Etiopathogenesis of Trismus in Patients With Head and Neck Cancer: An Exploratory Literature Review
Abstract
:Introduction
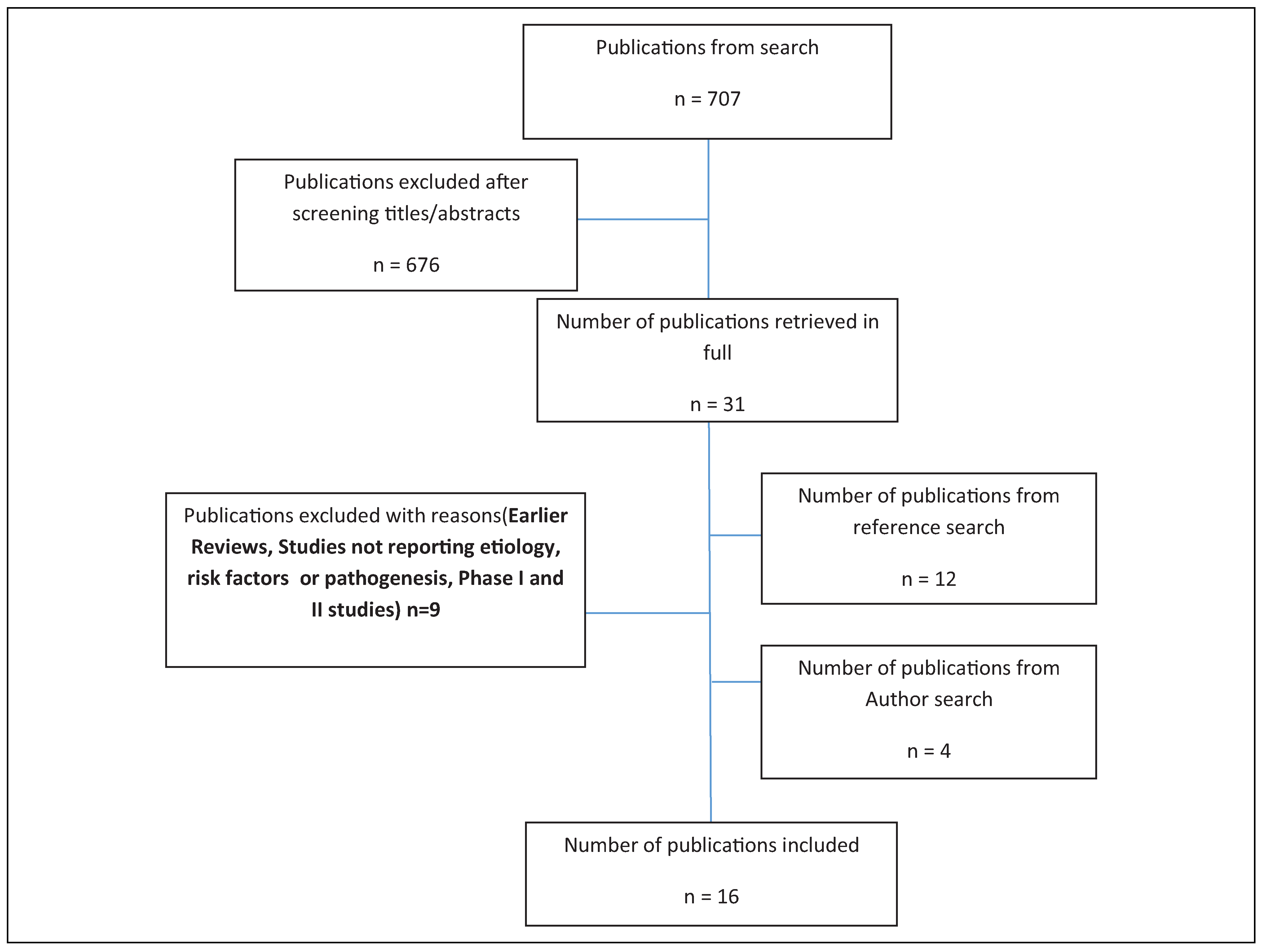
Materials and Methods
Search Methods
Inclusion and Exclusion Criteria
Results
Discussion
- Trismus due to tumor extension: The primary sites of malignant tumors that induce trismus are commonly the tongue, maxillary sinus, nasopharynx, retromolar trigone, infratemporal fossa, nasal cavity, hypopharynx and cervical esophagus, parotid gland, floor of the mouth, buccal mucosa, larynx, oropharynx, thyroid, palate, ear, and submandibular gland.[15] Among them, the patients with cancer of the tonsils are the one most prone to develop trismus.[16] The proximity of this site to the masticator space and infratemporal fossa could be the reason.
- Primary, benign: Benign neoplasms such as angioma, neurofibroma, neurolemmoma, osteoma, meningioma, and adamantinoma may arise in the infratemporal fossa or invade it from surrounding areas and give rise to trismus.[15] Osteochondromas, myxomas, osteomas, and hemangiomas have also been described as lesions which can cause limitation of mandibular movement.
- Primary, malignant: These are more likely to cause trismus. Carcinomas are tumors of epithelial origin. Epithelial tumors of the buccal mucosa, retromolar area, palate, pharynx, parotid, or maxillary sinus may infiltrate infratemporal fossa. Since carcinoma cells are genetically unstable, they start from an activated phenotype.
Postsurgical Trismus
Postirradiation Trismus
| Author | Year | Study Type | Findings |
|---|---|---|---|
| Chon-Jong Wang et al.[37] | 2005 | Prospective, single-armed measurement | Patients with nasopharyngeal cancer had a mean decrease in initial interincisal distance of 32% at 4 years after radiotherapy. It was rapid at 1 to 9 months after radiation therapy, then became slower and protracted over later years. |
| Bhatia et al.[35] | 2009 | Retrospective | The prevalence of trismus increases with increasing doses of RT, and levels in excess of 60 Gy are more likely to cause trismus. An abnormal proliferation of fibroblasts is an important initial event |
| Paul Okunieff et al.[38] | 2004 | Non-RCT | FGF2 is chemotactic and mitotic for fibroblasts, whereas TGFβ1 stimulates fibroblast proliferation and premature end differentiation, leading to excessive extracellular matrix glycoprotein production |
| Maria GebreMedhin et al.[39] | 2016 | Cohort retrospective | Mean radiation dose to the ipsilateral masseter muscle is an important risk factor for trismus development. |
| Hsuing et al.[40] | 2008 | Cohort prospective | Radiation-induced trismus results from postradiotherapy muscle fibrosis and scarring, fibrosis of the ligaments around the TMJ, and scarring of the pterygomandibular raphes. |
| Louise Kent et al.[41] | 2008 | Retrospective cohort | Direct cell damage combined with regional loss of vascular perfusion results in bone and soft tissue necrosis and finally leads to fibrosis to the muscles of mastication. |
| Marc Goldstein et al.[42] | 1999 | Prospective cohort | As dose to the temporomandibular joint and pterygoid muscles increases, maximal jaw opening decreases linearly. Doses as low as 1493 cGy to the TMJ resulted in functional impairment. |
| Lisette van der Molen et al.[45] | 2013 | Prospective longitudinal study | Dose parameters of masseter and pterygoid muscles were significant predictors of trismus at 10 weeks posttreatment. And at 1-year posttreatment therapy, dose parameters of all mastication structures are strong predictors for subjective mouth-opening problems. |
| Teguh et al.[43] | 2008 | Retrospective cohort | In radiation-induced fibrosis, there is presence of infiltrating inflammatory cells, atypical fibroblasts, and large amounts of various extracellular matrix components. |
| Ichimura and Tanaka[15] | 1993 | Retrospective | Benign neoplasms in the parapharyngeal space and simple compression of the muscles do not induce trismus. |
| George P. Katsantonis et al.[44] | 1989 | Retrospective cohort study | Lesions arising in the deep lobe of the parotid will naturally extend into the parapharyngeal space and the infratemporal fossa. Infratemporal fossa invasion is usually associated with trismus and pain along the distribution of V3 and occasionally V2 |
| Lisette van der Molen et al.[45] | 2013 | Prospective | Dose parameters of masseter and pterygoid muscles were significant predictors of trismus |
| Frank R. Miller et al.[46] | 1996 | Retrospective | Compared with benign parapharyngeal space neoplasms, the malignant tumors were much more likely to be associated with pain, trismus. |
| R. Lee et al.[47] | 2012 | Prospective | Current or previous heavy drinkers had a substantially smaller chance of developing trismus. |
| The alcohol also acts as a muscle relaxant and counteracts the laying down of collagen. | |||
| Joakim Johnson et al.[10] | 2010 | Retrospective | Trismus incidence (≤35 mm) was highest in the patients treated for parotid gland tumors, followed by patients treated for nasopharyngeal cancers and submandibular gland tumors |
| Christina Hague et al.[48] | 2018 | Prospective randomized trial | Restricted mouth opening developed when radiation mean doses was >40 Gy to the ipsilateral block. |
Conclusion
Funding
Conflicts of Interest
References
- Krogman, W.M. Illustrated Dictionary of Dentistry. By S. Jablonski; W.B. Saunders: Philadelphia, 1982; p. xvii + 919. [Google Scholar]
- Bensadoun, R.J.; Riesenbeck, D.; Lockhart, P.B.; et al. A systematic review of trismus induced by cancer therapies in head and neck cancer patients. Support Care Cancer. 2010, 18, 1033–1038. [Google Scholar] [PubMed]
- Santiago-Rosado, L.M.; Lewison, C.S. Trismus. In StatPearls; StatPearls Publishing: Treasure Island (FL). Available online: http://www.ncbi.nlm.nih.gov/books/NBK493203/ (accessed on 11 May 2019).
- Dijkstra, P.U.; Huisman, P.M.; Roodenburg, J.L. Criteria for trismus in head and neck oncology. Int J Oral Maxillofac Surg. 2006, 35, 337–342. [Google Scholar]
- Scott, B.; Butterworth, C.; Lowe, D.; Rogers, S.N. Factors associated with restricted mouth opening and its relationship to health-related quality of life in patients attending a Maxillofacial Oncology Clinic. Oral Oncol. 2008, 44, 430–438. [Google Scholar]
- Jen, Y.M.; Lin, Y.S.; Su, W.F.; et al. Dose escalation using twicedaily radiotherapy for nasopharyngeal carcinoma: does heavier dosing result in a happier ending? Int J Radiat Oncol Biol Phys. 2002, 54, 14–22. [Google Scholar]
- Nguyen, T.D.; Panis, X.; Froissart, D.; Legros, M.; Coninx, P.; Loirette, M. Analysis of late complications after rapid hyperfractionated radiotherapy in advanced head and neck cancers. Int J Radiat Oncol Biol Phys. 1988, 14, 23–25. [Google Scholar] [PubMed]
- Dijkstra, P.U.; Kalk, W.W.I.; Roodenburg, J.L.N. Trismus in head and neck oncology: a systematic review. Oral Oncol. 2004, 40, 879–889. [Google Scholar] [PubMed]
- Weber, C.; Dommerich, S.; Pau, H.W.; Kramp, B. Limited mouth opening after primary therapy of head and neck cancer. Oral Maxillofac Surg. 2010, 14, 169–173. [Google Scholar]
- Johnson, J.; van As-Brooks, C.J.; Fagerberg-Mohlin, B.; Finizia, C. Trismus in head and neck cancer patients in Sweden: incidence and risk factors. Med Sci Monit Int Med J Exp Clin Res. 2010, 16, CR278–CR282. [Google Scholar]
- Van Cann, E.M.; Dom, M.; Koole, R.; Merkx, M.A.; Stoelinga, P.J. Health related quality of life after mandibular resection for oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2005, 41, 687–693. [Google Scholar]
- Melchers, L.J.; Van Weert, E.; Beurskens, C.H.; et al. Exercise adherence in patients with trismus due to head and neck oncology: a qualitative study into the use of the Therabite. Int J Oral Maxillofac Surg. 2009, 38, 947–954. [Google Scholar]
- Wranicz, P.; Herlofson, B.B.; Evensen, J.F.; Kongsgaard, U.E. Prevention and treatment of trismus in head and neck cancer: a case report and a systematic review of the literature. Scand J Pain. 2010, 1, 84–88. [Google Scholar] [PubMed]
- Beekhuis, G.J.; Harrington, E.B. Trismus. Etiology and management of inability to open the mouth. Laryngoscope. 1965, 75, 1234–1258. [Google Scholar] [PubMed]
- Rapidis, A.D.; Dijkstra, P.U.; Roodenburg, J.L.; et al. Trismus in patients with head and neck cancer:etiopathogenesis, diagnosis and management. Clin Otolaryngol. 2015, 40, 516–526. [Google Scholar] [PubMed]
- Pauli, N.; Johnson, J.; Finizia, C.; Andréll, P. The incidence of trismus and long-term impact on health-related quality of life in patients with head and neck cancer. Acta Oncol. 2013, 52, 1137–1145. [Google Scholar]
- Irani, S. Metastasis to the jawbones: a review of 453 cases. J Int Soc Prev Community Dent. 2017, 7, 71–81. [Google Scholar]
- Varadarajan, V.V.; Pace, E.K.; Patel, V.; Sawhney, R.; Amdur, R.J.; Dziegielewski, P.T. Follicular thyroid carcinoma metastasis to the facial skeleton: a systematic review. BMC Cancer. 2017, 17, 225. [Google Scholar]
- Kveton, J.F.; Pillsbury, H.C. Breaking trismus to facilitate drainage of peritonsillar abscess. Laryngoscope. 1980, 90(Pt 1), 1892–1893. [Google Scholar]
- Ozyar, E.; Cengiz, M.; Gurkaynak, M.; Atahan, I.L. Trismus as a presenting symptom in nasopharyngeal carcinoma. Radiother Oncol. 2005, 77, 73–76. [Google Scholar]
- Scott, B.; D’Souza, J.; Perinparajah, N.; Lowe, D.; Rogers, S.N. Longitudinal evaluation of restricted mouth opening (trismus) in patients following primary surgery for oral and oropharyngeal squamous cell carcinoma. Br J Oral Maxillofac Surg. 2011, 49, 106–111. [Google Scholar]
- Harrison, J.D.; Stather, J.W. The assessment of doses and effects from intakes of radioactive particles. J Anat. 1996, 189(Pt 3), 521–530. [Google Scholar]
- Travis, E.L. Organizational response of normal tissues to irradiation. Semin Radiat Oncol. 2001, 11, 184–196. [Google Scholar] [PubMed]
- Terasaki, Y.; Ohsawa, I.; Terasaki, M.; et al. Hydrogen therapy attenuates irradiation-induced lung damage by reducing oxidative stress. Am J Physiol Lung Cell Mol Physiol. 2011, 301, L415–L426. [Google Scholar] [PubMed]
- Chaudière, J.; Ferrari-Iliou, R. Intracellular antioxidants: From chemical to biochemical mechanisms. Food Chem Toxicol Int J Publ Br Ind Biol Res Assoc. 1999, 37, 949–962. [Google Scholar]
- Boerma, M.; Hauer-Jensen, M. Potential targets for intervention in radiation-induced heart disease. Curr Drug Targets. 2010, 11, 1405–1412. [Google Scholar]
- Lefaix, J.L.; Daburon, F. Diagnosis of acute localized irradiation lesions: review of the French experimental experience. Health Phys. 1998, 75, 375–384. [Google Scholar] [CrossRef]
- Calveley, V.L.; Khan, M.A.; Yeung, I.W.T.; Vandyk, J.; Hill, R.P. Partial volume rat lung irradiation: Temporal fluctuations of in-field and out-of-field DNA damage and inflammatory cytokines following irradiation. Int J Radiat Biol. 2005, 81, 887–899. [Google Scholar]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012, 122, 787–795. [Google Scholar]
- Mathew, M.; Thomas, S.M. The Cellular Microenvironment of Head and Neck Squamous Cell Carcinoma. 2012. Available online: https://www.intechopen.com/books/squamous-cell-carcinoma/the-cellular-microenvironment-of-head-and-neck-squamous-cell-carcinoma (accessed on 23 July 2019).
- Yarnold, J.; Brotons, M.C. Pathogenetic mechanisms in radiation fibrosis. Radiother Oncol. 2010, 97, 149–161. [Google Scholar]
- Darby, I.A.; Hewitson, T.D. Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol. 2007, 257, 143–179. [Google Scholar]
- Pardo, A. Matrix metalloproteases in aberrant fibrotic tissue remodeling. Proc Am Thorac Soc. 2006, 3, 383–388. [Google Scholar]
- Toussaint, O.; Remacle, J.; Dierick, J.F.; et al. Approach of evolutionary theories of ageing, stress, senescence-like phenotypes, calorie restriction and hormesis from the view point of far-from-equilibrium thermodynamics. Mech Ageing Dev. 2002, 123, 937–946. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, K.S.S.; King, A.D.; Paunipagar, B.K.; et al. MRI findings in patients with severe trismus following radiotherapy for nasopharyngeal carcinoma. Eur Radiol. 2009, 19, 2586–2593. [Google Scholar] [CrossRef]
- Kraaijenga, S.A.; Hamming Vrieze, O.; Verheijen, S.; et al. Radiation dose to the masseter and medial pterygoid muscle in relation to trismus after chemoradiotherapy for advanced head and neck cancer. Head Neck. 2019, 41, 1387–1394. [Google Scholar] [PubMed]
- Wang, C.-J.; Huang, E.-Y.; Hsu, H.-C.; Chen, H.-C.; Fang, F.-M.; Hsiung, C.-Y. The degree and timecourse assessment of radiation-induced trismus occurring after radiotherapy for nasopharyngeal cancer. Laryngoscope. 2005, 115, 1458–1460. [Google Scholar]
- Okunieff, P.; Augustine, E.; Hicks, J.E.; et al. Pentoxifylline in the Treatment of Radiation-Induced Fibrosis. J Clin Oncol. 2004, 22, 2207–2213. [Google Scholar] [PubMed]
- Gebre-Medhin, M.; Haghanegi, M.; Robért, L.; Kjellén, E.; Nilsson, P. Dose-volume analysis of radiation-induced trismus in head and neck cancer patients. Acta Oncol. 2016, 55, 1313–1317. [Google Scholar]
- Hsiung, C.-Y.; Huang, E.-Y.; Ting, H.-M.; Huang, H.-Y. Intensitymodulated radiotherapy for nasopharyngeal carcinoma: The reduction of radiation-induced trismus. Br J Radiol. 2008, 81, 809–814. [Google Scholar]
- Louise Kent, M.; Brennan, M.T.; Noll, J.L.; et al. Radiationinduced trismus in head and neck cancer patients. Support Care Cancer. 2008, 16, 305–309. [Google Scholar]
- Goldstein, M.; Maxymiw, W.G.; Cummings, B.J.; Wood, R.E. The effects of antitumor irradiation on mandibular opening and mobility: A prospective study of 58 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontology. 1999, 88, 365–373. [Google Scholar] [CrossRef]
- Teguh, D.N.; Levendag, P.C.; Voet, P.; et al. Trismus in patients with oropharyngeal cancer: Relationship with dose in structures of mastication apparatus. Head Neck. 2008, 30, 622–630. [Google Scholar]
- Katsantonis, G.P.; Friedman, W.H.; Rosenblum, B.N. The surgical management of advanced malignancies of the parotid gland. Otolaryngol Head Neck Surg. 1989, 101, 633–640. [Google Scholar] [CrossRef] [PubMed]
- van der Molen, L.; Heemsbergen, W.D.; de Jong, R.; et al. Dysphagia and trismus after concomitant chemo-IntensityModulated Radiation Therapy (chemo-IMRT) in advanced head and neck cancer; dose-effect relationships for swallowing and mastication structures. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2013, 106, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.R.; Wanamaker, J.R.; Lavertu, P.; Wood, B.G. Magnetic resonance imaging and the management of parapharyngeal space tumors. Head Neck. 1996, 18, 67–77. [Google Scholar] [CrossRef]
- Lee, R.; Slevin, N.; Musgrove, B.; Swindell, R.; Molassiotis, A. Prediction of post-treatment trismus in head and neck cancer patients. Br J Oral Maxillofac Surg. 2012, 50, 328–332. [Google Scholar] [CrossRef]
- Hague, C.; Beasley, W.; Garcez, K.; et al. Prospective evaluation of relationships between radiotherapy dose to masticatory apparatus and trismus. Acta Oncol. 2018, 57, 1038–1042. [Google Scholar] [CrossRef]
© 2020 by the author. The Author(s) 2020.
Share and Cite
Raj, R.; Thankappan, K.; Janakiram, C.; Iyer, S.; Mathew, A. Etiopathogenesis of Trismus in Patients With Head and Neck Cancer: An Exploratory Literature Review. Craniomaxillofac. Trauma Reconstr. 2020, 13, 219-225. https://doi.org/10.1177/1943387520917518
Raj R, Thankappan K, Janakiram C, Iyer S, Mathew A. Etiopathogenesis of Trismus in Patients With Head and Neck Cancer: An Exploratory Literature Review. Craniomaxillofacial Trauma & Reconstruction. 2020; 13(3):219-225. https://doi.org/10.1177/1943387520917518
Chicago/Turabian StyleRaj, Radhu, Krishnakumar Thankappan, Chandrasekhar Janakiram, Subramania Iyer, and Anil Mathew. 2020. "Etiopathogenesis of Trismus in Patients With Head and Neck Cancer: An Exploratory Literature Review" Craniomaxillofacial Trauma & Reconstruction 13, no. 3: 219-225. https://doi.org/10.1177/1943387520917518
APA StyleRaj, R., Thankappan, K., Janakiram, C., Iyer, S., & Mathew, A. (2020). Etiopathogenesis of Trismus in Patients With Head and Neck Cancer: An Exploratory Literature Review. Craniomaxillofacial Trauma & Reconstruction, 13(3), 219-225. https://doi.org/10.1177/1943387520917518




