The Effect of Bone Marrow Mesenchymal Stem Cells Application on Distracted Bone Quality during Rapid Rate of Distraction Osteogenesis
Abstract
:Materials and Methods
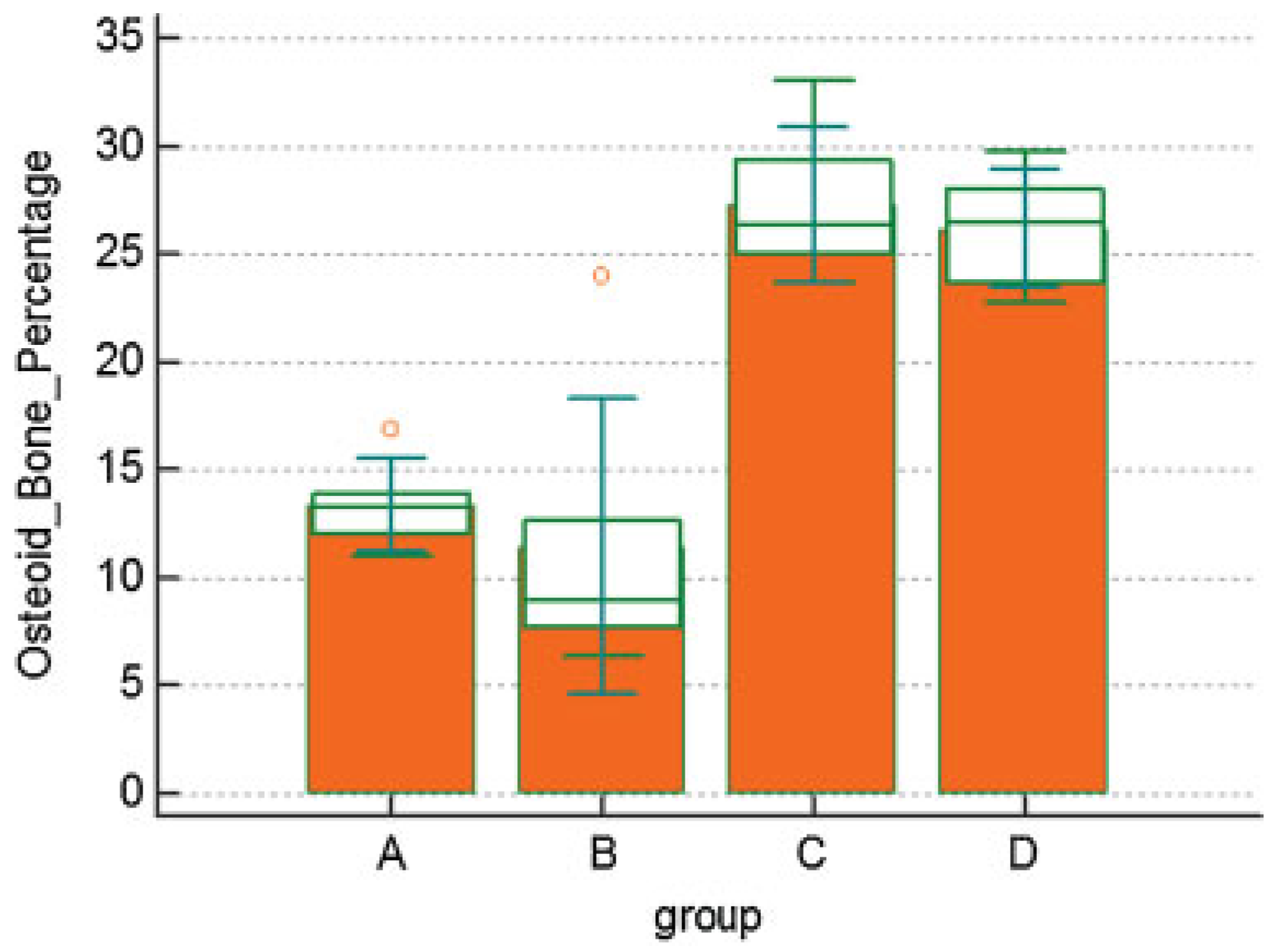
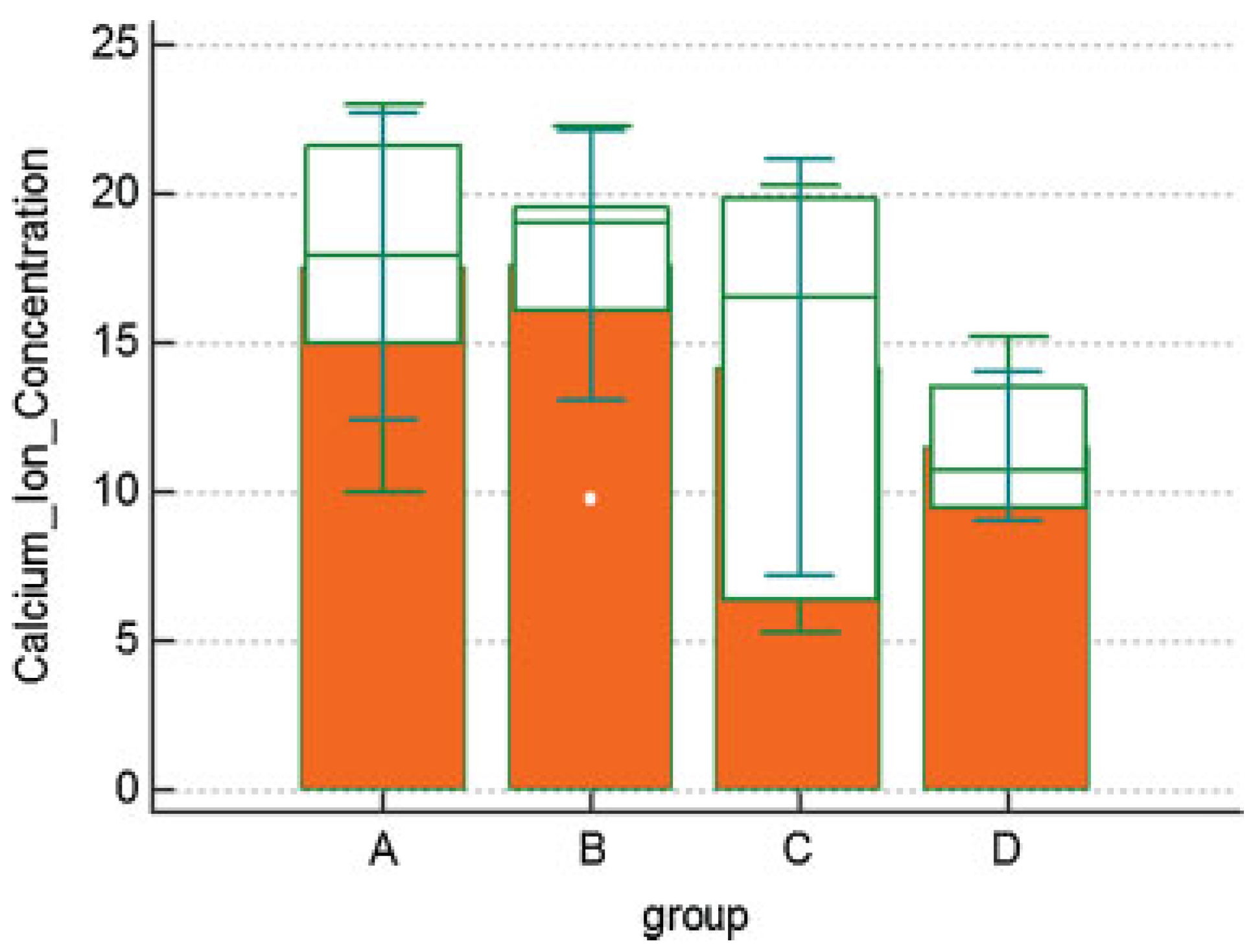
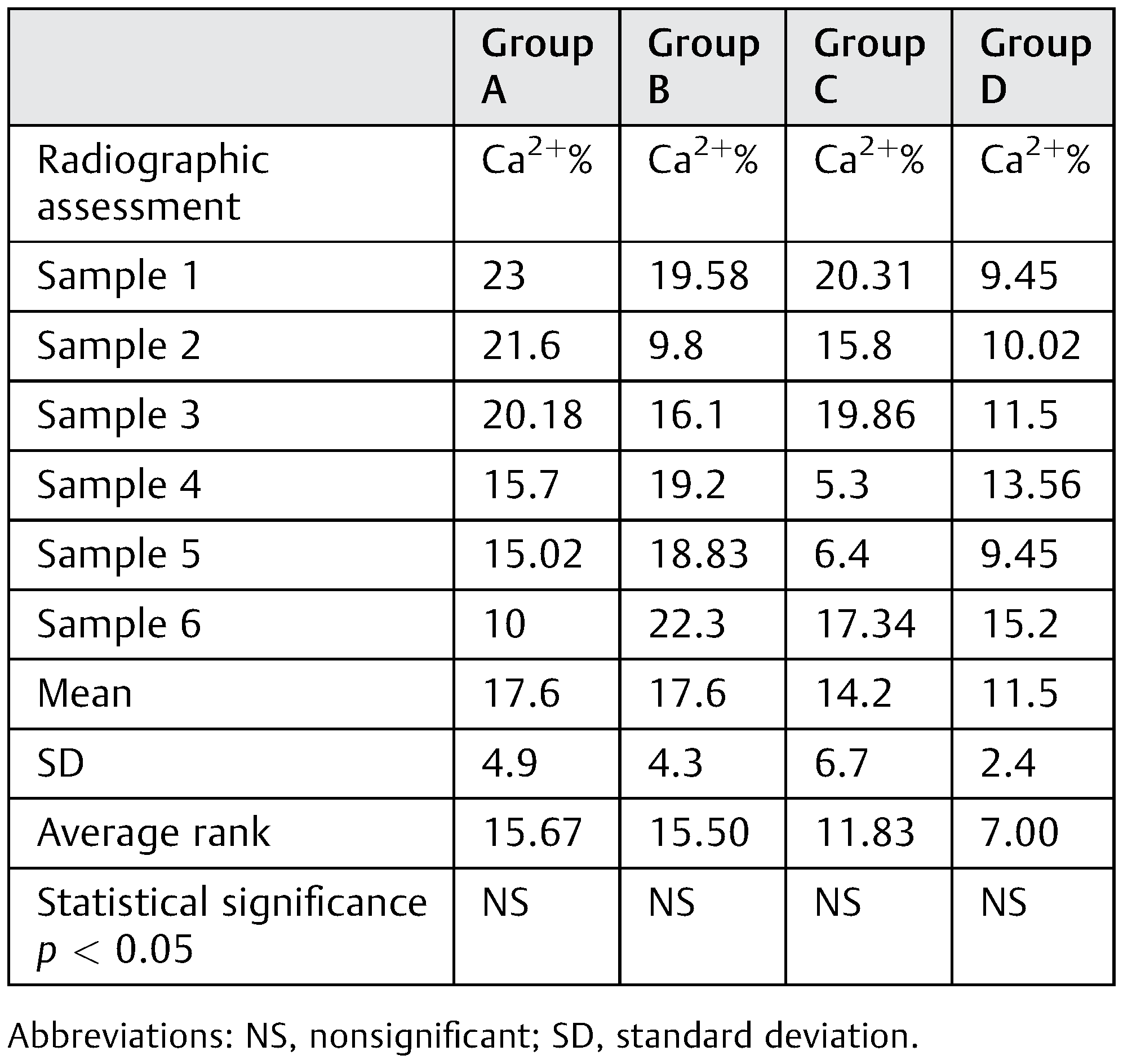
Results
Conflicts of Interest
References
- McCarthy, J.G.; Schreiber, J.; Karp, N.; Thorne, C.H.; Grayson, B.H. Lengthening the human mandible by gradual distraction. Plast Reconstr Surg 1992, 89, 1–8, discussion 9. [Google Scholar] [CrossRef] [PubMed]
- Djasim, U.M.; Wolvius, E.B.; van Neck, J.W.; Weinans, H.; van der Wal, K.G. Recommendations for optimal distraction protocols for various animal models on the basis of a systematic review of the literature. Int J Oral Maxillofac Surg 2007, 36, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.C.; Sun, Z. Mandibular distraction osteogenesis assisted by cell-based tissue engineering: a systematic review. Orthod Craniofac Res 2015, 18 (Suppl. S1), 39–49. [Google Scholar] [CrossRef]
- Kitoh, H.; Kitakoji, T.; Tsuchiya, H.; et al. Transplantation of marrowderived mesenchymal stem cells and platelet-rich plasma during distraction osteogenesis–a preliminary result of three cases. Bone 2004, 35, 892–898. [Google Scholar] [CrossRef]
- Qi, M.; Hu, J.; Zou, S.; Zhou, H.; Han, L. Mandibular distraction osteogenesis enhanced by bone marrow mesenchymal stem cells in rats. J Craniomaxillofac Surg 2006, 34, 283–289. [Google Scholar] [CrossRef]
- El Hadidi, Y.N.; El Kassaby, M.; El Fatah Ahmed, S.A.; Khamis, N.S. The effect of mesenchymal stem cells application on the distracted bone microstructure: an experimental study. J Oral Maxillofac Surg 2016, 74, 1463.e1–1463e11. [Google Scholar] [CrossRef]
- Spector, W.S. Handbook of Biological Data; W.B. Saunders: Philadelphia, PA, USA, 1956. [Google Scholar]
- Uccelli, A.; Moretta, L.; Pistoia, V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol 2006, 36, 2566–2573. [Google Scholar] [CrossRef]
- Brandi, M.L. Microarchitecture, the key to bone quality. Rheumatology (Oxford) 2009, 48 (Suppl. S4), iv3–iv8. [Google Scholar] [CrossRef]
- Field, A.P. Eta and Eta squared. Wiley StatsRef: Statistics Reference Online. Wiley Online Library; 2014.
- al Ruhaimi, K.A. Comparison of different distraction rates in the mandible: an experimental investigation. Int J Oral Maxillofac Surg 2001, 30, 220–227. [Google Scholar] [CrossRef]
- Stewart, K.J.; Lvoff, G.O.; White, S.A.; et al. Mandibular distraction osteogenesis: a comparison of distraction rates in the rabbit model. J Craniomaxillofac Surg 1998, 26, 43–49. [Google Scholar] [CrossRef]
- Schiller, J.R.; Moore, D.C.; Ehrlich, M.G. Increased lengthening rate decreases expression of fibroblast growth factor 2, platelet-derived growth factor, vascular endothelial growth factor, and CD31 in a rat model of distraction osteogenesis. J Pediatr Orthop 2007, 27, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Troulis, M.J.; Glowacki, J.; Perrott, D.H.; Kaban, L.B. Effects of latency and rate on bone formation in a porcine mandibular distraction model. J Oral Maxillofac Surg 2000, 58, 507–513, discussion 514. [Google Scholar] [CrossRef] [PubMed]
- Mofid, M.M.; Manson, P.N.; Robertson, B.C.; Tufaro, A.P.; Elias, J.J.; Vander Kolk, C.A. Craniofacial distraction osteogenesis: a review of 3278 cases. Plast Reconstr Surg 2001, 108, 1103–1114, discussion 1115. [Google Scholar] [CrossRef] [PubMed]
- Swennen, G.; Dempf, R.; Schliephake, H. Cranio-facial distraction osteogenesis: a review of the literature. Part II: Experimental studies. Int J Oral Maxillofac Surg 2002, 31, 123–135. [Google Scholar]
- Glowacki, J.; Shusterman, E.M.; Troulis, M.; Holmes, R.; Perrott, D.; Kaban, L.B. Distraction osteogenesis of the porcine mandible: histomorphometric evaluation of bone. Plast Reconstr Surg 2004, 113, 566–573. [Google Scholar] [CrossRef]
- Kaban, L.B.; Thurmüller, P.; Troulis, M.J.; et al. Correlation of biomechanical stiffness with plain radiographic and ultrasound data in an experimental mandibular distraction wound. Int J Oral Maxillofac Surg 2003, 32, 296–304. [Google Scholar] [CrossRef]
- Farhadieh, R.D.; Gianoutsos, M.P.; Dickinson, R.; Walsh, W.R. Effect of distraction rate on biomechanical, mineralization, and histologic properties of an ovine mandible model. Plast Reconstr Surg 2000, 105, 889–895. [Google Scholar] [CrossRef]
- Hasse, A.R.; Pörksen, M.; Zimmermann, C.E. Bilateral mandibular distraction in adult dogs with an epiperiosteal distractor. Br J Oral Maxillofac Surg 2005, 43, 105–112. [Google Scholar] [CrossRef]
- Zimmermann, C.E.; Harris, G.; Thurmüller, P.; et al. Assessment of bone formation in a porcine mandibular distraction wound by computed tomography. Int J Oral Maxillofac Surg 2004, 33, 569–574. [Google Scholar] [CrossRef]
- Zimmermann, C.E.; Thurmüller, P.; Troulis, M.J.; Perrott, D.H.; Rahn, B.; Kaban, L.B. Histology of the porcine mandibular distraction wound. Int J Oral Maxillofac Surg 2005, 34, 411–419. [Google Scholar] [CrossRef]
- Perrott, D.H.; Rahn, B.; Wahl, D.; et al. Development of a mechanical testing system for a mandibular distraction wound. Int J Oral Maxillofac Surg 2003, 32, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.I.; Mooney, M.P. Appropriate animal models for craniofacial biology. Cleft Palate J 1990, 27, 18–25. [Google Scholar] [PubMed]
- O’Loughlin, P.F.; Morr, S.; Bogunovic, L.; Kim, A.D.; Park, B.; Lane, J.M. Selection and development of preclinical models in fracture-healing research. J Bone Joint Surg Am 2008, Suppl. S1, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.A.; Simpson, A.H. In vivo models of bone repair. J Bone Joint Surg Br 2012, 94, 865–874. [Google Scholar] [CrossRef]
- Kroczek, A.; Park, J.; Birkholz, T.; Neukam, F.W.; Wiltfang, J.; Kessler, P. Effects of osteoinduction on bone regeneration in distraction: results of a pilot study. J Craniomaxillofac Surg 2010, 38, 334–344. [Google Scholar] [CrossRef]
- Castro-Govea, Y.; Cervantes-Kardasch, V.H.; Borrego-Soto, G.; et al. Human bone morphogenetic protein 2-transduced mesenchymal stem cells improve bone regeneration in a model of mandible distraction surgery. J Craniofac Surg 2012, 23, 392–396. [Google Scholar] [CrossRef]
- Sun, Z.; Tee, B.C.; Kennedy, K.S.; et al. Scaffold-based delivery of autologous mesenchymal stem cells for mandibular distraction osteogenesis: preliminary studies in a porcine model. PLoS One 2013, 8, e74672. [Google Scholar] [CrossRef]
- Aykan, A.; Ozturk, S.; Sahin, I.; et al. Biomechanical analysis of the effect of mesenchymal stem cells on mandibular distraction osteogenesis. J Craniofac Surg 2013, 24, e169–e175. [Google Scholar] [CrossRef]
- Song, S.H.; Yun, Y.P.; Kim, H.J.; Park, K.; Kim, S.E.; Song, H.R. Bone formation in a rat tibial defect model using carboxymethyl cellulose/BioC/ bone morphogenic protein-2 hybrid materials. BioMed Res Int 2014, 2014, 230152. [Google Scholar] [CrossRef]
- Hsu, J.T.; Wang, S.P.; Huang, H.L.; Chen, Y.J.; Wu, J.; Tsai, M.T. The assessment of trabecular bone parameters and cortical bone strength: a comparison of micro-CT and dental cone-beam CT. J Biomech 2013, 46, 2611–2618. [Google Scholar] [CrossRef]
- Parsa, A.; Ibrahim, N.; Hassan, B.; van der Stelt, P.; Wismeijer, D. Bone quality evaluation at dental implant site using multislice CT, micro-CT, and cone beam CT. Clin Oral Implants Res 2015, 26, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.T.; Chen, Y.J.; Tsai, M.T.; et al. Predicting cortical bone strength from DXA and dental cone-beam CT. PLoS One 2012, 7, e50008. [Google Scholar] [CrossRef]
- Pauwels, R.; Nackaerts, O.; Bellaiche, N.; et al. SEDENTEXCT Project Consortium. Variability of dental cone beam CT grey values for density estimations. Br J Radiol 2013, 86, 20120135. [Google Scholar] [PubMed]
- Busse, B.; Hahn, M.; Soltau, M.; et al. Increased calcium content and inhomogeneity of mineralization render bone toughness in osteoporosis: mineralization, morphology and biomechanics of human single trabeculae. Bone 2009, 45, 1034–1043. [Google Scholar] [CrossRef]
- Rachmiel, A.; Laufer, D.; Jackson, I.T.; Lewinson, D. Midface membranous bone lengthening: A one-year histological and morphological follow-up of distraction osteogenesis. Calcif Tissue Int 1998, 62, 370–376. [Google Scholar] [CrossRef]
- Tai, K.; Pelled, G.; Sheyn, D.; et al. Nanobiomechanics of repair bone regenerated by genetically modified mesenchymal stem cells. Tissue Eng Part A 2008, 14, 1709–1720. [Google Scholar] [CrossRef]




© 2017 by the author. The Author(s) 2017.
Share and Cite
El Kassaby, M.; El Kader, K.A.; Khamis, N.; Al Hammoud, A.; Talb, A.B.; el Hadidi, Y.N. The Effect of Bone Marrow Mesenchymal Stem Cells Application on Distracted Bone Quality during Rapid Rate of Distraction Osteogenesis. Craniomaxillofac. Trauma Reconstr. 2018, 11, 192-198. https://doi.org/10.1055/s-0037-1604070
El Kassaby M, El Kader KA, Khamis N, Al Hammoud A, Talb AB, el Hadidi YN. The Effect of Bone Marrow Mesenchymal Stem Cells Application on Distracted Bone Quality during Rapid Rate of Distraction Osteogenesis. Craniomaxillofacial Trauma & Reconstruction. 2018; 11(3):192-198. https://doi.org/10.1055/s-0037-1604070
Chicago/Turabian StyleEl Kassaby, Marwa, Khaled Abd El Kader, Nahed Khamis, Alaa Al Hammoud, Alaa Ben Talb, and Yasser Nabil el Hadidi. 2018. "The Effect of Bone Marrow Mesenchymal Stem Cells Application on Distracted Bone Quality during Rapid Rate of Distraction Osteogenesis" Craniomaxillofacial Trauma & Reconstruction 11, no. 3: 192-198. https://doi.org/10.1055/s-0037-1604070
APA StyleEl Kassaby, M., El Kader, K. A., Khamis, N., Al Hammoud, A., Talb, A. B., & el Hadidi, Y. N. (2018). The Effect of Bone Marrow Mesenchymal Stem Cells Application on Distracted Bone Quality during Rapid Rate of Distraction Osteogenesis. Craniomaxillofacial Trauma & Reconstruction, 11(3), 192-198. https://doi.org/10.1055/s-0037-1604070



