | 40 | 24.70% |
| Tongue | 33 | 20.40% | |
| Floor of mouth | 35 | 21.60% | |
| Mandibular alveolus | 32 | 19.80% | |
| Retro molar trigone | 22 | 13.60% | |
| pT stage: | T2 | 12 | 7.4% |
| T3 | 63 | 38.90% | |
| T4a | 76 | 46.90% | |
| T4b | 11 | 6.8% | |
| pN stage: | N0 | 94 | 58.02% |
| N+ | 68 | 42% | |
| Mandibular Invasion: Histopathology | No involvement | 76 | 46.9% |
| Cortical involvement | 19 | 11.73% | |
| Medullary involvement | 67 | 41.40% | |
| Erosive | 30 | 34.88% | |
| Infiltrative | 56 | 65.11% | |
| Grading. | Well | 72 | 44.40% |
| Moderate | 47 | 29.0% | |
| Poor | 43 | 26.5% | |
| LVI | Absent | 101 | 62.3% |
| Present | 61 | 37.7% | |
| PNI | Absent | 115 | 71% |
| Present | 47 | 29% | |
| Pattern of invasion | Cohesive | 92 | 56.8% |
| Aggressive | 70 | 43.2% | |
| DOI (mm) | ≤6.5 | 95 | 58.6% |
| >6.5 | 67 | 41.4% | |
| ECS | Absent | 122 | 75.3% |
| Present | 40 | 24.7% | |
| Lymph node size (cm) | ≤2.95 | 116 | 71.6% |
| >2.95 | 46 | 28.4% | |
| Mandibulectomy: | Marginal | 42 | 25.9% |
| Segmental | 120 | 74.1% | |
| Management of neck: | Ipsilateral SND | 130 | 80.24% |
| Ipsilateral MRND | 28 | 17.3% | |
| Ipsilateral MRND + contralateral SND | 4 | 2.5% | |
| Adjuvant treatment: | No adjuvant therapy | 72 | 44.4% |
| Radiotherapy | 70 | 43.2% | |
| Chemoradiation | 20 | 12.3% | |
| Univariate Regression | OR | 95% C.I. | p Value | OR | 95% C.I | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||||
| BM | multivariate regression | Site | 0.830 | 0.639 | 1.079 | 0.165 | ||||
| FOM | 2.746 | 0.782 | 9.643 | 0.115 | size | 0.940 | 0.537 | 1.645 | 0.828 | |
| Mn alveolus | 1.701 | 0.501 | 5.782 | 0.395 | DOI | 2.810 | 1.200 | 6.580 | 0.017 | |
| RMT | 1.266 | 0.295 | 5.439 | 0.751 | GRADING | 1.353 | 0.854 | 2.141 | 0.197 | |
| TONGUE | 0.359 | 0.101 | 1.276 | 0.114 | LVI | 0.865 | 0.273 | 2.738 | 0.805 | |
| pT 2 | PNI | 2.162 | 0.607 | 7.706 | 0.234 | |||||
| pT 3 | 1.140 | 0.199 | 6.544 | 0.883 | PATTERN | 8.870 | 3.825 | 20.570 | 0.000 | |
| pT4 | 0.788 | 0.141 | 4.409 | 0.787 | LN size | 1.907 | 0.573 | 6.352 | 0.293 | |
| DOI ≤ 6.5 | ||||||||||
| DOI > 6.5 mm | 3.273 | 1.283 | 8.348 | 0.013 | ||||||
| Well differentiated | ||||||||||
| Moderate | 0.702 | 0.253 | 1.946 | 0.496 | ||||||
| Poor | 1.843 | 0.700 | 4.853 | 0.216 | ||||||
| LVI absent | ||||||||||
| LVI present | 0.810 | 0.237 | 2.772 | 0.738 | ||||||
| PNI absent | ||||||||||
| PNI present | 2.955 | 0.738 | 11.830 | 0.126 | ||||||
| Cohesive pattern | ||||||||||
| Aggressive pattern | 10.748 | 4.222 | 27.357 | <0.001 | ||||||
| LN size ≤ 2.95 | ||||||||||
| LN size > 2.95 cm | 3.544 | 0.917 | 13.705 | 0.067 | ||||||
| Univariate Regression | OR | 95% C.I. | p Value | OR | 95% C.I. | p Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||||
| BM | multivariate regression | Site | 0.804 | 0.604 | 1.071 | 0.136 | ||||
| FOM | 1.586 | 0.431 | 5.842 | 0.488 | pT | 1.525 | 0.797 | 2.917 | 0.203 | |
| Mn alveolus | 0.767 | 0.199 | 2.956 | 0.700 | DOI | 6.486 | 2.453 | 17.144 | <0.001 | |
| Tongue | 0.916 | 0.159 | 5.288 | 0.922 | GRADING | 1.455 | 0.860 | 2.461 | 0.162 | |
| Site_1(4) | 0.492 | 0.139 | 1.743 | 0.272 | LVI | 2.156 | 0.591 | 7.864 | 0.245 | |
| pT2 | 0.753 | PNI | 1.419 | 0.326 | 6.173 | 0.641 | ||||
| pT3 | 0.946 | 0.131 | 6.827 | 0.956 | PATTERN | 11.448 | 4.414 | 29.693 | <0.001 | |
| pT4a | 1.504 | 0.223 | 10.145 | 0.675 | ||||||
| pT4b | 2.319 | 0.138 | 38.897 | 0.559 | ||||||
| DOI ≤ 6.5 | ||||||||||
| DOI > 6.5 | 7.155 | 2.547 | 20.099 | <0.001 | ||||||
| Well differentiated | ||||||||||
| Moderate | 1.866 | 0.635 | 5.483 | 0.257 | ||||||
| Poor | 1.993 | 0.682 | 5.823 | 0.207 | ||||||
| LVI absent | ||||||||||
| LVI present | 2.109 | 0.569 | 7.820 | 0.265 | ||||||
| PNI absent | ||||||||||
| PNI present | 1.475 | 0.324 | 6.711 | 0.615 | ||||||
| Cohesive pattern | ||||||||||
| Aggressive pattern | 11.516 | 4.332 | 30.611 | <0.001 | ||||||
| Univariate Regression | OR | 95% C.I. | p Value | Multivariate Regression | OR | 95% C.I. | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | ||||||
| BM | Site | 0.318 | 0.073 | 1.383 | 0.127 | ||||
| FOM | 0.371 | 0.091 | 1.510 | 0.166 | pT | 1.120 | 0.592 | 2.118 | 0.728 |
| Mn alveolus | 0.296 | 0.067 | 1.311 | 0.109 | DOI | 0.845 | 0.357 | 2.005 | 0.703 |
| RMT | 0.955 | 0.193 | 4.723 | 0.955 | GRADING | 0.858 | 0.533 | 1.381 | 0.528 |
| Tongue | 3.684 | 0.908 | 14.941 | 0.068 | LVI | 1.503 | 0.483 | 4.674 | 0.481 |
| pT2 | PNI | 0.575 | 0.153 | 2.158 | 0.412 | ||||
| pT3 | 8.302 | 0.490 | 140.752 | 0.143 | PATTERN | 1.293 | 0.406 | 4.120 | 0.663 |
| pT4a | 9.276 | 0.558 | 154.324 | 0.120 | LN size | 19.152 | 5.827 | 62.946 | <0.001 |
| pT4b | 5.540 | 0.217 | 141.512 | 0.300 | |||||
| DOI ≤ 6.5 | |||||||||
| DOI > 6.5 | 0.563 | 0.215 | 1.478 | 0.244 | |||||
| Well differentiated | |||||||||
| Moderate | 1.317 | 0.344 | 5.041 | 0.688 | |||||
| Poor | 0.977 | 0.338 | 2.829 | 0.966 | |||||
| LVI absent | |||||||||
| LVI present | 1.491 | 0.433 | 5.140 | 0.527 | |||||
| PNI absent | |||||||||
| PNI present | 0.703 | 0.165 | 3.003 | 0.634 | |||||
| Cohesive pattern | |||||||||
| Aggressive pattern | 1.689 | 0.471 | 6.053 | 0.421 | |||||
| LN size ≤ 2.95 | |||||||||
| LN size > 2.95 | 27.795 | 6.953 | 111.119 | 0.000 | |||||
| Mean and Median Survival Time (in Months) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PATTERN | Mean | Median | ||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
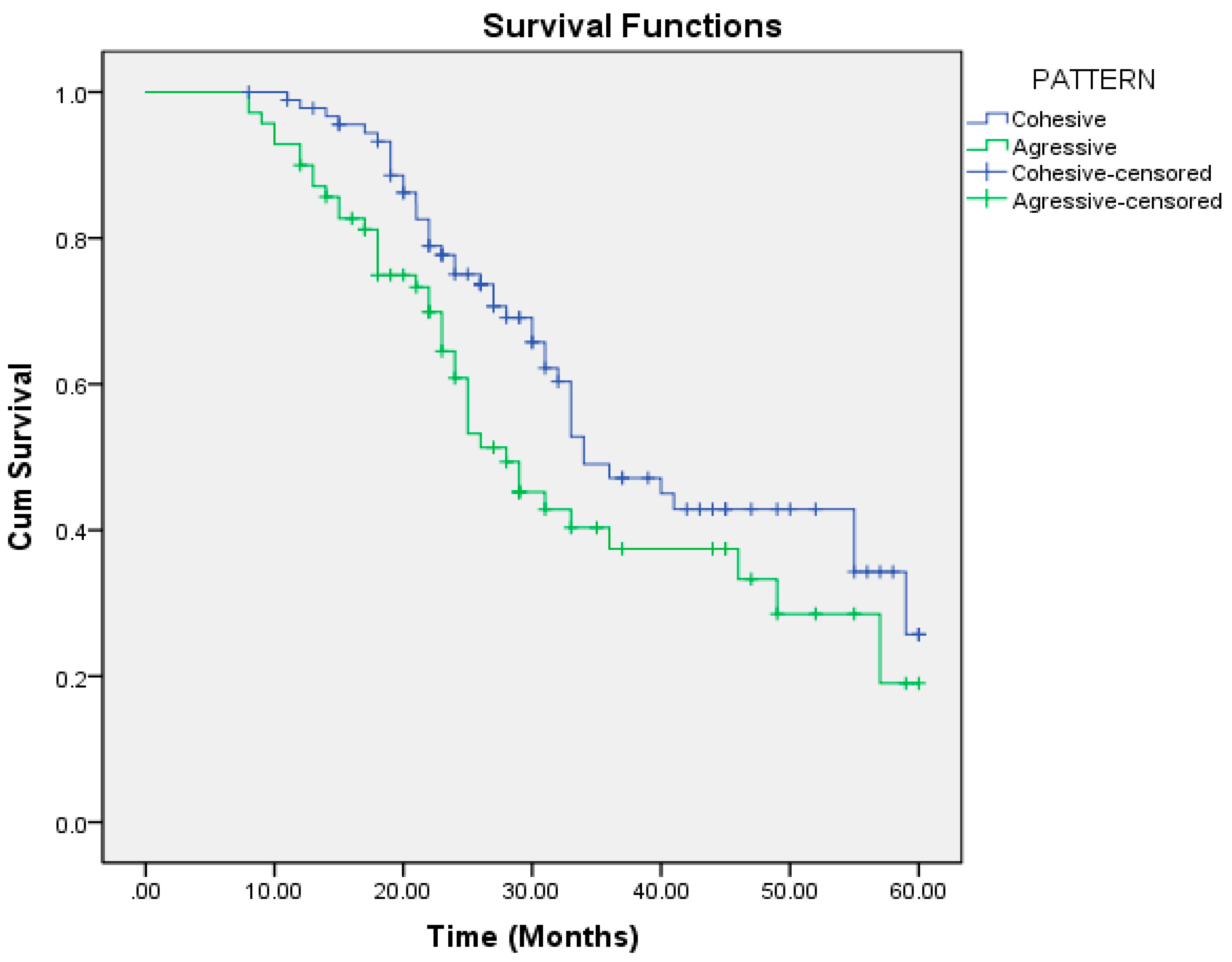
| Cohesive | 40.521 | 2.057 | 36.489 | 44.553 | 34.000 | 3.294 | 27.544 | 40.456 |
| Aggressive | 34.430 | 2.472 | 29.585 | 39.275 | 28.000 | 2.564 | 22.974 | 33.026 |
| pT | ||||||||
| T2 | 47.932 | 5.165 | 37.808 | 58.056 | ||||
| T3 | 38.072 | 2.559 | 33.057 | 43.086 | 34.000 | 8.772 | 16.808 | 51.192 |
| T4a | 37.629 | 2.344 | 33.034 | 42.223 | 33.000 | 0.581 | 31.862 | 34.138 |
| T4b | 26.364 | 3.718 | 19.077 | 33.651 | 24.000 | 2.752 | 18.605 | 29.395 |
| GRADING | ||||||||
| Well | 51.016 | 2.214 | 46.676 | 55.356 | ||||
| Moderate | 34.065 | 2.353 | 29.452 | 38.677 | 33.000 | 1.301 | 30.450 | 35.550 |
| Poor | 26.022 | 2.362 | 21.391 | 30.652 | 21.000 | 1.634 | 17.798 | 24.202 |
| LVI | ||||||||
| Absent | 41.337 | 2.117 | 37.188 | 45.487 | 41.000 | 8.703 | 23.943 | 58.057 |
| Present | 33.374 | 2.359 | 28.751 | 37.997 | 30.000 | 2.279 | 25.533 | 34.467 |
| PNI | ||||||||
| Absent | 40.710 | 1.959 | 36.871 | 44.549 | 40.000 | 6.489 | 27.281 | 52.719 |
| Present | 31.908 | 2.657 | 26.701 | 37.115 | 28.000 | 1.424 | 25.209 | 30.791 |
| MANDIBULAR INVASION | ||||||||
| Absent | 45.664 | 2.298 | 41.160 | 50.169 | 55.000 | 4.125 | 46.914 | 63.086 |
| Present | 31.279 | 1.879 | 27.596 | 34.962 | 27.000 | 1.845 | 23.383 | 30.617 |
| LYMPH NODE METASTASIS | ||||||||
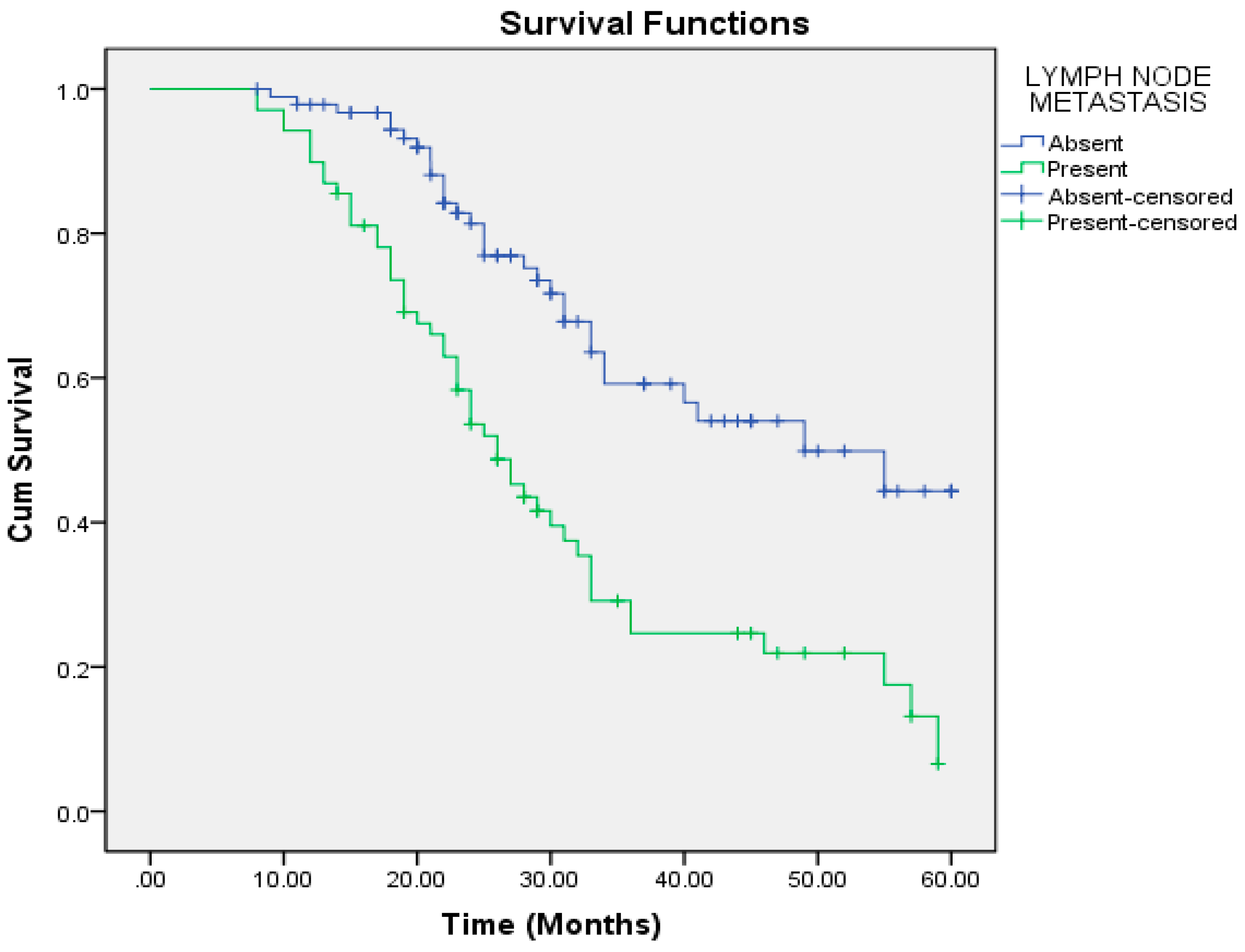
| Absent | 44.089 | 2.146 | 39.883 | 48.296 | 49.000 | 9.114 | 31.136 | 66.864 |
| Present | 30.720 | 2.140 | 26.526 | 34.914 | 26.000 | 2.087 | 21.909 | 30.091 |
| ECS | ||||||||
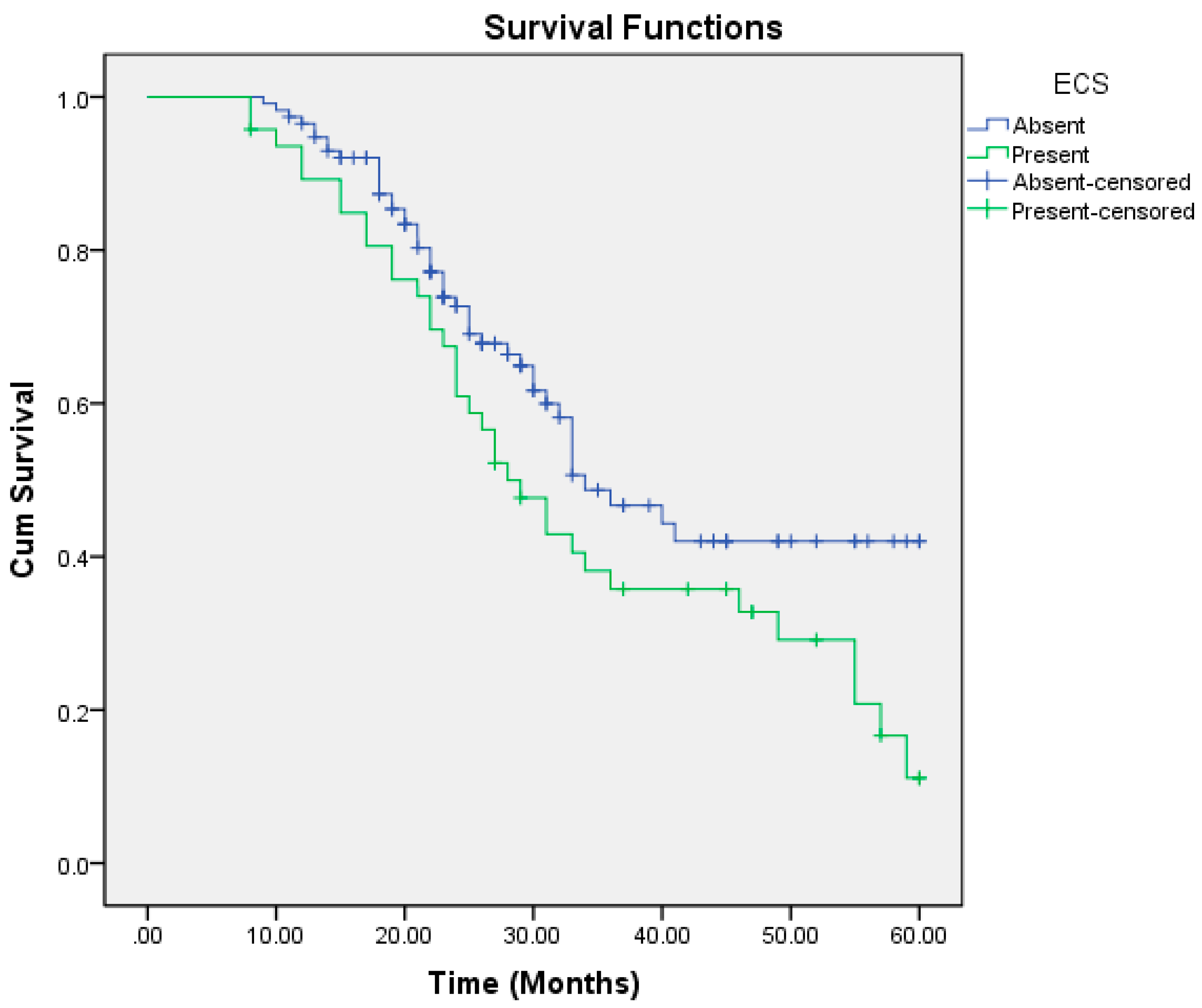
| Absent | 40.082 | 2.066 | 36.032 | 44.132 | 34.000 | 3.486 | 27.168 | 40.832 |
| Present | 34.337 | 2.624 | 29.193 | 39.481 | 28.000 | 2.703 | 22.702 | 33.298 |
| DOI_1 | ||||||||
| ≤6.5 | 44.898 | 2.160 | 40.664 | 49.132 | ||||
| >6.5 | 30.123 | 1.989 | 26.225 | 34.020 | 26.000 | 2.237 | 21.615 | 30.385 |
| LN_size | ||||||||
| ≤2.95 | 42.952 | 1.878 | 39.271 | 46.633 | 49.000 | 6.286 | 36.680 | 61.320 |
| >2.95 | 26.657 | 2.263 | 22.221 | 31.092 | 24.000 | 1.270 | 21.512 | 26.488 |
| Site | ||||||||
| BM | 35.459 | 2.904 | 29.767 | 41.151 | 30.000 | 3.839 | 22.475 | 37.525 |
| FOM | 39.326 | 3.498 | 32.469 | 46.182 | 49.000 | 14.004 | 21.553 | 76.447 |
| Mandibular alveolus | 38.319 | 4.182 | 30.122 | 46.515 | 36.000 | 8.472 | 19.395 | 52.605 |
| RMT | 36.857 | 3.409 | 30.176 | 43.538 | 33.000 | 2.455 | 28.188 | 37.812 |
| TONGUE | 39.126 | 3.635 | 32.002 | 46.250 | 34.000 | 6.270 | 21.711 | 46.289 |
| Overall | 37.918 | 1.604 | 34.774 | 41.063 | 33.000 | 1.982 | 29.116 | 36.884 |
| PATTERN | Frequency | p Value | OR | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|
| Cohesive | 92 | ||||
| Aggressive | 70 | 0.046 | 1.560 | 1.008 | 2.414 |
| pT | |||||
| T2 | 12 | ||||
| T3 | 63 | 0.063 | 3.915 | 0.928 | 16.511 |
| T4a | 76 | 0.048 | 4.207 | 1.014 | 17.451 |
| T4b | 11 | 0.004 | 9.407 | 2.074 | 42.659 |
| GRADING | |||||
| Well | 72 | ||||
| Moderate | 47 | <0.001 | 3.902 | 1.983 | 7.680 |
| Poor | 43 | <0.001 | 7.629 | 3.987 | 14.598 |
| LVI | |||||
| Absent | 101 | ||||
| Present | 61 | 0.019 | 1.688 | 1.091 | 2.610 |
| PNI | |||||
| Absent | 115 | ||||
| Present | 47 | 0.008 | 1.816 | 1.165 | 2.831 |
| MANDIBULAR INVASION | |||||
| Absent | 76 | ||||
| Present | 86 | <0.001 | 8.467 | 4.234 | 16.934 |
| LYMPH NODE METASTASIS | |||||
| Absent | 94 | ||||
| Present | 68 | <0.001 | 12.172 | 5.596 | 26.475 |
| ECS | |||||
| Absent | 122 | ||||
| Present | 40 | <0.001 | 8.411 | 3.862 | 18.317 |
| DOI | |||||
| ≤6.5 | 95 | ||||
| >6.5 | 67 | <0.001 | 2.924 | 1.860 | 4.597 |
| LN size | |||||
| ≤2.95 | 116 | ||||
| >2.95 | 46 | <0.001 | 4.476 | 2.838 | 7.058 |
| SITE | |||||
| BM | 40 | ||||
| FOM | 35 | 0.478 | 0.793 | 0.419 | 1.503 |
| Mandibular alveolus | 32 | 0.901 | 0.959 | 0.493 | 1.864 |
| RMT | 22 | 0.832 | 0.928 | 0.464 | 1.856 |
| TONGUE | 33 | 0.514 | 0.798 | 0.405 | 1.571 |
| Total | 162 |
| OR | 95.0% CI for Exp(B) | p Value | ||
|---|---|---|---|---|
| Lower | Upper | |||
| DOI | 0.987 | 0.565 | 1.722 | 0.963 |
| GRADING | 1.284 | 0.889 | 1.853 | 0.182 |
| LVI | 0.834 | 0.392 | 1.776 | 0.638 |
| PNI | 0.730 | 0.337 | 1.581 | 0.425 |
| pT | 1.213 | 0.882 | 1.668 | 0.235 |
| MANDIBULAR INVASION | 2.274 | 1.006 | 5.144 | 0.048 |
| ECS | 2.762 | 1.106 | 6.898 | 0.030 |
| PATTERN | 2.065 | 1.152 | 3.703 | 0.015 |
| LN size | 1.903 | 1.037 | 3.491 | 0.038 |
| LYMPHNODE METASTASIS | 2.790 | 1.056 | 7.367 | 0.038 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bera, R.N.; Tripathi, R. Factors Influencing Mandibular Invasion, Lymph Node Metastasis and Extracapsular Spread in Squamous Cell Carcinoma of the Oral Cavity. Craniomaxillofac. Trauma Reconstr. 2025, 18, 30. https://doi.org/10.3390/cmtr18030030
Bera RN, Tripathi R. Factors Influencing Mandibular Invasion, Lymph Node Metastasis and Extracapsular Spread in Squamous Cell Carcinoma of the Oral Cavity. Craniomaxillofacial Trauma & Reconstruction. 2025; 18(3):30. https://doi.org/10.3390/cmtr18030030
Chicago/Turabian StyleBera, Rathindra Nath, and Richik Tripathi. 2025. "Factors Influencing Mandibular Invasion, Lymph Node Metastasis and Extracapsular Spread in Squamous Cell Carcinoma of the Oral Cavity" Craniomaxillofacial Trauma & Reconstruction 18, no. 3: 30. https://doi.org/10.3390/cmtr18030030
APA StyleBera, R. N., & Tripathi, R. (2025). Factors Influencing Mandibular Invasion, Lymph Node Metastasis and Extracapsular Spread in Squamous Cell Carcinoma of the Oral Cavity. Craniomaxillofacial Trauma & Reconstruction, 18(3), 30. https://doi.org/10.3390/cmtr18030030






