Abstract
Post-traumatic reconstruction of the orbit can pose a challenge due to inherent intraoperative problems. Intra-orbital adipose tissue is difficult to manipulate and retract making visualization of the posterior orbital contents difficult. Rapid prototyping (RP) is a cost-effective method of anatomical model production allowing the surgeon to produce a patient specific implant (PSI) which can be pre-surgically adapted to the orbital defect with exact reconstruction. Intraoperative imaging allows immediate assessment of reconstruction at the time of surgery. Utilization and combination of both technologies improves accuracy of reconstruction with orbital implants and reduces cost, surgical time, and the rate of revision surgery.
Introduction
Reconstruction of the orbital trauma patient poses significant challenges to the maxillofacial surgeon due to the complex anatomy and inherent difficulties of orbital content and struc- ture visualization. These include the posterior structures such as the posterior ledge and peripheries of the traumatic defect. Orbital soft tissue such as fat is difficult to retract and often visualization of one anatomical landmark makes assessment of another landmark difficult or impossible. Reconstruction of the orbit has several options which include placement of a titanium implant to reconstruct orbital walls. Accurate implant positioning is mandatory to restore optimal anatomy and function. Malposition can lead to several complications such as enophthalmos, restricted globe movement, injury, and entrapment of key structures such as muscles and associated soft tissue. A classic complication is placement of the orbital plate below the posterior ledge into the fracture defect.
Preformed titanium plates such as those by Synthes® (Matrix MIDFACE, West Chester, PA) and Leibinger® (Stryker Craniomaxillofacial, Kalamazoo, MI) have been used to mitigate these risks however even with these, opti- mal anatomic reconstruction can be difficult.
Recently, the introduction of technologies such as rapid prototyping (RP) and intraoperative imaging have been employed to accurately reconstruct the traumatized orbit.[1-7] RP in orbital trauma involves pre-operative man- ufacture of a three dimensional (3D) physical model of the patients affected orbit based on computed tomography (CT) data. This is used for operative planning and serves as a template for adaptation of a preformed titanium plate, creating a patient specific implant (PSI). Several papers demonstrate that combining RP and intraoperative imaging technologies significantly improves post-operative out- comes.[2,3] This paper is a review of the current literature. The workflow utilized at the authors unit is also presented.
Rationale for Patient Specific Implants (PSIs)
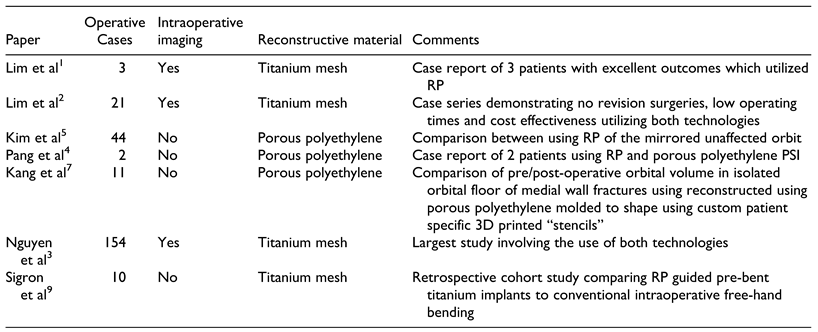
There are many advantages of PSIs such as decreased oper- ating time, optimal adaptation to the defect site and likely reduced rates of revision surgery due to malposition.[2-5] PSIs allow exact reconstruction of the bony anatomy. A typical protocol has previously been discussed by Lim et al (Figure 1).[2] The use of PSIs can be used for orbital wall fractures with or without involvement of the zygo- matic complex. The protocol for those involving the zygo- matic complex differs slightly from pure orbital wall fractures and involves a “reflected” model of the contral- ateral orbit. In the past, 3D modeling of anatomy and the production of stereolithographic models for use in surgery has previously been underutilized most likely due to pro- duction cost. RP emerged in recent years and has become a popular tool in maxillofacial surgery to generate accurate anatomic models. RP biomodels can be available within the same working day in institutions who have access to a 3D printer.[2,4] Advantages include rapid production time and cost effectiveness.[2-5] The generation of a traumatized orbit model allows pre-operative adaptation of a titanium implant to the fracture which adequately restores the defect and bony volume. Like other PSIs such as the customized TMJ prosthesis by TMJ concepts (Ventura, CA, USA), there is little ambiguity with anatomical fit and implant position.
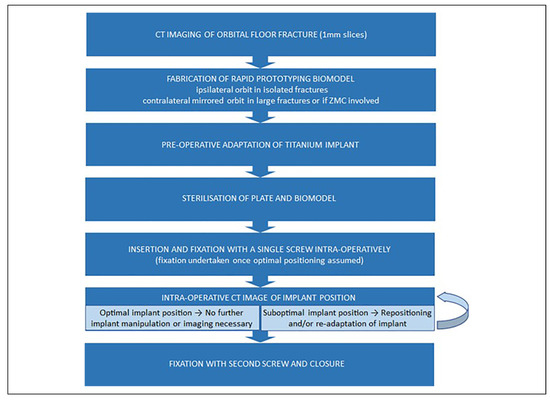
Figure 1.
Workflow for orbital reconstruction utilizing rapid prototyping, PSIs, and intraoperative imaging (adapted from Lim et al with permission).[2].
Intraoperative Imaging
Prior to the era of intraoperative imaging, the anatomy of an orbital defect was estimated from two-dimensional (2D) CT images with post-operative CT evaluating implant posi- tion. The obvious disadvantage with post-operative CT is a secondary procedure if revision is necessary with further surgical trauma and additional theater and staff cost con- siderations. Hoelzle et al were among the first to document the benefits of intraoperative imaging.[8] Intraoperative radiographic assessment of implant position has obvious advantages of allowing immediate correction of implant position during the same surgical setting, thus optimizing fracture repair and reducing additional surgical interven- tions and anesthesia.[3]
An early case series of 3 patients utilizing RP for orbital reconstruction was provided by Lim et al.[1] In this report, RP was suggested as an economic and anatomically accu- rate method to assess and facilitate reconstruction of the isolated orbital floor. All 3 cases presented with enophthal- mos and all had excellent adaption of implants to the orbital walls with no ocular dysfunction post-reconstruction. Fur- ther follow up was reported the year after with a case series of 21 patients utilizing both intraoperative imaging and RP.[2] There were no revision surgeries in this cohort and all implants were placed in the optimal anatomic position in less than 10 minutes (Table 1).

Table 1.
Reports Utilizing RP With or Without Intraoperative Imaging as Adjuncts.
Pang et al have recently described 2 cases in which 3D printing technology was also utilized to facilitate pre- operative planning and implant adaptation.[4] Porous poly- ethylene (MEDPOR®, Stryker, MI, USA) was used and adapted to the fracture defect rather than titanium mesh implants. The reported time required to prepare the 3D printed model was 1 hour, with 8-10 hours printing time and a subsequent 1 hour to adapt the porous polyethylene to the fracture defect. There was no mention of intraoperative imaging with these cases, however, both were reported to have satisfactory results with resolution of diplopia, enophthalmos and normal extra-ocular eye movements at the two-month post-operative period. The authors also reported decreased operating time of up to 30 min com- pared to comparable orbital floor defects done without the aid of a 3D printed model in the same unit.
Nguyen et al reviewed 154 orbital reconstructions from 2013 through 2016.[3] The same surgical protocol mentioned in Figure 1 was used for those patients deemed to meet operative criteria with most patients utilizing both technol- ogies.[2] Five cases required revision surgery for implant malposition. All 5 cases did not use intraoperative imaging (P ¼ 0.0016), and 4 did not have a RP biomodel (P ¼ 0.006). This is the largest cohort to date that reviewed the use of both RP and intraoperative imaging in combina- tion. There was a statistically significant difference in the rate of revision surgery required for those with and without RP and/or intraoperative imaging.
Kim et al examined the accuracy of RP anatomical mod- els in 82 patients.[6] Forty-four of these patients were treated with a RP adjunct, with 38 in the control group. There were several differences in protocol as compared to the Nguyen et al paper which included adapting the plate intraopera- tively and utilizing a reflected model rather than the orig- inal trauma defect for reconstruction. Intraoperative implant adaptation would increase the operative time and therefore costs. Another issue to consider is that each indi- vidual may not necessarily be exactly symmetrical, and some distortions may occur when the reflected RP tech- nique is used. If the exact anatomy is used for reconstruc- tion in an orbital fracture that does not include the orbital rims, an exact adaption of the implant can be prepared that covers the defect and rests on critical anatomical points such as the posterior ledge and the medial wall when involved. Nevertheless, the accuracy of implant placement was assessed using post-operative CT and it was found that orbital fractures reconstructed using RP lead to more accu- rate anatomic reconstruction compared to conventional intraoperative adaptation. Patients who underwent recon- struction with intraoperative adaptation of the orbital implant were also more likely to experience post- operative enophthalmos and limited ocular motility. In 2 of these cases, revision surgery was required, and it was found that the orbital implant did not fully cover the bone defect. Arguably, intraoperative imaging may have negated this.
Kang et al utilized custom fabricating 3D printed “stencils” that were used intraoperatively to anatomically contour and mold porous polyethylene with embedded tita- nium in 6 cases of orbital floor and 5 cases of medial wall reconstruction.[7] Pre- and post-operative orbital volumes were compared using CT scan data. No statistically significant difference was found between the volume of the unaffected orbit and the reconstructed traumatized orbit, indicating good volumetric reconstruction. While this data shows that the “stencil” method restores orbital volume appropriately, the time to design and fabricate the custom 3D printed orbital “stencils” and operative time was omitted from this paper, so it is difficult to draw conclu- sions regarding the efficiency of this method.
Sigron et al recently conducted a retrospective cohort study to compare operative time and post-operative orbital volume between cases of isolated orbital floor fractures.[9] The utilization of 3D printed bone models and patient spe- cific titanium mesh implants (intervention group) (n ¼ 10) were compared to conventional intraoperative adaptation (n ¼ 12). A statistically significant volume difference (unfractured orbit vs reconstructed fractured orbit) was found in the conventional group (mean ¼ 1.6 mL, P ¼ 0.002), while there was no statistically significant difference (mean ¼ 1 .0 mL, P ¼ 0.276) in the intervention group. Although, a small number of patients were reviewed, this indicates that RP and PSIs may lead to more accurate reconstruction of orbital anatomy. Additionally, there was a statistically significant reduction in operative time when utilizing RP and PSIs (difference of 42.5 min, P ¼ 0.001). Lim et al also found relatively low operating times when RP and PSIs were used for isolated orbital floor fractures (n ¼ 21), with the average time for plate place- ment and fixation with a single screw being less than 10 mins.[2] Of note, most of these cases only required a single intraoperative O-arm “spin”, highlighting the accuracy of initial plate position.
As seen in this review, there are relatively few reports regarding intraoperative imaging to guide orbital implant placement. The only report to combine RP and intraopera- tive imaging are from the author’s unit in Christchurch, New Zealand.[2,3] In the largest clinical study of orbital reconstruction using RP, Nguyen et al found that of the 5 cases (3.3%) requiring revision surgery, none utilized intraoperative imaging. None of the 110 cases that used intraoperative imaging required revision surgery.[3] These findings, in conjunction with the other reports discussed, indicate that a RP biomodel and PSI leads to more accurate orbital reconstruction and a reduction in post-operative complications.
In the authors experience, 3D RP biomodels are not exact replicas of natural anatomy and dimensional error for their construction has been reported between 0.18% and 0.55%.[10] In the authors experience, this dimensional dis- crepancy is minimal and precise adaptation to fracture mar- gins is achievable pre-operatively. Mispositioning seen with intraoperative imaging usually arises from an inade- quately adapted implant or poor surgical technique and hence justifies its utilization.
Intraoperative imaging comes with the obvious burden of initial set-up cost. Most intraoperative CT scanners are more than $1 million NZD. In the authors hospital, an intraoperative CT scanner is shared with orthopedic spine and neurosurgical services which has allowed cost justifi- cation. These machines are also expensive to maintain and repair. The use of intraoperative CT such as the Medtronic O-Arm requires additional trained staff. Once staff are trained, a single “spin” typically adds less than 10 mins to operating time. Comparable times (14.5 min) have been reported by Shaye et al.[11]
The results of the Nguyen et al review demonstrates intraoperative imaging reduces the likelihood of needing revision surgery.[3] With the cost of theater time in NZ being up to $107 NZD per minute, intraoperative imaging no doubt reduces the economic burden to the public health system, particularly in surgical units treating high volumes of orbital fractures. Similar costs have been reported in other units.[9] Intraoperative imaging also negates necessity for post-operative imaging. With the cost of a CT scan being *$900 NZD this provides additional cost-saving.
A reasonable concern is radiation exposure to patients undergoing intraoperative imaging. Lim et al provides a summary of the radiation exposure levels found with intraoperative O-arm imaging versus conventional post- operative cone-beam CT (i-CAT) scans.[2] The radiation exposure to the head from O-arm scanning ranges from 0.48 mSv to 0.78 mSv, whereas that from post-operative i-CAT scans range from 0.068 mSv to 1.073 mSv.[12] The radiation dose of the O-arm in 3D scan acquisition mode has also been shown to be approximately half the dose of a 64-slice CT, and at least one-third or less than exposure from a conventional CT, depending on the organ.[13,14] The use of post-operative cone-beam or medical grade CT, combined with the documented increased rates of revision surgery and anesthetic time when intraoperative imaging is not used, likely negates the deleterious effects of radiation exposure from intraoperative O-arm utilization.
Cost of RP
RP has a significantly lower cost when compared to tradi- tional stereolithographic models. In 2013, the 3D printer used in the authors unit cost less than $1,500 NZD. A recently purchased UpBox 3D printer (Beijing Tiertime Technology Co. Ltd) has greater printing capacity and was purchased for approximately $3,700 NZD. Pang et al esti- mated the cost of producing a RP biomodel to be $20-30 USD per print.[4] In 2015, the material cost of attaining a RP biomodel (25-35 g of polylactic acid [PLA] or acrylonitrile butadiene styrene [ABS] polymer) in Christchurch, New Zealand was approximately $10 NZD. Now in 2020 this is approximately $2-3 NZD.
Christchurch Hospital Maxillofacial Surgery Unit Workflow
A summary of the workflow utilized in the authors unit is provided in Figure 1. Using pre-operative CT data, a stereolithographic model of the traumatized orbit is designed using a 3D Slicer platform (www.slicer.org) and built with the UpBox 3D printer (Beijing Tiertime Tech- nology Co. Ltd). Designing the model takes approximately 45 mins and printing times average 1.5 hours. “Prints” are fabricated with PLA or ABS polymer. If the zygomatico- maxillary complex is involved, a mirror of the contralateral orbit is printed. Preformed titanium mesh reconstruction plates from either Synthes Matrix MIDFACE (West Che- ster, PA) or Leibinger, Stryker Craniomaxillofacial, (Kala- mazoo, MI) are selected and adapted to the 3D model prior to sterilization. Alternatively, “in-house” custom milled mesh plates can be fabricated within the ISO-certified (ISO 9001) bioengineering facility at Christchurch Hospital (Figure 2). The “in-house” plates are fabricated using 0.5 mm thick B-265 grade 2 sheet titanium alloy certified by ASTM International (American Society for Testing and Materials) (West Conshohocken, PA). During surgery, the PSI is inserted in the optimal position and secured with a single screw at the infra-orbital rim. The patient’s orbit is scanned using the O-Arm 2D/3D surgical imaging system (Medtronic, Minneapolis, MN) (Figure 3). In the authors experience, this generally adds an additional 10 mins to operating time. The position of the PSI is assessed in 3 planes and adjusted if required followed by a subsequent intraoperative scan (Figure 4 and Figure 5). Once favorable implant position and contour is seen, a second screw can be used to secure the PSI prior to closure. Post-operative imaging is not required.

Figure 2.
Representative pictures of a milled orbital implant press to mold the custom fabricated titanium mesh plate to the desired anatomical contours.
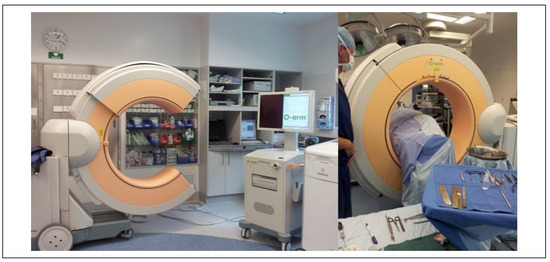
Figure 3.
Medtronic O-arm 2D/3D surgical imaging system (Minneapolis, MN) utilized for intraoperative imaging at Canterbury DHB.
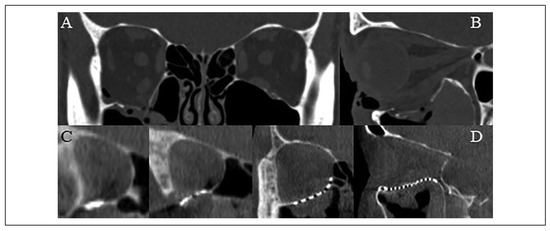
Figure 4.
(A) Coronal and (B) sagittal slices of the pre-operative computed tomography scan demonstrating an isolated right orbital floor blow-out fracture. (C) Coronal and (D) sagittal slices of the intraoperative O-arm “spin” demonstrating favorable position and contour of an “in-house” custom milled titanium mesh plate to reconstruct the orbit.
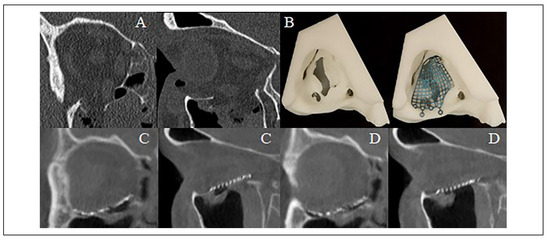
Figure 5.
(A) Isolated right orbital floor fracture with herniation of the inferior rectus muscle into the fracture defect. (B) 3D printed rapid prototype biomodel and pre-bent Leibinger reconstruction plate prior to sterilization. (C) First O-arm “spin” demonstrating plate malposition within the orbit and (D) second O-arm “spin” demonstrating favorable reconstruction after adjusting plate position.
Conclusion
RP and PSIs facilitate accurate anatomic reconstruction of orbital fractures and restoration of orbital volume. This relatively low-cost technology significantly reduces oper- ating time and the number of patients that require revision surgery and is further enhanced by intraoperative ima- ging. This ultimately leads to improved patient outcomes. In units equipped with this technology, RP and PSIs in combination with intraoperative imaging is now emerging as the standard of care in the management of orbital fractures.
Author Contributions
D.O.G. and C.G.T.L. contributed equally to the literature review and writing of the manuscript. J.E. oversaw the review and contrib- uted to developing the current protocol used at Canterbury DHB.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Acknowledgments
The authors wish to acknowledge the Biomechanical Engineering Department at Christchurch Hospital for their assistance in devel- oping the presented orbit reconstruction protocol.
Conflicts of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Lim C, Campbell D, Clucas D. Rapid prototyping technology in orbital floor reconstruction: application in three patients. Craniomaxillofac Trauma Reconstr.
- Lim CG, Campbell DI, Cook N, Erasmus J. A case series of rapid prototyping and intraoperative imaging in orbital recon- struction. Craniomaxillofac Trauma Reconstr.
- Nguyen E, Lockyer J, Erasmus J, Lim C. Improved outcomes of orbital reconstruction with intraoperative imaging and rapid prototyping. J Oral Maxillofac Surg, 1211.
- Pang SS, Fang C, Chan JY. Application of three-dimensional printing technology in orbital floor fracture reconstruction. Trauma Case Rep.
- Kim YC, Min KH, Choi JW, Koh KS, Oh TS, Jeong WS. Patient-specific puzzle implant preformed with 3D-printed rapid prototype model for combined orbital floor and medial wall fracture. J Plast Reconstr Aesthetic Surg.
- Kim YC, Jeong WS, Park T, Choi JW, Koh KS, Oh TS. The accuracy of patient specific implant prebented with 3D- printed rapid prototype model for orbital wall reconstruction. J Cranio-Maxillofacial Surg.
- Kang S, Kwon J, Ahn CJ, et al. Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction. Eye, 1864.
- Hoelzle F, Klein M, Schwerdtner O, et al. Intraoperative computed tomography with the mobile CT Tomoscan M dur- ing surgical treatment of orbital fractures. Int J Oral Maxil- lofac Surg.
- Sigron GR, Ru¨edi N, Chammartin F, et al. Three-dimensional analysis of isolated orbital floor fractures pre- and post- reconstruction with standard titanium Meshes and “Hybrid” patient-specific implants. J Clin Med, 1579.
- Salmi M, Paloheimo KS, Tuomi J, Wolff J, Ma¨kitie A. Accu- racy of medical models made by additive manufacturing (rapid manufacturing). J Craniomaxillofac Surg, 6: 41(7).
- Shaye DA, Tollefson TT, Strong EB. Use of intraoperative computed tomography for maxillofacial reconstructive sur- gery. JAMA Facial Plast Surg.
- Ludlow JB, Ivanovic M. Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg, Oral Med Oral Pathol Oral Radiol Endod.
- Zhang J, Weir V, Fajardo L, Lin J, Hsiung H, Ritenour ER. Dosimetric characterization of a cone-beam O-armTM ima- ging system. J Xray Sci Technol.
- Pitteloud N, Gamulin A, Barea C, Damet J, Racloz G, Sans- Merce M. Radiation exposure using the O-arm® surgical ima- ging system. Eur Spine J.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the AO Foundation. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).