Repair of Occipital Bone Defects in Neurofibromatosis Type 1 by Means of CAD/CAM Prefabricated Titanium Plates
Abstract
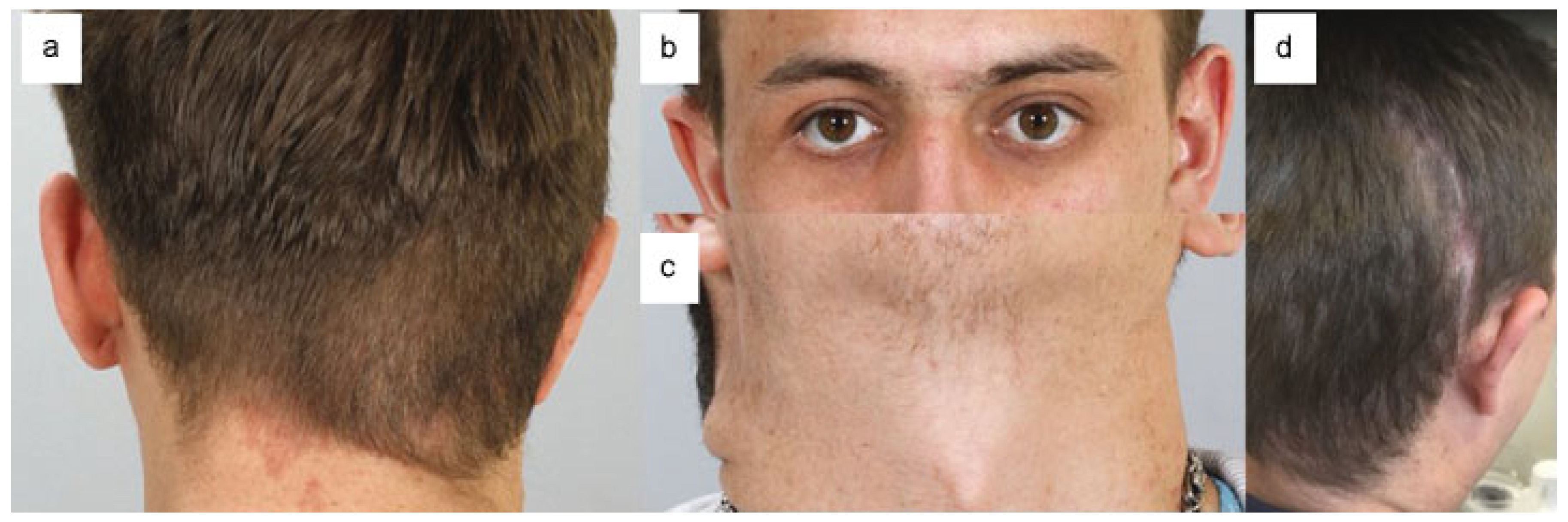
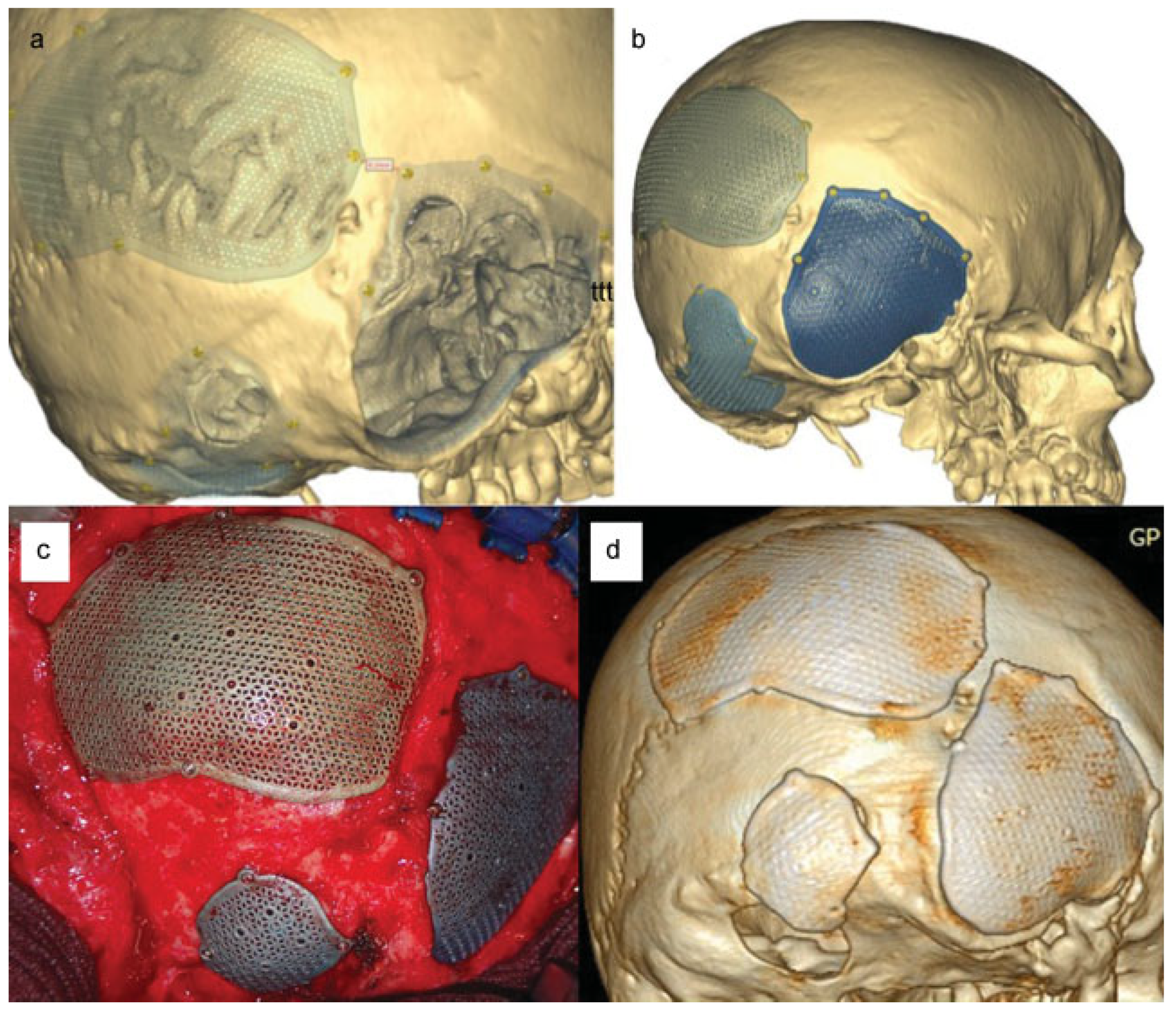
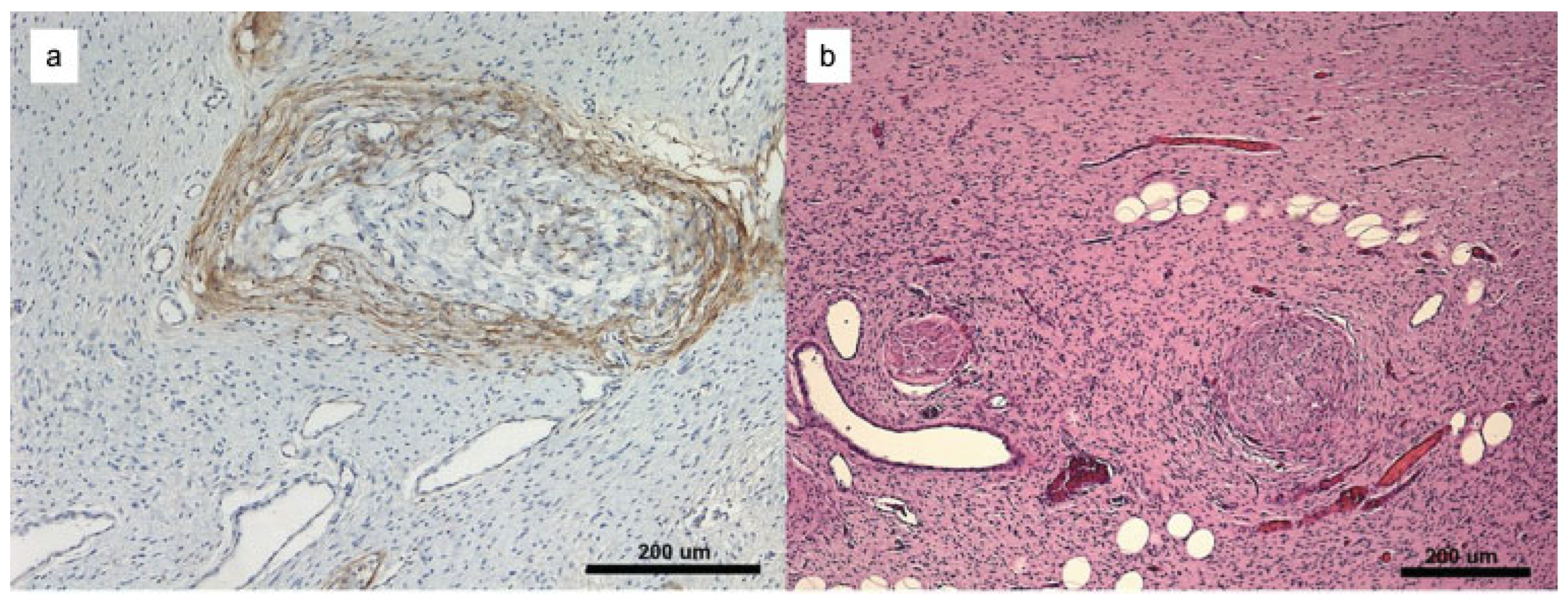
:Case Report
Discussion
Conclusion
References
- Gutmann, D.H.; Ferner, R.E.; Listernick, R.H.; Korf, B.R.; Wolters, P.L.; Johnson, K.J. Neurofibromatosis type 1. Nat Rev Dis Primers 2017, 3, 17004. [Google Scholar] [CrossRef] [PubMed]
- Chernoff, K.A.; Schaffer, J.V. Cutaneous and ocular manifestations of neurocutaneous syndromes. Clin Dermatol 2016, 34, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Joffe, N. Calcarial bone defects involving the lambdoid suture in neurofibromatosis. Br J Radiol 1965, 38, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Arrington, D.K.; Danehy, A.R.; Peleggi, A.; Proctor, M.R.; Irons, M.B.; Ullrich, N.J. Calvarial defects and skeletal dysplasia in patients with neurofibromatosis Type 1. J Neurosurg Pediatr 2013, 11, 410–416. [Google Scholar] [CrossRef]
- Davidson, K.C. Cranial and intracranial lesions in neurofibromatosis. Am J Roentgenol Radium Ther Nucl Med 1966, 98, 550–556. [Google Scholar] [CrossRef]
- Handa, J.; Koyama, T.; Shimizu, Y.; Yoneda, S. Skull defect involving the lambdoid suture in neurofibromatosis. Surg Neurol 1975, 3, 119–121. [Google Scholar]
- Weber, K.; Grauthoff, H.J. Defect of lambda suture: Typical change in Recklinghausen’s neurofibromatosis [in German]. Fortschr Geb Rontgenstr Nuklearmed 1973, 118, 230–231. [Google Scholar] [CrossRef]
- Ohaegbulam, S.C. “Congenital” plexiform neurofibroma of the occipital scalp. Case report. J Neurosurg 1977, 46, 245–247. [Google Scholar] [CrossRef]
- Mann, H.; Kozic, Z.; Medinilla, O.R. Computed tomography of lambdoid calvarial defect in neurofibromatosis. A case report. Neuroradiology 1983, 25, 175–176. [Google Scholar] [CrossRef]
- Solanki, C.; Ramachandran, S.; Devi, B.I.; Sharma, R. Calvarial defects in the region of the lambdoid suture in neurofibromatosis type-1 patients. J Pediatr Neurosci 2015, 10, 22–24. [Google Scholar]
- Renshaw, A.; Borsetti, M.; Nelson, R.J.; Orlando, A. Massive plexiform neurofibroma with associated meningo-encephalocoele and occipital bone defect presenting as a cervical mass. Br J Plast Surg 2003, 56, 514–517. [Google Scholar] [CrossRef] [PubMed]
- Dadlani, R.; Sadanand, V.; Ghosal, N.; Hegde, A.S. Congenital giant plexiform neurofibroma with occipital calvarial dysplasia in association with meningoencephalocele in neurofibromatosis Type 1 and segmental neurofibromatosis: Report of 2 cases. J Neurosurg Pediatr 2013, 12, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Helmholz, H.R.; Cushing, H. Elephantiasis nervorum of the scalp, a manifestation of Von Recklinghausen’s disease. Am J Med Sci 1906, 132, 355–378. [Google Scholar] [CrossRef]
- Shack, R.B.; Reilley, A.F.; Lynch, J.B. Neurofibromas of the head and neck. South Med J 1985, 78, 801–804. [Google Scholar] [CrossRef]
- Mislow, J.M.; Proctor, M.R.; McNeely, P.D.; Greene, A.K.; Rogers, G.F. Calvarial defects associated with neurofibromatosis type 1. Report of two cases. J Neurosurg 2007, 106, 484–489. [Google Scholar] [CrossRef]
- Ismail, N.J.; Shehu, B.B.; Lasseini, A.; et al. Solitary giant neurofibroma of the scalp with calvarial defect in a child. J Surg Tech Case Rep 2010, 2, 24–26. [Google Scholar] [CrossRef]
- Saha, M.M.; Agarwal, K.N.; Bhardwaj, O.P. Calvarial bone defects in neurofibromatosis. Am J Roentgenol Radium Ther Nucl Med 1969, 105, 319–321. [Google Scholar] [CrossRef]
- Kankane, V.K.; Jaiswal, G.; Gupta, T.K. Congenital extra calvarial plexiform neurofibroma in occipito-cervical region with occipital bone defect with neurofibromatosis type 1 and segmental neurofibromatosis: Case report and review of literature. J Pediatr Neurosci 2016, 11, 295–297. [Google Scholar] [CrossRef]
- Mohamed, K.N. Neurofibromatosis and multiple skull defects: A case report. Singapore Med J 1986, 27, 450–452. [Google Scholar]
- Sheikh, B.Y. Huge occipital myxomatous plexiform neurofibroma in the absence of neurofibromatosis. Neurosciences (Riyadh) 2003, 8, 195–197. [Google Scholar]
- Kumar, S.; Chaurasia, P.; Singh, D.; Batra, V.V.; Aher, R. Solitary giant diffuse neurofibroma of the scalp with calvarial defect. Asian J Neurosurg 2017, 12, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Maroun, F.B.; Jacob, J.C.; Markesteyn, P.H.; Mercer, D.R. Congenital venous malformation of the scalp associated with plexiform neurofibroma and cranial defect. Case report. J Neurosurg 1969, 31, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, M.; Mizumaki, Y.; Fukuda, O.; Hayashi, N.; Kuwayama, N.; Endo, S. Giant plexiform neurofibroma and suboccipital meningocele manifesting as segmental neurofibromatosis. Neurol Med Chir (Tokyo) 2008, 48, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, V.; Mahore, A.; Patil, M.; et al. Brain herniation in neurofibromatosis with dysplasia of occipital bone and posterior skull base. Case Rep Neurol Med 2015, 2015, 816079. [Google Scholar] [CrossRef]
- Duchateau, J.; Lejour, M. Neurofibromatose du scalp associée à une aplasie osseuse occipitale [in French]. Ann Chir Plast Esthet 1986, 31, 73–78. [Google Scholar]
- Kurimoto, M.; Hirashima, Y.; Hayashi, N.; et al. Suboccipital meningocele presenting as a huge retropharyngeal mass in a patient with neurofibromatosis Type 1. Case report. J Neurosurg 1999, 91, 503–505. [Google Scholar] [CrossRef]
- Scott, M. Massive plexiform neurofibroma of the occipital scalp. Case report. J Neurosurg 1966, 25, 81–82. [Google Scholar] [CrossRef]
- Heine, J. Über ungewöhnliche Missbildungen bei Neurofbromatose. Beitr Pathol Anat 1927, 78, 122–159. [Google Scholar]
- Opitz, H.; Petersen, D.; Heiss, E.; Duffner, F.; Meyermann, R. Giant cell tumor of the occipital bone in a case of von Recklinghausen neurofibromatosis. Clin Neuropathol 1996, 15, 226–230. [Google Scholar]
- Simsek, E.; Yavuz, C.; Ustundag, N. Atypical meningioma and extensive calvarium defects in neurofibromatosis type 1. Pediatr Radiol 2003, 33, 551–553. [Google Scholar] [CrossRef]
- Barnowsky, L.; Dalal, R. Lacunarlike skull in neurofibromatosis. AJNR Am J Neuroradiol 1990, 11, 1253. [Google Scholar] [PubMed]
- Lam, S.; Kuether, J.; Fong, A.; Reid, R. Cranioplasty for large-sized calvarial defects in the pediatric population: A review. Craniomaxillofac Trauma Reconstr 2015, 8, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y. Hemorrhage into a plexiform neurofibroma induced by trauma: A rare complication of von Recklinghausen’s disease. J Dermatol 1987, 14, 378–381. [Google Scholar] [CrossRef]
- Tung, T.C.; Chen, Y.R.; Chen, K.T.; Chen, C.T.; Bendor-Samuel, R. Massive intratumor hemorrhage in facial plexiform neurofibroma. Head Neck 1997, 19, 158–162. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Kazekawa, K.; Tanaka, A.; et al. Rapid expansion of benign scalp neurofibroma caused by massive intratumoral hemorrhage– case report. Neurol Med Chir (Tokyo) 2002, 42, 338–340. [Google Scholar] [CrossRef]
- Kwak, S.W.; Han, Y.M.; Park, Y.S. Diffuse neurofibroma presenting with spontaneous intra-tumoral hemorrhage. J Korean Neurosurg Soc 2006, 39, 459–463. [Google Scholar]
- Reconstruction of craniofacial bone defects with individual alloplastic implants based on CAD/CAM-manipulated CT-data. J Craniomaxillofac Surg 1995, 23, 175–181.
- Eickhoff, U.; Fischer, W. Eine seltene kombinierte Dysplasie bei der Neurofibromatosis Recklinghausen. Fortschr Röntgenstr 1972, 116, 776–781. [Google Scholar] [CrossRef]
- Wakuta, Y.; Mitani, T. Giant epicranial neurofibroma–case report. Neurol Med Chir (Tokyo) 1988, 28, 690–694. [Google Scholar] [CrossRef]
- García-Uría, J.; Sola, R.G.; Carrillo, R.; Bravo, G. Epicranial plexiform neurofibroma. Surg Neurol 1979, 11, 390–392. [Google Scholar]
- Snyder, B.J.; Hanieh, A.; Trott, J.A.; David, D.J. Transcranial correction of orbital neurofibromatosis. Plast Reconstr Surg 1998, 102, 633–642. [Google Scholar] [CrossRef]

© 2017 by the author. The Author(s) 2017.
Share and Cite
Friedrich, R.E.; Emami, P.; Hagel, C.; Wikner, J.; Hanken, H. Repair of Occipital Bone Defects in Neurofibromatosis Type 1 by Means of CAD/CAM Prefabricated Titanium Plates. Craniomaxillofac. Trauma Reconstr. 2018, 11, 324-330. https://doi.org/10.1055/s-0037-1608699
Friedrich RE, Emami P, Hagel C, Wikner J, Hanken H. Repair of Occipital Bone Defects in Neurofibromatosis Type 1 by Means of CAD/CAM Prefabricated Titanium Plates. Craniomaxillofacial Trauma & Reconstruction. 2018; 11(4):324-330. https://doi.org/10.1055/s-0037-1608699
Chicago/Turabian StyleFriedrich, Reinhard E., Pedram Emami, Christian Hagel, Johannes Wikner, and Henning Hanken. 2018. "Repair of Occipital Bone Defects in Neurofibromatosis Type 1 by Means of CAD/CAM Prefabricated Titanium Plates" Craniomaxillofacial Trauma & Reconstruction 11, no. 4: 324-330. https://doi.org/10.1055/s-0037-1608699
APA StyleFriedrich, R. E., Emami, P., Hagel, C., Wikner, J., & Hanken, H. (2018). Repair of Occipital Bone Defects in Neurofibromatosis Type 1 by Means of CAD/CAM Prefabricated Titanium Plates. Craniomaxillofacial Trauma & Reconstruction, 11(4), 324-330. https://doi.org/10.1055/s-0037-1608699


