Reconstruction of Composite Mandible Defects Using a Cellular Bone Allograft and Soft Tissue Free Flap Coverage
Abstract
Introduction
Methods
Patient Selection and Management
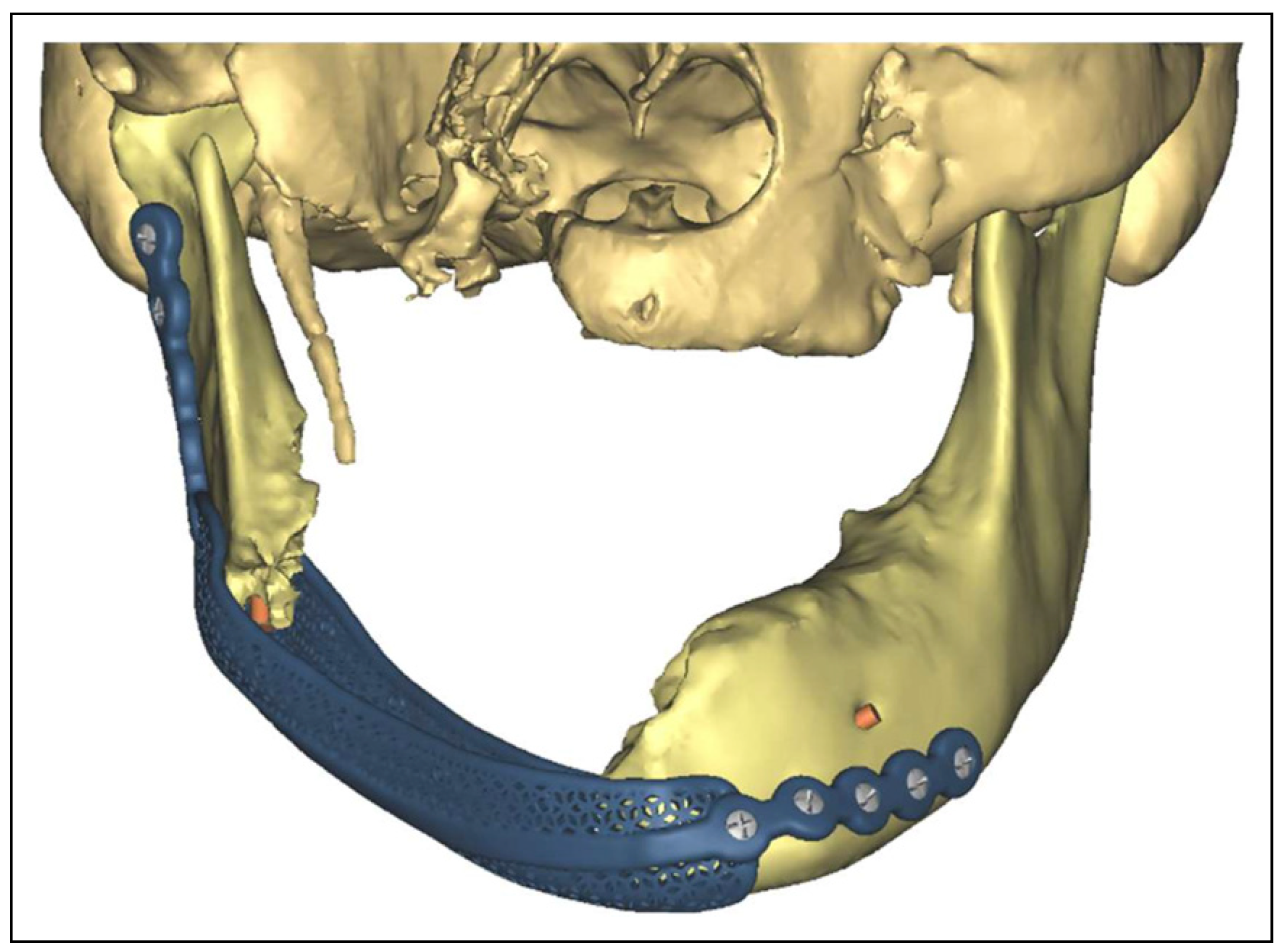
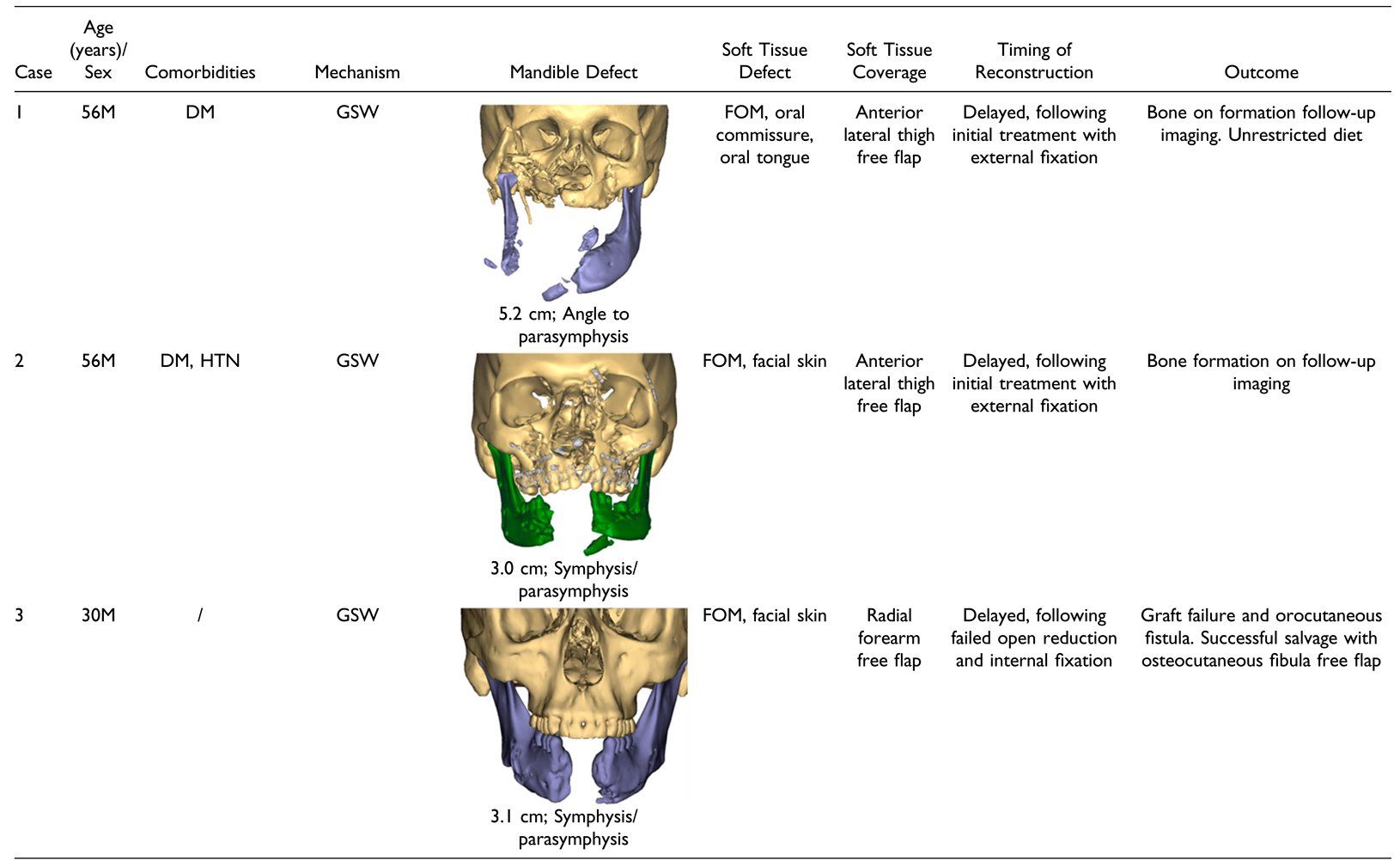
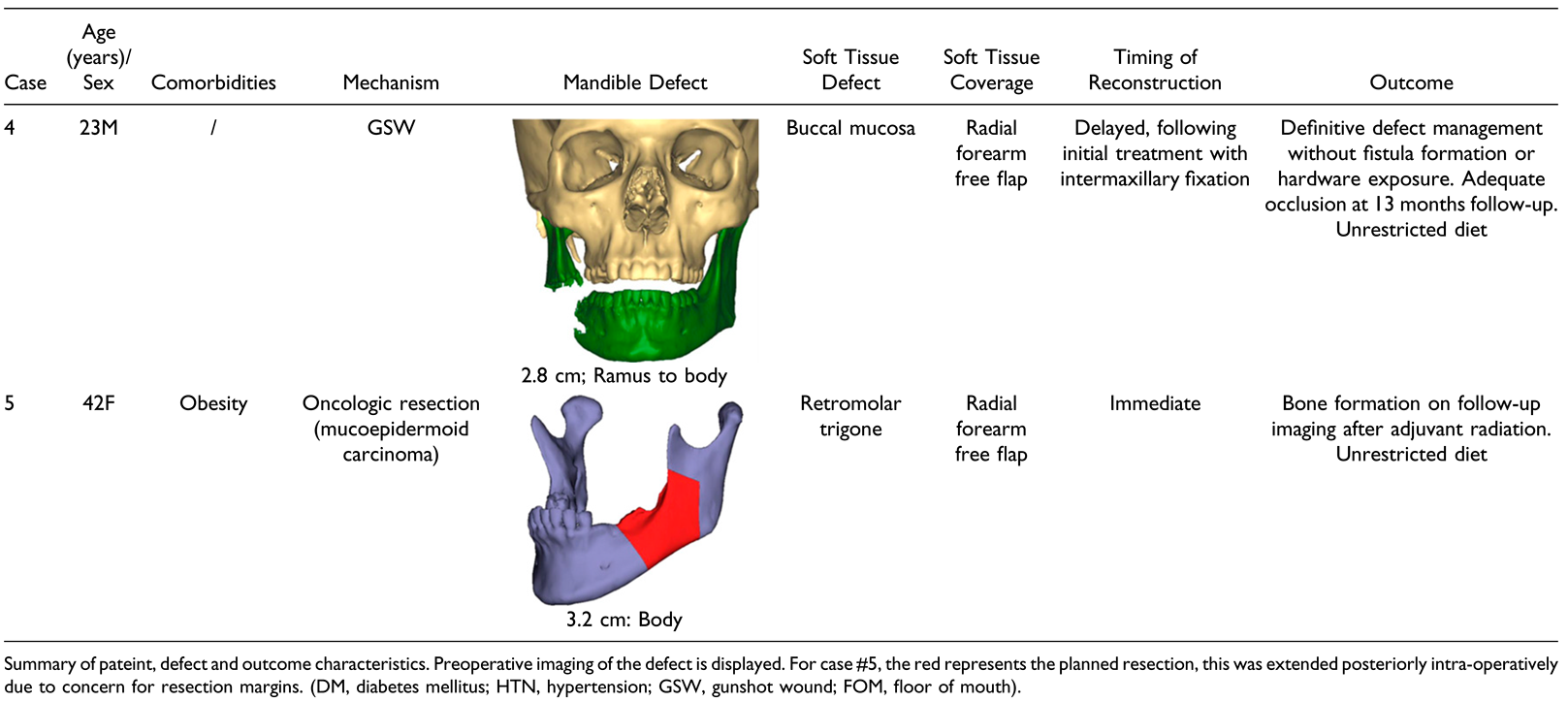
Results
Discussion
Conclusion
Funding
Acknowledgments
Conflicts of Interest
References
- Bak, M.; Jacobson, A.S.; Buchbinder, D.; Urken, M.L. Contemporary reconstruction of the mandible. Oral Oncol. 2010, 46, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.J.; Hakim, S.G.; Melville, J.C.; Su, Y.X. Current trends in the reconstruction and rehabilitation of jaw following ablative surgery. Cancers. 2022, 14, 3308. [Google Scholar] [CrossRef] [PubMed]
- Elgafy, H.; Wetzell, B.; Gillette, M.; et al. Lumbar spine fusion outcomes using a cellular bone allograft with lineage-committed bone-forming cells in 96 patients. BMC Musculoskelet Disord. 2021, 22, 699. [Google Scholar] [CrossRef] [PubMed]
- Moran, T.E.; Sequeira, S.; Cooper, M.T.; Park, J. A retrospective analysis of outcomes from foot and ankle arthrodesis and open reduction and internal fixation using cellular bone allograft augmentation. Foot Ankle Spec. 2022, 15, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.P.; Loveland, J.; Atkinson, B.L.; Ryaby, J.T.; Linovitz, R.J.; Nunley, J.A. Prospective, multicenter evaluation of allogeneic bone matrix containing viable osteogenic cells in foot and/or ankle arthrodesis. Foot Ankle Int. 2015, 36, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Pinter, Z.W.; Elder, B.D.; Kaye, I.D.; et al. A review of commercially available cellular-based allografts. Clin Spine Surg. 2022, 35, E77–E86. [Google Scholar] [CrossRef] [PubMed]
- Ryu, B.; Abraham, C.; Polido, W.D. Treatment of mandibular non-union using patient specific crib cage plates and cellular bone allograft: A case report. Craniomaxillofac Trauma Reconstr Open 2021, 6, 247275122110059. [Google Scholar] [CrossRef]
- Alfi, D.M.; Hassan, A.; East, S.M.; Gianulis, E.C. Immediate mandibular reconstruction using a cellular bone allograft following tumor resection in a pediatric patient. FACE. 2021, 2, 490–495. [Google Scholar] [CrossRef]
- Yang, W.F.; Choi, W.S.; Wong, M.C.; et al. Three-dimensionally printed patient-specific surgical plates increase accuracy of oncologic head and neck reconstruction versus conventional surgical plates: A comparative study. Ann Surg Oncol. 2021, 28, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Chin, S.J.; Muecke, T.; et al. Increasing the accuracy of mandibular reconstruction with free fibula flaps using functionalized selective laser-melted patient-specific implants: A retrospective multicenter analysis. J CranioMaxillo-Fac Surg. 2017, 45, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Stoor, P.; Suomalainen, A.; Mesima¨ki, K.; Kontio, R. Rapid prototyped patient specific guiding implants in critical mandibular reconstruction. J Cranio-Maxillo-Fac Surg. 2017, 45, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Moura, L.B.; Carvalho, P.H.A.; Xavier, C.B.; et al. Autogenous non-vascularized bone graft in segmental mandibular reconstruction: A systematic review. Int J Oral Maxillofac Surg. 2016, 45, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Ramezanzade, S.; Aeinehvand, M.; Ziaei, H.; et al. Reconstruction of critical sized maxillofacial defects using composite allogeneic tissue engineering: Systematic review of current literature. Biomimetics 2023, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.M.A.; Hassan, S.A. The feasibility of rib grafts in long span mandibular defects reconstruction: A long term follow up. J Cranio-Maxillo-Fac Surg. 2019, 47, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Melville, J.C.; Tran, H.Q.; Bhatti, A.K.; Manon, V.; Young, S.; Wong, M.E. Is reconstruction of large mandibular defects using bioengineering materials effective? J Oral Maxillofac Surg. 2020, 78, 661. [Google Scholar] [CrossRef] [PubMed]
- Gianulis, E.; Wetzell, B.; Scheunemann, D.; et al. Characterization of an advanced viable bone allograft with preserved native bone-forming cells. Cell Tissue Bank. 2023, 24, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Schlund, M.; Nicot, R.; Depeyre, A.; Alkasbi, J.; Ferri, J. Reconstruction of a large posttraumatic mandibular defect using bone tissue engineering with fresh-frozen humeral allograft seeded with autologous bone marrow aspirate and vascularized with a radial forearm flap. J Craniofac Surg. 2019, 30, 2085–2087. [Google Scholar] [CrossRef] [PubMed]




  |
© 2024 by the author. The Author(s) 2024.
Share and Cite
Carlson, K.J.; Liebman, R.M.; Bak, M.J.; Dougherty, W.M.; Mark, J.R. Reconstruction of Composite Mandible Defects Using a Cellular Bone Allograft and Soft Tissue Free Flap Coverage. Craniomaxillofac. Trauma Reconstr. 2024, 17, 63. https://doi.org/10.1177/19433875241237920
Carlson KJ, Liebman RM, Bak MJ, Dougherty WM, Mark JR. Reconstruction of Composite Mandible Defects Using a Cellular Bone Allograft and Soft Tissue Free Flap Coverage. Craniomaxillofacial Trauma & Reconstruction. 2024; 17(4):63. https://doi.org/10.1177/19433875241237920
Chicago/Turabian StyleCarlson, Kevin J., Robert M. Liebman, Matthew J. Bak, William M. Dougherty, and Jonathan R. Mark. 2024. "Reconstruction of Composite Mandible Defects Using a Cellular Bone Allograft and Soft Tissue Free Flap Coverage" Craniomaxillofacial Trauma & Reconstruction 17, no. 4: 63. https://doi.org/10.1177/19433875241237920
APA StyleCarlson, K. J., Liebman, R. M., Bak, M. J., Dougherty, W. M., & Mark, J. R. (2024). Reconstruction of Composite Mandible Defects Using a Cellular Bone Allograft and Soft Tissue Free Flap Coverage. Craniomaxillofacial Trauma & Reconstruction, 17(4), 63. https://doi.org/10.1177/19433875241237920




