Surgical Treatment of Fibroosseous Lesion in Young Patient with Reduced Mouth Opening
Abstract
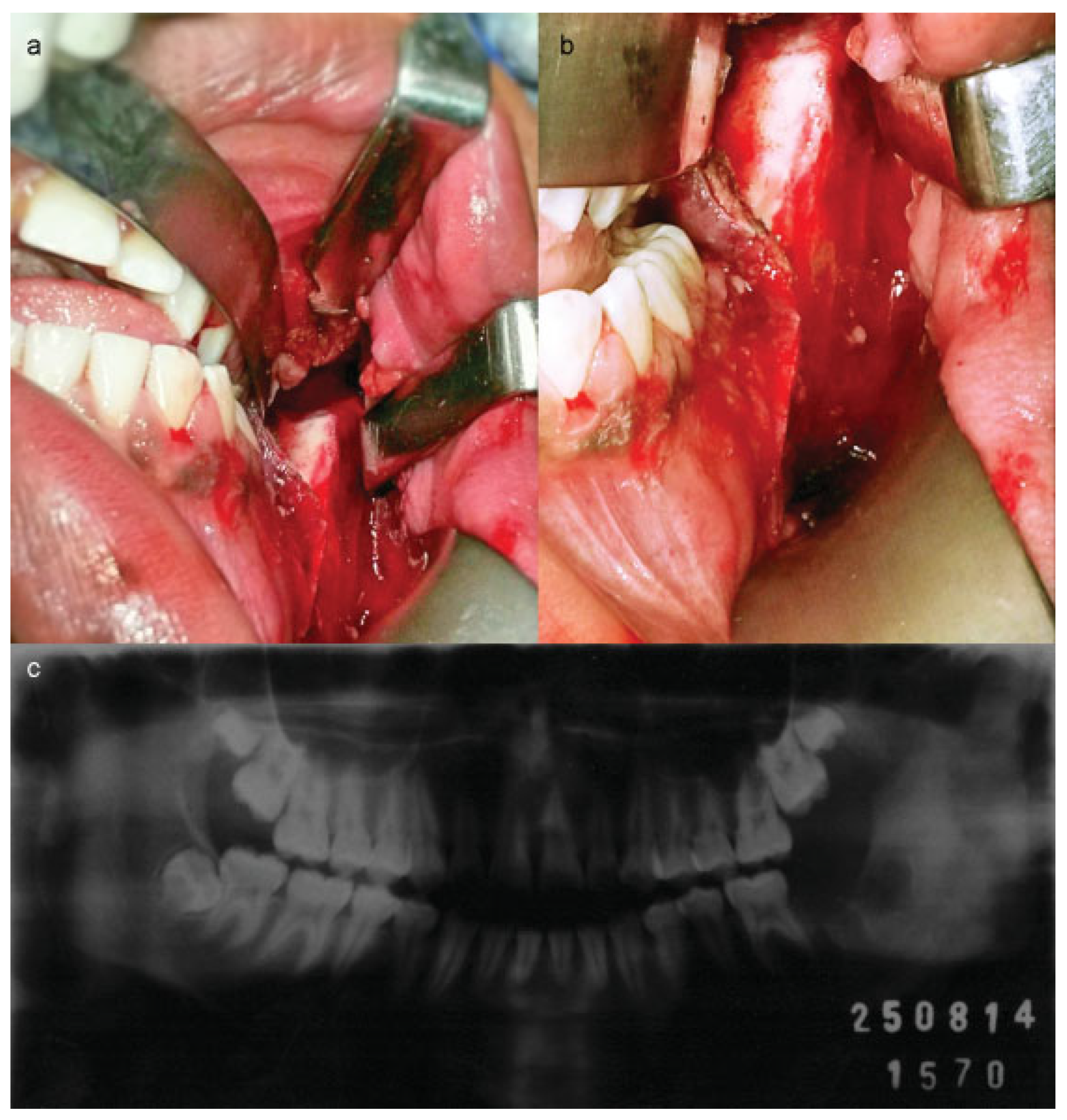
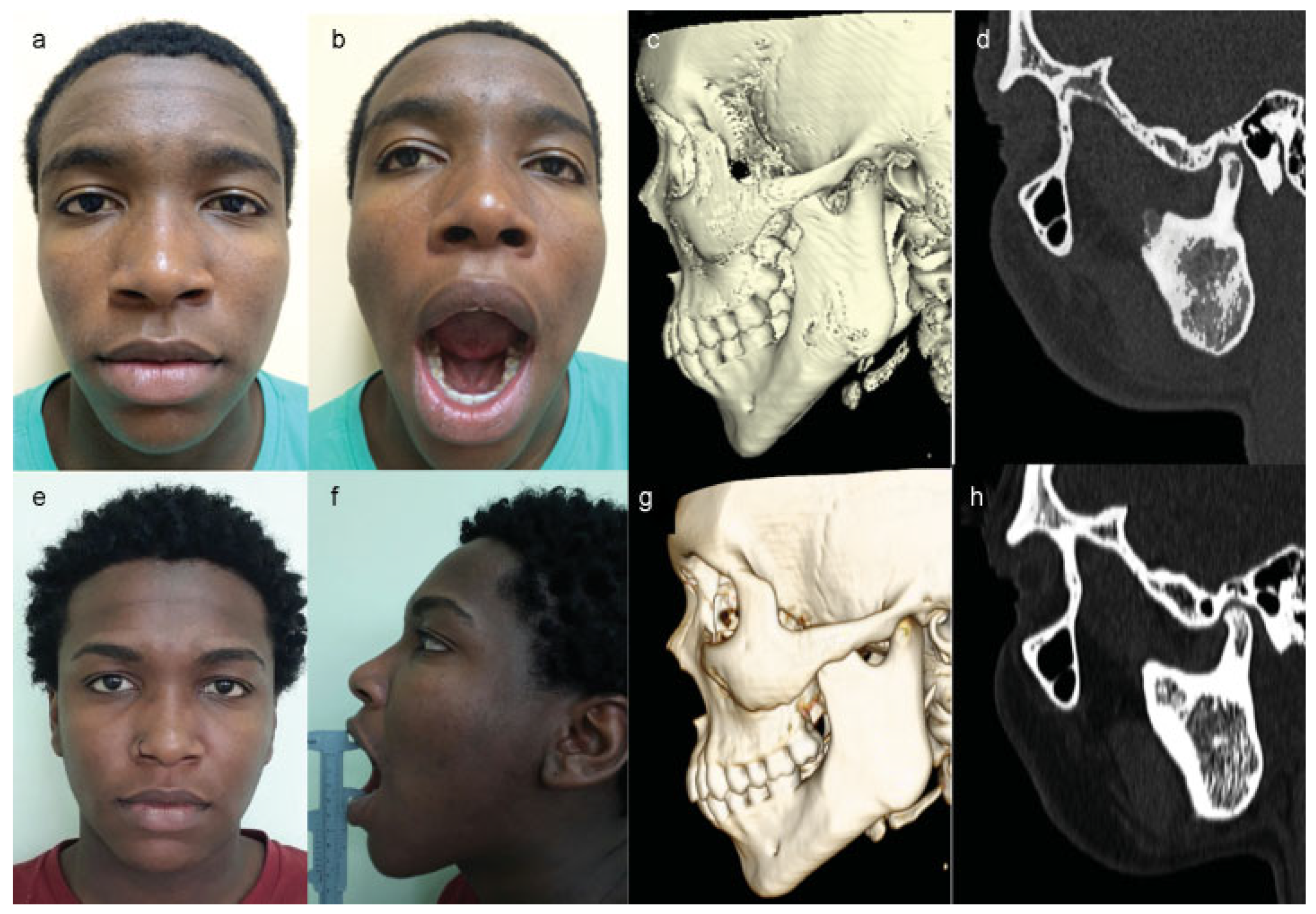
:Case Report
Discussion
Final Considerations
Acknowledgments
Conflicts of Interest
References
- Waldron, C.A. Fibro-osseous lesions of the jaws. J Oral Maxillofac Surg 1993, 51, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Kishore, P.K.; Mohan, A.P.; Venkatesh, V.; Kumar, B.P.; Gandla, D. Management and treatment outcomes of maxillofacial fibroosseous lesions: a retrospective study. J Maxillofac Oral Surg 2015, 14, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Soto, A.; Baquero-Ruiz de la Hermosa, M.C.; Minguez-Martínez, I.; et al. Management of fibro-osseous lesions of the craniofacial area. Presentation of 19 cases and review of the literature. Med Oral Patol Oral Cir Bucal 2013, 18, 479–485. [Google Scholar] [CrossRef]
- MacDonald, D.S. Maxillofacial fibro-osseous lesions. Clin Radiol 2015, 70, 25–36. [Google Scholar] [CrossRef] [PubMed]
- MacDonald-Jankowski, D.S. Fibro-osseous lesions of the face and jaws. Clin Radiol 2004, 59, 11–25. [Google Scholar] [CrossRef]
- McCarthy, E.F. Fibro-osseous lesions of the maxillofacial bones. Head Neck Pathol 2013, 7, 5–10. [Google Scholar] [CrossRef]
- Tabareau-Delalande, F.; Collin, C.; Gomez-Brouchet, A.; et al. Diagnostic value of investigating GNAS mutations in fibro-osseous lesions: a retrospective study of 91 cases of fibrous dysplasia and 40 other fibro-osseous lesions. Mod Pathol 2013, 26, 911–921. [Google Scholar] [CrossRef]
- Akintoye, S.O.; Boyce, A.M.; Collins, M.T. Dental perspectives in fibrous dysplasia and McCune-Albright syndrome. Oral Surg Oral Med Oral Pathol Oral Radiol 2013, 116, e149–e155. [Google Scholar] [CrossRef]
- Prabhu, S.; Sharanya, S.; Naik, P.M.; et al. Fibro-osseous lesions of the oral and maxillo-facial region: Retrospective analysis for 20 years. J Oral Maxillofac Pathol 2013, 17, 36–40. [Google Scholar] [CrossRef]
- Eversole, R.; Su, L.; ElMofty, S. Benign fibro-osseous lesions of the craniofacial complex. A review. Head Neck Pathol 2008, 2, 177–202. [Google Scholar] [CrossRef]
- Chapurlat, R.D. Fibrous dysplasia of bone and McCune-Albright syndrome. IBMS BoneKEy 2010, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Nardone, V.; D’Asta, F.; Brandi, M.L. Pharmacological management of osteogenesis. Clinics (Sao Paulo) 2014, 69, 438–446. [Google Scholar] [CrossRef]
- Chapurlat, R.D.; Gensburger, D.; Jimenez-Andrade, J.M.; Ghilardi, J.R.; Kelly, M.; Mantyh, P. Pathophysiology and medical treatment of pain in fibrous dysplasia of bone. Orphanet J Rare Dis 2012, 7 (Suppl 1), S3. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.M.; Khan, S.N.; O’Connor, W.J.; et al. Bisphosphonate therapy in fibrous dysplasia. Clin Orthop Relat Res 2001, 382, 6–12. [Google Scholar] [CrossRef]
- Lala, R.; Matarazzo, P.; Bertelloni, S.; Buzi, F.; Rigon, F.; de Sanctis, C. Pamidronate treatment of bone fibrous dysplasia in nine children with McCune-Albright syndrome. Acta Paediatr 2000, 89, 188–193. [Google Scholar] [CrossRef]
- Petrocelli, M.; Kretschmer, W. Conservative treatment and implant rehabilitation of the mandible in a case of craniofacial fibrous dysplasia: a case report. J Oral Maxillofac Surg 2014, 72, e1–902.e6. [Google Scholar] [CrossRef]
- Bajwa, M.S.; Ethunandan, M.; Flood, T.R. Oral rehabilitation with endosseous implants in a patient with fibrous dysplasia (McCune-Albright syndrome): a case report. J Oral Maxillofac Surg 2008, 66, 2605–2608. [Google Scholar] [CrossRef]
- Villar-Puchades, R.; Ramos-Medina, B. Virtual surgical planning for extensive fibrous dysplasia in the mandible. Aesthetic Plast Surg 2014, 38, 941–945. [Google Scholar] [CrossRef]
- Mendonça Caridad, J.J.; Platas, F., Jr. Fibrous dysplasia of the mandible: Surgical treatment with platelet-rich plasma and a corticocancellous iliac crest graft-report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008, 105, e12–e18. [Google Scholar] [CrossRef]
- Metwally, T.; Burke, A.; Tsai, J.Y.; Collins, M.T.; Boyce, A.M. Fibrous dysplasia and medication-related osteonecrosis of the jaw. J Oral Maxillofac Surg 2016, 74, 1983–1999. [Google Scholar] [CrossRef]
- Eisenberg, E.; Eisenbud, L. Benign fibro-osseous disease: current concepts in historical perspective. Oral Maxillofac Surg Clin North Am 1997, 9, 551–562. [Google Scholar] [CrossRef]
- Abdelkarim, A.; Green, R.; Startzell, J.; Preece, J. Craniofacial polyostotic fibrous dysplasia: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008, 106, e49–e55. [Google Scholar] [CrossRef] [PubMed]
- Majoor, B.C.; Appelman-Dijkstra, N.M.; Fiocco, M.; van de Sande, M.A.; Dijkstra, P.S.; Hamdy, N.A. Outcome of long-term bisphosphonate therapy in McCune-Albright syndrome and polyostotic fibrous dysplasia. J Bone Miner Res 2017, 32, 264–276. [Google Scholar] [CrossRef] [PubMed]
- De Melo, W.M.; Sonoda, C.K.; Hochuli-Vieira, E. Monostotic fibrous dysplasia of the mandible. J Craniofac Surg 2012, 23, e452–e454. [Google Scholar] [CrossRef]
- Zeng, H.F.; Lu, J.J.; Teng, L.; et al. Surgical treatment of craniomaxillofacial fibrous dysplasia: functionally or aesthetically? J Craniofac Surg 2013, 24, 758–762. [Google Scholar] [CrossRef]
- Macdonald-Jankowski, D.S.; Li, T.K. Fibrous dysplasia in a Hong Kong community: the clinical and radiological features and outcomes of treatment. Dentomaxillofac Radiol 2009, 38, 63–72. [Google Scholar] [CrossRef]


© 2017 by the author. The Author(s) 2017.
Share and Cite
de Azambuja Carvalho, P.H.; Torriani, M.A.; Post, L.K.; Chagas, O.L., Júnior. Surgical Treatment of Fibroosseous Lesion in Young Patient with Reduced Mouth Opening. Craniomaxillofac. Trauma Reconstr. 2018, 11, 314-319. https://doi.org/10.1055/s-0037-1608697
de Azambuja Carvalho PH, Torriani MA, Post LK, Chagas OL Júnior. Surgical Treatment of Fibroosseous Lesion in Young Patient with Reduced Mouth Opening. Craniomaxillofacial Trauma & Reconstruction. 2018; 11(4):314-319. https://doi.org/10.1055/s-0037-1608697
Chicago/Turabian Stylede Azambuja Carvalho, Pedro Henrique, Marcos Antonio Torriani, Letícia Kirst Post, and Otacílio Luiz Chagas, Júnior. 2018. "Surgical Treatment of Fibroosseous Lesion in Young Patient with Reduced Mouth Opening" Craniomaxillofacial Trauma & Reconstruction 11, no. 4: 314-319. https://doi.org/10.1055/s-0037-1608697
APA Stylede Azambuja Carvalho, P. H., Torriani, M. A., Post, L. K., & Chagas, O. L., Júnior. (2018). Surgical Treatment of Fibroosseous Lesion in Young Patient with Reduced Mouth Opening. Craniomaxillofacial Trauma & Reconstruction, 11(4), 314-319. https://doi.org/10.1055/s-0037-1608697


