The Advancement of Herbal-Based Nanomedicine for Hair
Abstract
:1. Introduction
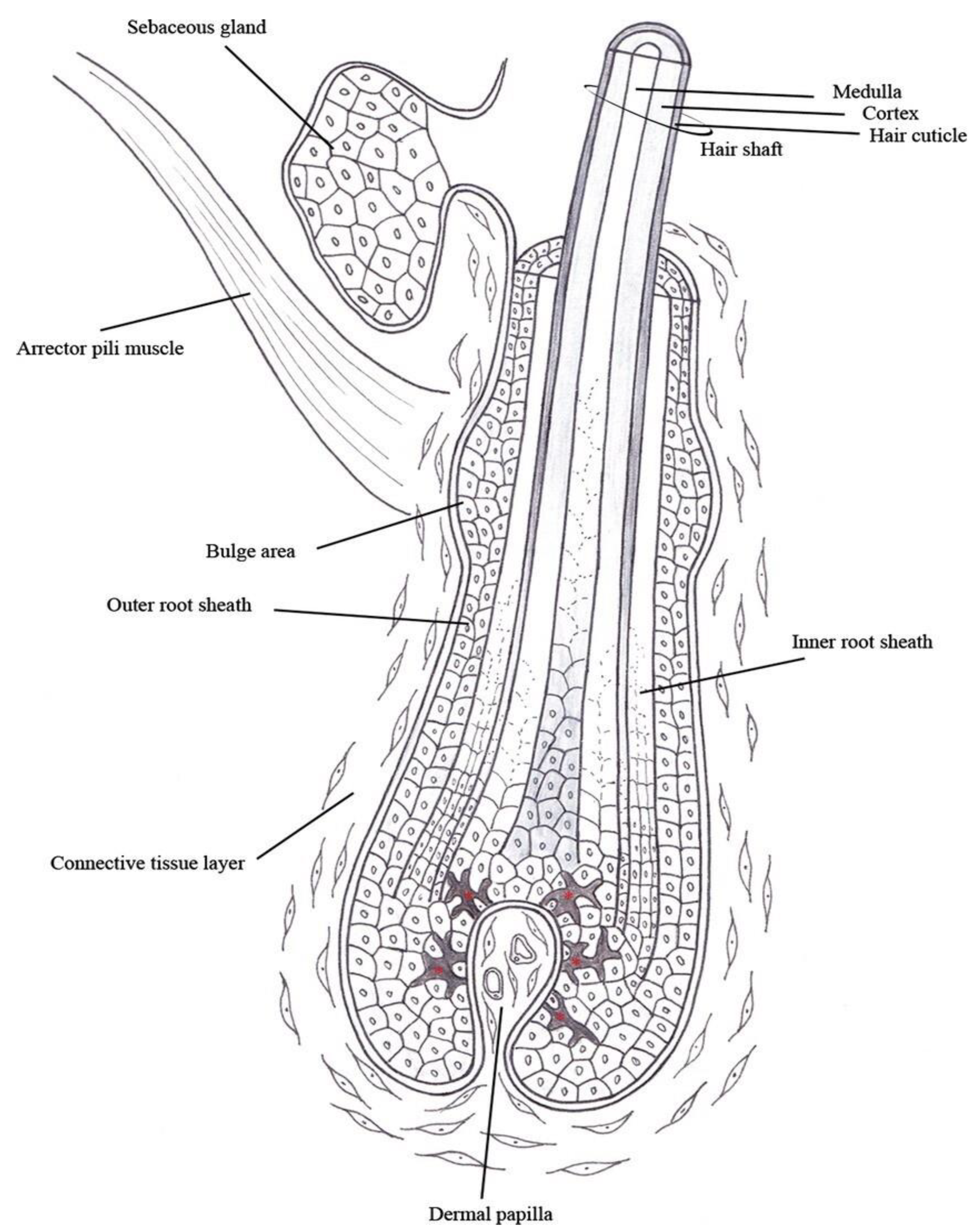
2. Hair Morphology
2.1. Hair Follicle
2.2. Shaft
2.2.1. Medulla
2.2.2. Cortex
2.2.3. Cuticle
- Layer A, which is formed of cross-linked cysteine, is a very resistant layer. This connection provides mechanical and physical resistance [8].
- Exocuticle: This structure is also known as the B layer. Though not quite as hard as the A layer, it is physically rigid and high in cysteine concentration.
- Epicuticle: The 8-methyleicosonic acid-containing epicuticle is a hydrophobic lipid layer that covers the exterior of the hair shaft [14]. Approximately 3% of the epicuticle is likewise made up of cystine. As a soft layer, it expands and turns brittle when exposed to water, which explains why wet hair breaks when combed [8].
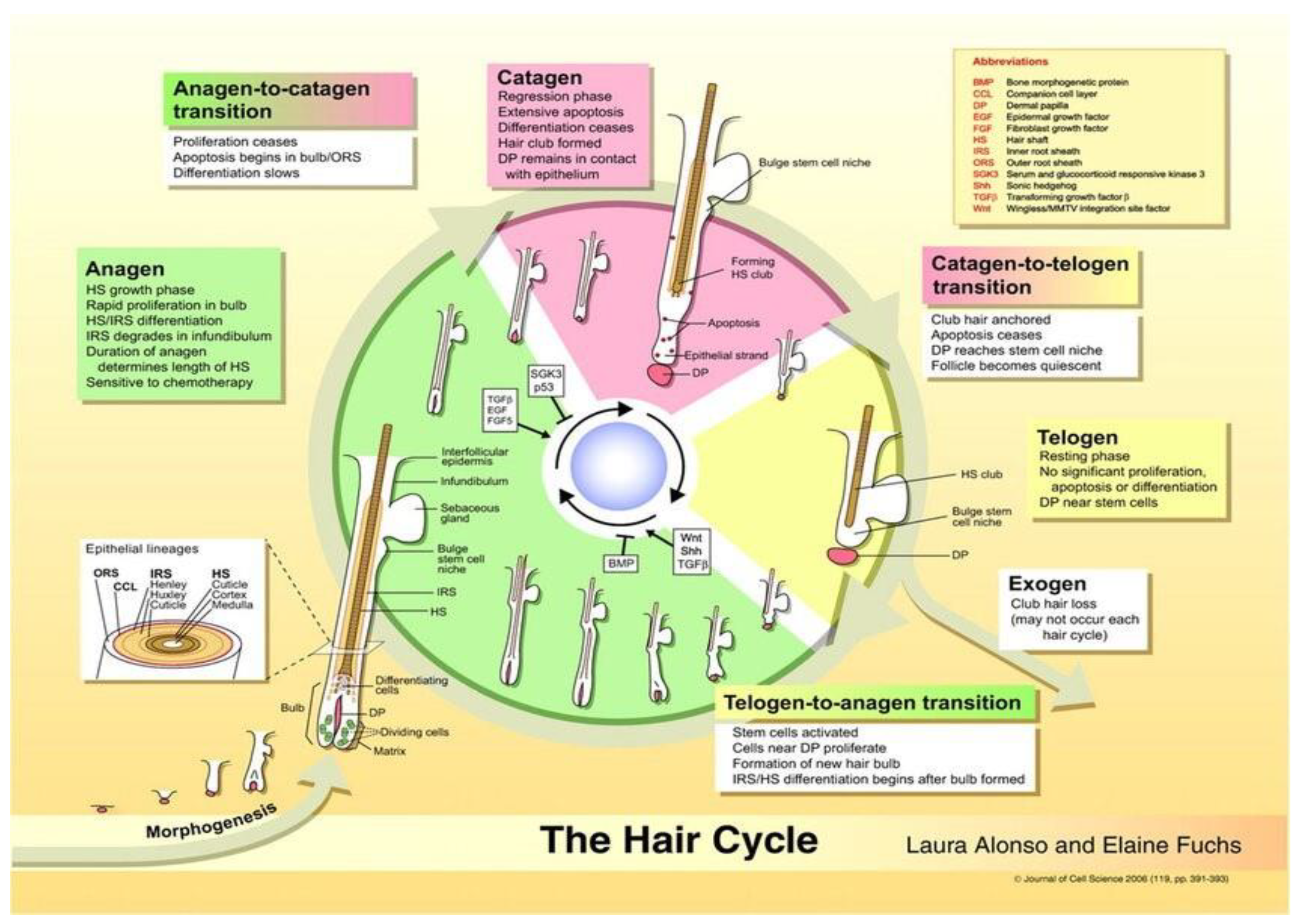
2.3. Hair Cycle
2.3.1. Anagen Phase
2.3.2. Catagen Phase
2.3.3. Telogen Phase
2.3.4. Exogen
3. Role of Stratum Corneum Barriers in the Design of Nanocarriers
3.1. Stratum Corneum Barriers
3.2. Design of Nanotechnology-Based Nanocarriers
3.3. Nano-Formulations for Herbal Hair Care
3.3.1. Liposomes
3.3.2. Phytosome
3.3.3. Ethosome
3.3.4. Cubosome
3.3.5. Polymeric Nanoparticles
3.3.6. Melatonin Nanostructured Lipid Carrier
3.3.7. Solid Lipid Nanoparticles
| Biological Source | Technique | Outcomes | Reference |
|---|---|---|---|
| Carthamus tinctorius florets extract | Loaded nanostructured lipid carriers | Particle size around 100 nm, zeta potential −40 to −49 mv. NLC promoted hair growth in the mice better than minoxidil. Good physical properties and stabilities. | [52] |
| Quercetin | Phospholipid-polymer hybrid nanoparticle-mediated trans-follicular delivery | Particle size 339 ± 0.6. Zeta potential −32.6 ± 0.5. Entrapment efficiency 78 ± 5.5 Treatment of androgenic alopecia. | [47] |
| Mixtures of P. linteus, C. militaris, P. multiflorum, F. carica, and C. nucifera oil | Poly(γ-glutamic acid)/chitosan hydrogel nanoparticles | Control release. Prolong growth-promoting effect. Enlarge in hair bulbs. Induction of hair growth. Delivery phytoconstituents at hair follicles. | [48] |
| Chitosan | Surface-deacetylated chitin nanofibers | Promoted hair growth. Upregulated levels of FGF−7 and sonic hedgehog hair follicles. | [49] |
| Almond oil, g primrose oil, olive oil, and soybean oil | Nanostructured lipid carriers | Nanometer size. Negatively charged surface. High entrapment efficiency. High anti-oxidant potential. Sustained release for 6 h. Good storage stability. Increased hair density and thickness. Decreased hair loss. | [55] |
| Pueraria mirifica ethanolic extract | Solid lipid nanoparticles | Particle size (93.83 ± 0.32 nm). Entrapment efficiency (42.64 ± 0.47%) Good safety herbal extract.SLN containing 5% (w/v) extract can pass through the skin. | [51] |
| Fenugreek seed extract | Solid lipid nanoparticles-based hydrogels | The particle size of 223.36 nm. PDI of 0.33. Entrapment efficiency 74.56 ± 0.2%. Management of alopecia. Reduce the systemic side effects. | [53] |
| Carthamus tinctorius (safflower) florets extract | Nanostructured lipid carriers (NLC) | Particle size around 00 nm. Zeta potential (−40 to −49) mv. NLC promoted hair growth in the mice better than minoxidil. | [52] |
| A natural-based formula containing γ linolenic acid, β-sitosterol, epigallocatechin gallate, and genistein | Β-cyclodextrin inclusion complex | Nine-month uncontrolled, open-label case series, 0 day -low vertex scalp hair 90 day—negative hair growth cycle altered. 80 days—hair thickening on the scalp. 270 day—hair observed with a full thickness on the scalp. | [54] |
| Moringa oleifera | Ethosomes | Better skin penetration and Hair growth-enhancing activity | [57] |
| Black tea | Ethosomes | Increases transdermal absorption rates and Hair dye | [58] |
3.4. Regulatory Aspects of Herbal Nanomedicine for Hair Care
4. Hair Problems
4.1. Hair Loss
4.2. Gray Hair
4.3. Dandruff
4.4. Frizzy Hair
4.5. Dull Hair
4.6. Androgenic Alopecia
4.7. Alopecia Areta
4.8. Telogen Effluvium
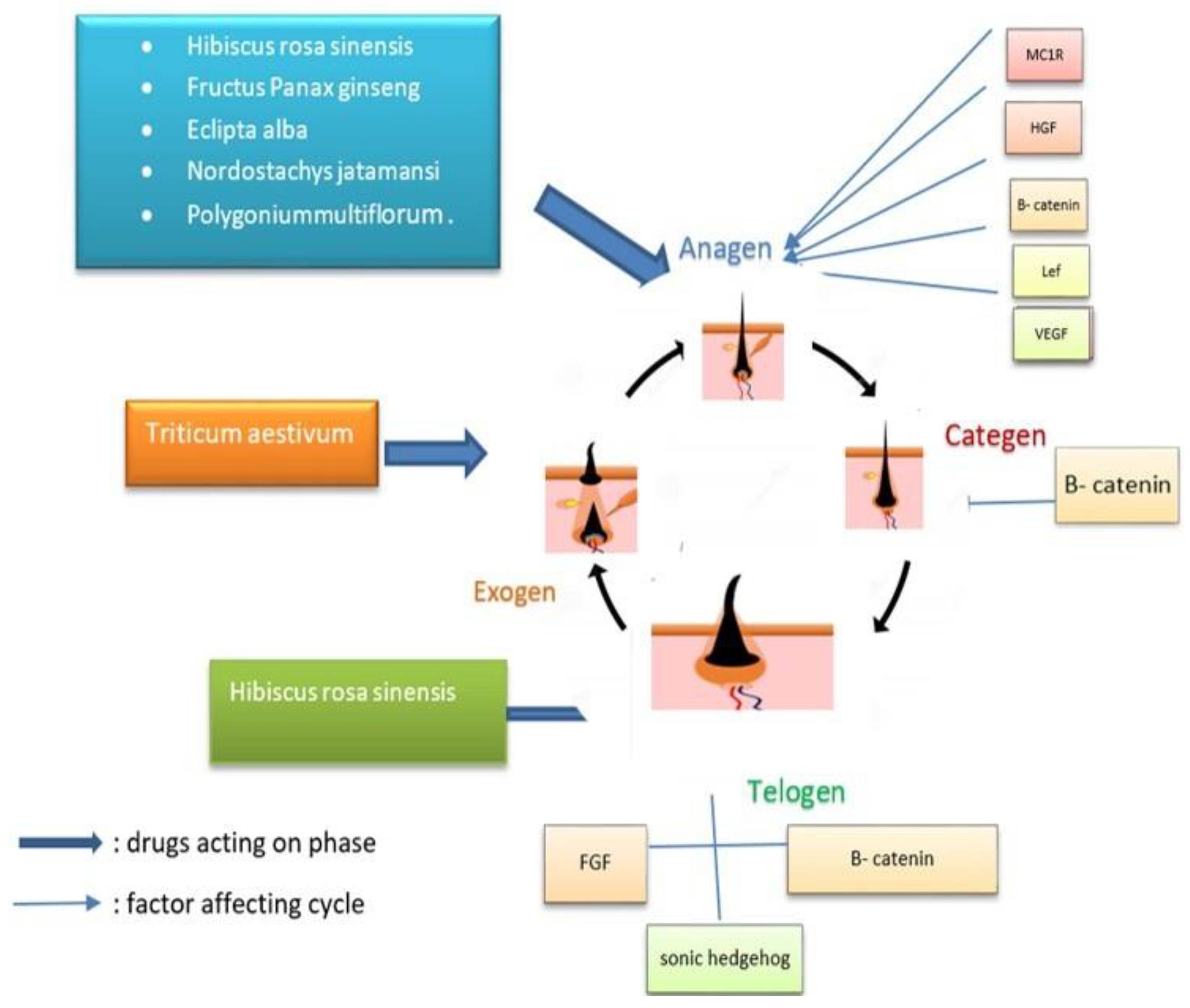
5. Mechanism of Action of Herbal Medicines on Hair
5.1. Modulation in the Hair Cycle by Alteration in Signaling Pathways
5.2. Hair Cycle Modulation
5.2.1. Anagen Phase
5.2.2. Hair Follicle Telogen Phase
5.2.3. Hair Follicle Exogen Event
5.2.4. Dystrophic Anagen Growth Phase
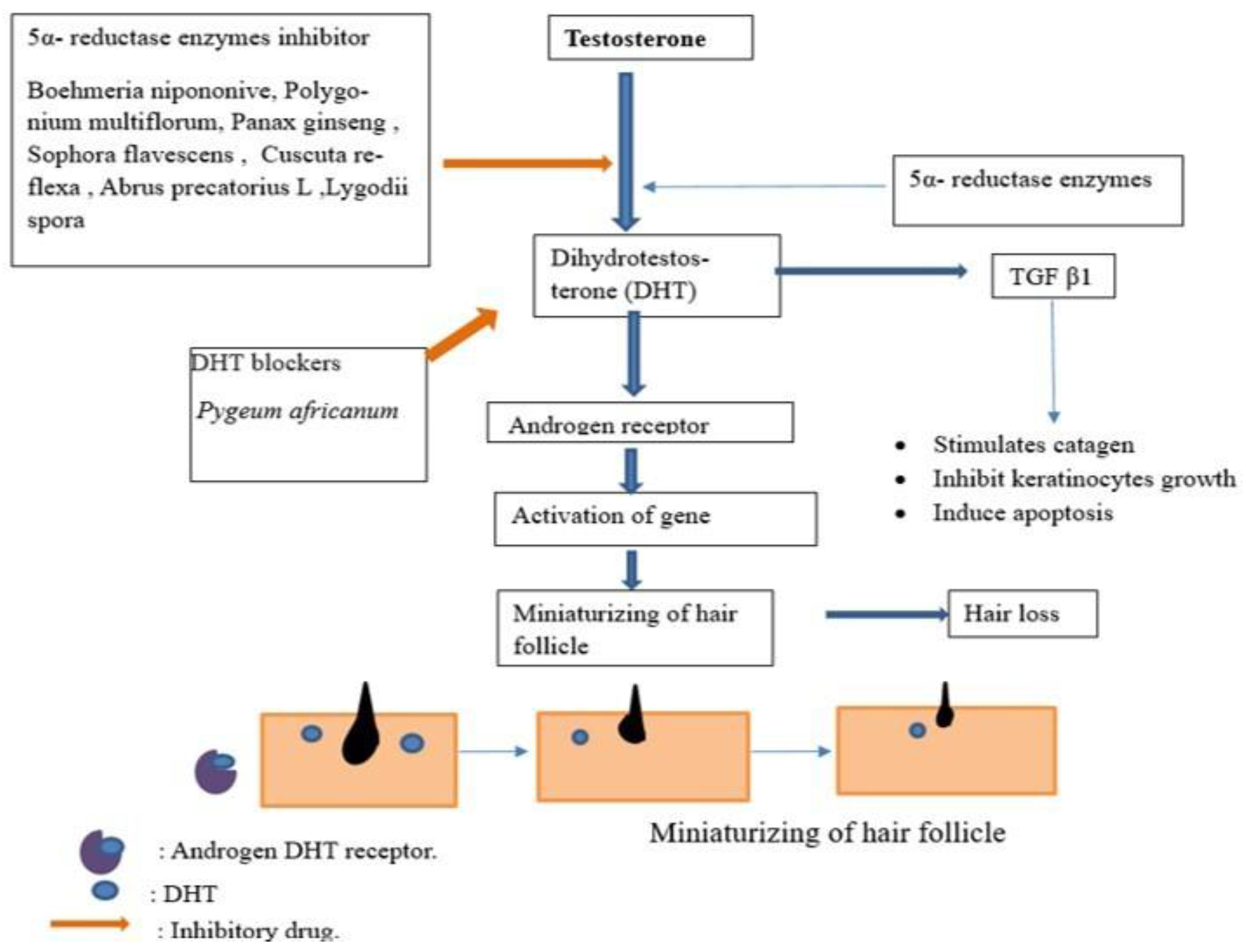
5.3. Hormones
5.4. Protein Kinase
5.5. IGF
6. Herbal Treatment
7. Herbal Plants for Hair Care
7.1. Panax ginseng
7.2. Eclipta alba
7.3. Camellia sinensis (Green Tea)
7.4. Solanum nigrum
7.5. Hibiscus rosa-sinensis
7.6. Serenoa repens
7.7. Trigonella foenum-graecum
7.8. Emblica officinalis
7.9. Pueraria thunbergiana
7.10. Capsicum annum
7.11. Asiasari radix
7.12. Punica granatum (Pomegranate)
7.13. Nardostachys jatamansi
7.14. Cuscuta reflexa
7.15. Polygonum multiflorum
7.16. Acanthopanax koreanum
7.17. Crataegus pinnatifida
7.18. Malus pumila (Apple)
7.19. Allium sativum (Garlic)
7.20. Coffea arabica (Coffee)
7.21. Rosmarinus officinalis (Rosemary)
7.22. Carthamus tinctorius (Safflower) Floret
7.23. Thuja orientalis
7.24. Nasturtium officinale (Watercress)
7.25. Sophora angustifolia
8. Patents in Herbal Extract
9. Conclusions and Future Scope
Author Contributions
Funding
Conflicts of Interest
References
- Buffoli, B.; Rinaldi, F.; Labanca, M.; Sorbellini, E.; Trink, A.; Guanziroli, E.; Rezzani, R.; Rodella, L.F. The human hair: From anatomy to physiology. Int. J. Dermatol. 2013, 53, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, A.D.; Devasagayam, T.P. Current Status of Herbal Drugs in India: An Overview. J. Clin. Biochem. Nutr. 2007, 41, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, J.F. New Hair Freedom? 1990s Hair Care Marketing. In Proceedings of the Conference on Historical Analysis and Research in Marketing, Online Conference, 4–5 June 2021; pp. 31–40. [Google Scholar]
- Jacobson, K.B.; Rao, M.; Bonilla, H.; Subramanian, A.; Hack, I.; Madrigal, M.; Singh, U.; Jagannathan, P.; Grant, P. Patients with Uncomplicated Coronavirus Disease 2019 (COVID-19) Have Long-Term Persistent Symptoms and Functional Impairment Similar to Patients with Severe COVID-19: A Cautionary Tale During a Global Pandemic. Clin. Infect. Dis. 2021, 73, e826–e829. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.; Tosti, A. Alopecia in patients with COVID-19: A systematic review and meta-analysis. JAAD Int. 2022, 7, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.K.; Jain, P.K.; Das, D.; Ak, S. Alternative herbal drugs used for treating hair disease. Asian J. Pharm. Clin. Res. 2016, 9, 75–77. Available online: https://www.researchgate.net/publication/301601961 (accessed on 19 August 2022).
- Karimi, A.; Majlesi, M.; Rafieian-Kopaei, M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacol. 2015, 4, 27–30. [Google Scholar]
- Pundkar, A.S.; Murkute, P.M.; Wani, S.; Tathe, M. A review: Herbal therapy used in hair loss. Pharm. Reson. 2020, 3, 44–50. [Google Scholar]
- Prajapati, S.K.; Jain, D.; Parveen, S.; Maji, S.; Deb, P.K. Nanodelivery of Antioxidant Herbal Extracts, Spices, and Dietary Constituents. In Phytoantioxidants and Nanotherapeutics; Wiley: Hoboken, NJ, USA, 2022; pp. 145–171. [Google Scholar] [CrossRef]
- Anastassakis, K. The Morphology and Structure of the Hair Shaft. In Androgenetic Alopecia from A to Z; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Lanjewar, A.; Maurya, S.; Sharma, D.; Gaur, A. Review on Hair Problem and its Solution. J. Drug Deliv. Ther. 2020, 10, 322–329. [Google Scholar] [CrossRef]
- Sperling, L.C. Hair anatomy for the clinician. J. Am. Acad. Dermatol. 1991, 25, 1–17. [Google Scholar] [CrossRef]
- Taghiabadi, E.; Nilforoushzadeh, M.A.; Aghdami, N. Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods. Ski. Pharmacol. Physiol. 2020, 33, 280–292. [Google Scholar] [CrossRef]
- Erdoğan, B. Anatomy and Physiology of Hair. Hair Scalp Disord. 2017, 13, 1–7. [Google Scholar] [CrossRef]
- Rabadiya, E.; Soni, H.; Pandya, K.; Patel, G.; Zaveri, M.; Patel, S. A Detail Phyto-Chemical Evaluation of Ayurvedic Tablet Formulation Used for Hair Care. Available online: https://www.wjpr.net (accessed on 19 August 2022).
- Alonso, L. The hair cycle. J. Cell Sci. 2006, 119, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Khogta, S.; Patel, J.; Barve, K.; Londhe, V. Herbal nano-formulations for topical delivery. J. Herb. Med. 2020, 20, 100300. [Google Scholar] [CrossRef]
- Harwansh, R.K.; Deshmukh, R.; Rahman, A. Nanoemulsion: Promising nanocarrier system for delivery of herbal bioactives. J. Drug Deliv. Sci. Technol. 2019, 51, 224–233. [Google Scholar] [CrossRef]
- Menon, G.K.; Cleary, G.W.; Lane, M.E. The structure and function of the stratum corneum. Int. J. Pharm. 2012, 435, 3–9. [Google Scholar] [CrossRef]
- Gorzelanny, C.; Mess, C.; Schneider, S.W.; Huck, V.; Brandner, J.M. Skin Barriers in Dermal Drug Delivery: Which Barriers Have to Be Overcome and How Can We Measure Them? Pharmaceutics 2020, 12, 684. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin barrier and transdermal drug delivery. Dermatology 2012, 3, 2065–2073. [Google Scholar]
- Despotopoulou, D.; Lagopati, N.; Pispas, S.; Gazouli, M.; Demetzos, C.; Pippa, N. The technology of transdermal delivery nanosystems: From design and development to preclinical studies. Int. J. Pharm. 2021, 611, 121290. [Google Scholar] [CrossRef]
- Ali, F.; Neha, K.; Sharma, K.; Khasimbi, S.; Chauhan, G. Nanotechnology-based medicinal products and patents: A promising way to treat psoriasis. Curr. Drug Deliv. 2022, 19, 587–599. [Google Scholar] [CrossRef]
- Dhawan, S.; Sharma, P.; Nanda, S. Chapter 8—Cosmetic nanoformulations and their intended use. In Nanocosmetics; Nanda, A., Nanda, S., Nguyen, T.A., Rajendran, S., Slimani, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 141–169. [Google Scholar] [CrossRef]
- Dhawan, S.; Hooda, P.; Nanda, S. Herbal Nano Formulations: Patent and Regulatory Overview. Appl. Clin. Res. Clin. Trials Regul. Aff. 2018, 5, 159–180. [Google Scholar] [CrossRef]
- Gopi, S.; Amalraj, A. Introduction of Nanotechnology in Herbal Drugs and Nutraceutical: A Review. J. Nanomed. Biother. Discov. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Sunil, D.; Shraddha, P.; Vaishnav, P.; Avinash, N.; Swapnil, S.; Jaswanth, G.; Prashant, K. Microparticulate and nanotechnology mediated drug delivery system for the delivery of herbal extracts. J. Biomater. Sci. Polym. Ed. 2022, 33, 1531–1554. [Google Scholar] [CrossRef]
- Mishra, P.; Handa, M.; Ujjwal, R.R.; Singh, V.; Kesharwani, P.; Shukla, R. Potential of nanoparticulate based delivery systems for effective management of alopecia. Colloids Surf. B Biointerfaces 2021, 208, 112050. [Google Scholar] [CrossRef]
- Ansari, S.H.; Sameem, M.; Islam, F. Influence of nanotechnology on herbal drugs: A Review. J. Adv. Pharm. Technol. Res. 2012, 3, 142. [Google Scholar] [CrossRef]
- Jadhav, N.R.; Powar, T.; Shinde, S.; Nadaf, S. Herbal nanoparticles: A patent review. Asian J. Pharm. 2014, 8, 58. [Google Scholar] [CrossRef]
- Alexander, A.; Dwivedi, S.; Ajazuddin; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for breaking the barriers of drug permeation through transdermal drug delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef]
- Sagar, R.; Pandey, S.P.; Sah, A.K. Nanotherapeutics of Phytoantioxidants for Viral Infections. In Phytoantioxidants and Nanotherapeutics; Wiley: Hoboken, NJ, USA, 2022; pp. 317–350. [Google Scholar] [CrossRef]
- Chakraborty, K.; Shivakumar, A.; Ramachandran, S. Nano-technology in herbal medicines: A review. Int. J. Herb. Med. 2016, 4, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Okole, B.; Pillai, S.K.; Ndzotoyi, P.; Phasha, V. 8-Use of herbal extract-based nanoemulsions for hair care application. In Nanotechnology for the Preparation of Cosmetics Using Plant-Based Extracts; Setapar, S.H.M., Ahmad, A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–233. [Google Scholar] [CrossRef]
- Patel, R.; Thakore, S.D.; Patel, M. Recent Survey on Patents of Nanoemulsions. Curr. Drug Deliv. 2015, 13, 857–881. [Google Scholar] [CrossRef] [PubMed]
- Hamdi, N.; Dima, K.; Usama, S.; Fadwa, O.; Abeer, B.; Walhan, A. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Vaishampayan, P.; Rane, M.M. Herbal nanocosmecuticals: A review on cosmeceutical innovation. J. Cosmet. Dermatol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Bombardelli, E.; Curri, S.B.; della Loggia, R.; del Negro, P.; Gariboldi, P.; Tubaro, A. Complexes between phospholipids and vegetal derivates of biological interest. Fitooterapia 1989, 1–9. [Google Scholar]
- Ajazuddin; Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Sandhya, S.; Chandra, S.J.; Vinod, K.; Rao, K.; Banji, D. Preclinical studies of a novel polyherbal phyto–complex hair growth promoting cream. Asian Pac. J. Trop. Biomed. 2012, 2, S296–S304. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef] [Green Version]
- Salim, S.; Kamalasanan, K. Controlled drug delivery for alopecia: A review. J. Control. Release 2020, 325, 84–99. [Google Scholar] [CrossRef]
- Nafisi, S.; Maibach, H.I. Chapter 22—Nanotechnology in Cosmetics. In Cosmetic Science and Technology: Theoretical Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Koli, J.R.; Lin, S. Development of anti oxidant ethosomes for topical delivery utilizing the synergistic properties of Vit A palmitate, Vit E and Vit C. AAPS Pharm. Sci. Tec. 2009, 11, 1–8. [Google Scholar]
- Pan, X.; Han, K.; Peng, X.; Yang, Z.; Qin, L.; Zhu, C.; Huang, X.; Shi, X.; Dian, L.; Lu, M. Nanostructured cubosomes as advanced drug delivery system. Curr. Pharm. Des. 2013, 19, 6290–6297. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Mechanism of action of herbs and their active constituents used in hair loss treatment. Fitoterapia 2016, 114, 18–25. [Google Scholar] [CrossRef]
- Das, L.; Kaurav, M.; Pandey, R.S. Phospholipid–polymer hybrid nanoparticle-mediated transfollicular delivery of quercetin: Prospective implement for the treatment of androgenic alopecia. Drug Dev. Ind. Pharm. 2019, 45, 1654–1663. [Google Scholar] [CrossRef]
- Kim, H.S.; Kwon, H.-K.; Lee, D.H.; Le, T.N.; Park, H.-J.; Kim, M.I. Poly(γ-Glutamic Acid)/Chitosan Hydrogel Nanoparticles for Effective Preservation and Delivery of Fermented Herbal Extract for Enlarging Hair Bulb And Enhancing Hair Growth. Int. J. Nanomed. 2019, 14, 8409–8419. [Google Scholar] [CrossRef] [Green Version]
- Azuma, K.; Koizumi, R.; Izawa, H.; Morimoto, M.; Saimoto, H.; Osaki, T.; Ito, N.; Yamashita, M.; Tsuka, T.; Imagawa, T.; et al. Hair growth-promoting activities of chitosan and surface-deacetylated chitin nanofibers. Int. J. Biol. Macromol. 2018, 126, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Guryanov, I.; Naumenko, E.; Fakhrullin, R. Hair surface engineering: Combining nanoarchitectonics with hair topical and beauty formulations. Appl. Surf. Sci. Adv. 2022, 7, 100188. [Google Scholar] [CrossRef]
- Tansathien, K.; Nuntharatanapon, N.; Jaewjira, S.; Pizon, J.R.L.; Opanasopit, P.; Rangsimawong, W. Solid Lipid Nanoparticles Containing Pueraria mirifica Ethanolic Extract for Hair Growth Promotion. Key Eng. Mater. 2019, 819, 175–180. [Google Scholar] [CrossRef]
- Kumar, N.; Chaiyasut, C. Hair Growth Promoting Activity of Carthamus Tinctorius Florets Extract-Loaded Nanostructured Lipid Carriers. Int. J. Pharm. Pharm. Sci. 2015, 7, 252–257. Available online: https://innovareacademics.in/journals/index.php/ijpps/article/view/3927 (accessed on 10 January 2021).
- Ananth, P.; Koland, M. Topical Delivery of Fenugreek Seed Extract Loaded Solid Lipid Nanoparticles Based Hydrogels for Alopecia. J. Pharm. Res. Int. 2021, 33, 231–241. [Google Scholar] [CrossRef]
- Marcovici, G.; Bauman, A. An Uncontrolled Case Series Using a Botanically Derived, β-Cyclodextrin Inclusion Complex in Two Androgenetic Alopecia-Affected Male Subjects. Cosmetics 2020, 7, 65. [Google Scholar] [CrossRef]
- Hatem, S.; Nasr, M.; Moftah, N.H.; Ragai, M.H.; Geneidi, A.S.; Elkheshen, S.A. Clinical cosmeceutical repurposing of melatonin in androgenic alopecia using nanostructured lipid carriers prepared with antioxidant oils. Expert Opin. Drug Deliv. 2018, 15, 927–935. [Google Scholar] [CrossRef]
- Jadhav, N.R.; Nadaf, S.J.; Lohar, D.A.; Ghagare, P.S.; Powar, T.A. Phytochemicals formulated as nanoparticles: Inventions, recent patents and future prospects. Recent Pat. Drug Deliv. Formul. 2017, 11, 173–186. [Google Scholar] [CrossRef]
- Builders, P.F.; Mbah, C.C.; Iwu, I.W.; Builders, M.I.; Audu, M.M. Moringa Oleifera Ethosomes a Potential Hair Growth Activator: Effect on Rats. J. Pharm. Biomed. Sci. 2014, 4. Available online: www.jpbms.info (accessed on 2 February 2022).
- Yeh, M.-I.; Huang, H.-C.; Liaw, J.-H.; Huang, M.-C.; Wu, T.-H.; Huang, K.-F.; Hsu, F.-L. Ethosomes in hair dye products as carriers of the major compounds of black tea extracts. Int. J. Dermatol. 2013, 52, 868–875. [Google Scholar] [CrossRef]
- Tinkle, S.; McNeil, S.E.; Mühlebach, S.; Bawa, R.; Borchard, G.; Barenholz, Y.; Tamarkin, L.; Desai, N. Nanomedicines: Addressing the scientific and regulatory gap. Ann. N. Y. Acad. Sci. 2014, 1313, 35–56. [Google Scholar] [CrossRef] [PubMed]
- Astier, A.; Pai, A.B.; Bissig, M.; Crommelin, D.J.; Flühmann, B.; Hecq, J.-D.; Knoeff, J.; Lipp, H.-P.; Morell-Baladrón, A.; Mühlebach, S. How to select a nanosimilar. Ann. N. Y. Acad. Sci. 2017, 1407, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Mühlebach, S.; Borchard, G.; Yildiz, S. Regulatory challenges and approaches to characterize nanomedicines and their follow-on similars. Nanomedicine 2015, 10, 659–674. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.; Sousa, J.; Pais, A.; Vitorino, C. Nanomedicine: Principles, Properties, and Regulatory Issues. Front. Chem. 2018, 6, 360. [Google Scholar] [CrossRef] [PubMed]
- Sainz, V.; Conniot, J.; Matos, A.I.; Peres, C.; Zupanǒiǒ, E.; Moura, L.; Silva, L.C.; Florindo, H.F.; Gaspar, R.S. Regulatory aspects on nanomedicines. Biochem. Biophys. Res. Commun. 2015, 468, 504–510. [Google Scholar] [CrossRef]
- Ramkrishna, C.; Reddy, J.P. Impact factor: 4.295 Current Regulations for Herbal Products. 2017. Available online: www.IJARIIT.com (accessed on 19 August 2022).
- Teja, P.K.; Mithiya, J.; Kate, A.S.; Bairwa, K.; Chauthe, S.K. Herbal nanomedicines: Recent advancements, challenges, opportunities and regulatory overview. Phytomedicine 2022, 96, 153890. [Google Scholar] [CrossRef]
- França, K.; Rodrigues, T.S.; Ledon, J.; Savas, J.; Chacon, A. Comprehensive Overview and Treatment Update on Hair Loss. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ashique, S.; Sandhu, N.K.; Haque, S.N.; Koley, K. A Systemic Review on Topical Marketed Formulations, Natural Products, and Oral Supplements to Prevent Androgenic Alopecia: A Review. Nat. Prod. Bioprospect. 2020, 10, 345–365. [Google Scholar] [CrossRef]
- Wolff, H.; Fischer, T.W.; Blume-Peytavi, U. Diagnostik und Therapie von Haar- und Kopfhauterkrankungen. Dtsch. Arztebl. Int. 2016, 113, 377–386. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Han, M.; Lin, P.; He, Y.; Yu, J.; Zhao, R. Hair Growth Promotion Activity and Its Mechanism of Polygonum multiflorum. Evid.-Based Complement. Altern. Med. 2015, 2015, 517901. [Google Scholar] [CrossRef] [Green Version]
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-Derived Sonic Hedgehog Defines a Niche for Hair Follicle Stem Cells Capable of Becoming Epidermal Stem Cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jindo, T.; Tsuboi, R.; Takamori, K.; Ogawa, H. Local Injection of Hepatocyte Growth Factor/Scatter Factor (HGF/SF) Alters Cyclic Growth of Murine Hair Follicles. J. Investig. Dermatol. 1998, 110, 338–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsatmali, M.; Ancans, J.; Thody, A.J. Melanocyte Function and Its Control by Melanocortin Peptides. J. Histochem. Cytochem. 2002, 50, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Sho, C.; Kawano, K.; Kurata, R.; Yoshimoto, M.; Okuno, H. Hair growth-promoting activity of components derived from sweet potato shochu. J. Biosci. Bioeng. 2021, 131, 405–411. [Google Scholar] [CrossRef]
- Li, Y.; Sheng, Y.; Liu, J.; Xu, G.; Yu, W.; Cui, Q.; Lu, X.; Du, P.; An, L. Hair-growth promoting effect and anti-inflammatory mechanism of Ginkgo biloba polysaccharides. Carbohydr. Polym. 2021, 278, 118811. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, G.; Li, X.; Sun, W.; Liang, Y.; Gan, D.; Liu, G.; Song, W.; Wang, Z. Study on the chemical constituents of nut oil from Prunus mira Koehne and the mechanism of promoting hair growth. J. Ethnopharmacol. 2020, 258, 112831. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Wang, J.; Gao, Y.; Yang, F.; Li, G.; Shi, Q. Ginsenoside Rb1 promotes the growth of mink hair follicle via PI3K/AKT/GSK-3β signaling pathway. Life Sci. 2019, 229, 210–218. [Google Scholar] [CrossRef]
- Wen, T.-C.; Li, Y.-S.; Rajamani, K.; Harn, H.-J.; Lin, S.-Z.; Chiou, T.-W. Effect of Cinnamomum osmophloeum Kanehira Leaf Aqueous Extract on Dermal Papilla Cell Proliferation and Hair Growth. Cell Transplant. 2018, 27, 256–263. [Google Scholar] [CrossRef] [Green Version]
- Saansoomchai, P.; Limmongkon, A.; Surangkul, D.; Chewonarin, T.; Srikummool, M. Enhanced VEGF Expression in Hair Follicle Dermal Papilla Cells by Centella asiatica Linn. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 25–37. [Google Scholar] [CrossRef]
- Kang, J.-I.; Kim, S.-C.; Hyun, J.-H.; Park, D.-B.; Lee, Y.-J.; Yoo, E.-S.; Kang, H.-K. Promotion effect of Schisandra nigra on the growth of hair. Eur. J. Dermatol. 2009, 19, 119–125. [Google Scholar] [CrossRef]
- Patel, S.; Sharma, V.; Chauhan, N.S.; Thakur, M.; Dixit, V.K. Hair Growth: Focus on Herbal Therapeutic Agent. Curr. Drug Discov. Technol. 2015, 12, 21–42. [Google Scholar] [CrossRef] [PubMed]
- Breitkopf, T.; Leung, G.; Yu, M.; Wang, E.; McElwee, K.J. The Basic Science of Hair Biology. What Are the Causal Mechanisms for the Disordered Hair Follicle. Dermatol. Clin. 2013, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V. New Activators and Inhibitors in the Hair Cycle Clock: Targeting Stem Cells’ State of Competence. J. Investig. Dermatol. 2012, 132, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [Green Version]
- Bhinge, S.D.; Bhutkar, M.A.; Randive, D.S.; Wadkar, G.H.; Todkar, S.S.; Savali, A.S.; Chittapurkar, H.R. Screening of hair growth promoting activity of Punica granatum L. (pomegranate) leaves extracts and its potential to exhibit antidandruff and anti-lice effect. Heliyon 2021, 7, e06903. [Google Scholar] [CrossRef]
- Rose, L.C.; Rusdi NN, S.; Asari, A.; Wahid ME, A.; Suhaimi, H. Potential hair growth of crude extract from Hibiscus rosa-sinensis Linn. Arch. Pharm. Pract. 2020, 1, 13–19. [Google Scholar]
- Adhirajan, N.; Kumar, T.R.; Shanmugasundaram, N.; Babu, M. In vivo and in vitro evaluation of hair growth potential of Hibiscus rosa-sinensis Linn. J. Ethnopharmacol. 2003, 88, 235–239. [Google Scholar] [CrossRef]
- Singh, V.; Ali, M.; Upadhyay, S. Study of colouring effect of herbal hair formulations on graying hair. Pharmacogn. Res. 2015, 7, 259–262. [Google Scholar] [CrossRef] [Green Version]
- Gottumukkala, V.R.; Annamalai, T.; Mukhopadhyay, T. Phytochemical investigation and hair growth studies on the rhizomes of Nardostachys jatamansi DC. Pharmacogn. Mag. 2011, 7, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.N.; Cui, L.; Li, W.; Yan, X.T.; Yang, S.Y.; Kang, J.I.; Kang, H.K.; Kim, Y.H. Promotion effect of constituents from the root of Polygonum multiflorum on hair growth. Bioorganic Med. Chem. Lett. 2013, 23, 4801–4805. [Google Scholar] [CrossRef]
- Shin, H.-S.; Lee, J.-M.; Park, S.-Y.; Yang, J.-E.; Kim, J.-H.; Yi, T.-H. Hair Growth Activity of Crataegus pinnatifida on C57BL/6 Mouse Model. Phytother. Res. 2012, 27, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S. Effect of Origanum vulgare extracts on hair regeneration. Korean J. Pharmacogn. 2013, 44, 275–280. [Google Scholar]
- Park, S.; Shin, W.-S.; Ho, J. Fructus panax ginseng extract promotes hair regeneration in C57BL/6 mice. J. Ethnopharmacol. 2011, 138, 340–344. [Google Scholar] [CrossRef]
- Park, P.-J.; Moon, B.-S.; Lee, S.-H.; Kim, S.-N.; Kim, A.-R.; Kim, H.-J.; Park, W.-S.; Choi, K.-Y.; Cho, E.-G.; Lee, T.R. Hair growth-promoting effect of Aconiti Ciliare Tuber extract mediated by the activation of Wnt/β-catenin signaling. Life Sci. 2012, 91, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Han, J.; Kchouk, M.E.; Isoda, H. Hair growth regulation by the extract of aromatic plant Erica multiflora. J. Nat. Med. 2009, 63, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-H.; Choi, J.-H.; Song, Y.-S.; Kim, G.-C.; Hong, J.-W. Ethanol extract of asiasari radix preferentially induces apoptosis in G361 human melanoma cells by differential regulation of p53. BMC Complement. Altern. Med. 2019, 19, 231. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, M.; Kim, S.-J. Phytochemical, Toxicological and Pharmacological Studies of Asiasari Radix et Rhizoma: A Review. Trop. J. Pharm. Res. 2015, 14, 545. [Google Scholar] [CrossRef] [Green Version]
- Bassino, E.; Gasparri, F.; Munaron, L. Protective Role of Nutritional Plants Containing Flavonoids in Hair Follicle Disruption: A Review. Int. J. Mol. Sci. 2020, 21, 523. [Google Scholar] [CrossRef] [Green Version]
- Datta, K.; Singh, A.T.; Mukherjee, A.; Bhat, B.; Ramesh, B.; Burman, A.C. Eclipta alba extract with potential for hair growth promoting activity. J. Ethnopharmacol. 2009, 124, 450–456. [Google Scholar] [CrossRef]
- Patel, S.; Nag, M.K.; Sharma, V.; Chauhan, N.S.; Dixit, V. A comparative in vivo and in vitro evaluation of hair growth potential of extracts and an isolate from petroleum ether extract of Cuscuta reflexa Roxb. Beni-Suef Univ. J. Basic Appl. Sci. 2014, 3, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Kondo, R.; Sakai, K.; Shoyama, Y.; Sato, H.; Ueno, T. Steroid 5α-Reductase Inhibitory Activity and Hair Regrowth Effects of an Extract from Boehmeria nipononivea. Biosci. Biotechnol. Biochem. 2000, 64, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpon, S.; Nimmannit, U.; Pongrakhan, V.; Chanvorach, P. Emblica (Phyllanthus emblica Linn.) Fruit Extract Promotes Proliferation in Dermal Papilla Cells of Human Hair Follicle. Res. J. Med. Plant 2011, 5, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Noguchi, K.; Kondo, M.; Onishi, M.; Watanabe, N.; Okamura, K.; Matsuda, H. Promotion of hair growth by Rosmarinus officinalis leaf extract. Phytother. Res. 2013, 27, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Kim, S.N.; Jung, I.K.; Kim, H.H.; Park, Y.H.; Park, W.S. The inhibitory effect of Scutellaria baicalensis extract and its active compound, baicalin, on the translocation of the androgen receptor with implications for preventing androgenetic alopecia. Planta Med. 2014, 80, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Chauhan, N.S.; Dixit, V.K. Effect of Cuscuta reflexa Roxb on androgen-induced alopecia. J. Cosmet. Dermatol. 2008, 7, 199–204. [Google Scholar] [CrossRef]
- Takahashi, T.; Kamimura, A.; Shirai, A.; Yokoo, Y. Several Selective Protein Kinase C Inhibitors Including Procyanidins Promote Hair Growth. Skin. Pharmacol. Appl. Skin. Physiol. 2000, 13, 133–142. [Google Scholar] [CrossRef]
- Jain, P.; Das, D.; Jain, P.; Jain, P. Pharmacognostic and Pharmacological Aspect of Bacopa Monnieri: A Review. Int. J. Pharm. Pharm. Sci. 2016, 4, 7–11. [Google Scholar]
- Harada, N.; Okajima, K.; Arai, M.; Kurihara, H.; Nakagata, N. Administration of capsaicin and isoflavone promotes hair growth by increasing insulin-like growth factor-I production in mice and in humans with alopecia. Growth Horm. IGF Res. 2007, 17, 408–415. [Google Scholar] [CrossRef]
- An, S.M.R.; El, M.; Philpott, P.; Thon1as, A.; Ealey, T.K. The Role of IGF-1 in Human Skin and its Appendages: Morphogen as Well as Mitogen? J. Investig. Dermatol. 1997, 109, 770–777. [Google Scholar]
- Inui, S.; Itami, S. Induction of insulin-like growth factor-I by cepharanthine from dermal papilla cells: A novel potential pathway for hair growth stimulation. J. Dermatol. 2013, 40, 1054–1055. [Google Scholar] [CrossRef]
- Shafi, A.; Akram, K. Chapter 10—Herbal oil in healthcare. In Herbal Biomolecules in Healthcare Applications; Mandal, S.C., Nayak, A.K., Dhara, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 205–213. [Google Scholar] [CrossRef]
- Jain, P.K.; Das, D.; Das, C. Prospect of Herbs ss Hair Growth Potential. Innovare J. Med. Sci. 2017, 5, 25–33. [Google Scholar]
- Gavazzoni, D. Hair: Sn overview. Int. J. Trichol. 2015, 7, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.-J.; Hong, S.-G.; Lee, J.-W.; Jeong, W.-S. Red Ginseng Marc Oil Inhibits inos and COX-2 via NFκB and p38 Pathways in LPS-Stimulated RAW 264.7 Macrophages. Molecules 2012, 17, 13769–13786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truong, V.-L.; Keum, Y.-S.; Jeong, W.-S. Red ginseng oil promotes hair growth and protects skin against UVC radiation. J. Res. 2021, 45, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Park, G.H.; Park, K.Y.; Cho, H.I.; Lee, S.M.; Han, J.S.; Won, C.H.; Chang, S.E.; Lee, M.W.; Choi, J.H.; Moon, K.C.; et al. Red ginseng extract promotes the hair growth in cultured human hair follicles. J. Med. Food 2015, 18, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Roy, R.K.; Thakur, M.; Dixit, V.K. Hair growth promoting activity of Eclipta alba in male albino rats. Arch. Dermatol. Res. 2008, 300, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Lee, M.R.; Gu, L.J.; Hossain, J.; Sung, C.K. Exogenous stimulation with Eclipta alba promotes hair matrix keratinocyte proliferation and downregulates TGF-β1 expression in nude mice. Int. J. Mol. Med. 2015, 35, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Rungseevijitprapa, W.; Narkkhong, N.-A.; Suttajit, M.; Chaiyasut, C. 5α-reductase inhibition and hair growth promotion of some Thai plants traditionally used for hair treatment. J. Ethnopharmacol. 2012, 139, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Bíró, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-β2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar] [CrossRef]
- Lourith, N.; Kanlayavattanakul, M. Hair loss and herbs for treatment. J. Cosmet. Dermatol. 2013, 12, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Akroum, S. Antifungal activity of Camellia sinensis crude extracts against four species of Candida and Microsporum persicolor. J. Mycol. Med. 2018, 28, 424–427. [Google Scholar] [CrossRef] [PubMed]
- Hameed, I.H.; Cotos, M.R.C.; Hadi, M.Y. A Review: Solanum nigrum L. Antimicrobial, Antioxidant properties, Hepatoprotective effects and Analysis of Bioactive Natural Compounds. Res. J. Pharm. Technol. 2017, 10, 4063. [Google Scholar] [CrossRef]
- Chakraborty, A.; Bhattacharjee, A.; Sepay, N.; Sodani, A.; Jain, D.; Mukhopadhyay, G. Herbal Hair Gel Formulation having 5α-Reductase Inhibitory Activity and its Standardization by HPTLC. J. Anal. Bioanal. Tech. 2016, 7, 341. [Google Scholar] [CrossRef] [Green Version]
- Murugusundram, S. Serenoa repens: Does it have any role in the management of androgenetic alopecia? J. Cutan. Aesthetic Surg. 2009, 2, 31–32. [Google Scholar] [CrossRef] [PubMed]
- Schoen, C.; Bielfeldt, S.; Schulz, C.; Reimann, J. Fenugreek+micronutrients: Efficacy of a Food Supplement against Hair Loss Effects of Lemon Verbena Extract (Recoverben®) Supplementation on Muscle Strength and Recovery after Exhaustive Exercise: A Randomized, Placebo-Controlled Trial View Project Cosmetic Medicine Bockshornsamen + Mikronährstoffe: Wirksamkeit Eines Nahrungsergänzungsmittels Gegen Haarausfall Fenugreek+micronutrients: Efficacy of a Food Supplement against Hair Loss. Kosmet. Med. 2006, 27, 176–179. Available online: https://www.researchgate.net/publication/251923543 (accessed on 2 February 2022).
- Imtiaz, F.; Islam, M.; Saeed, H.; Saleem, B.; Asghar, M.; Saleem, Z. Impact of Trigonella foenum-graecum Leaves Extract on Mice Hair Growth. Pak. J. Zool. 2017, 49, 1405–1412. [Google Scholar] [CrossRef]
- Kor, N.M.; Didarshetaban, M.B.; Pour, H.R.S. Fenugreek (Trigonella foenum-graecum L.) as a Valuable Medicinal Plant. Int. J. Adv. Biol. Biomed. Res. 2013, 1, 922–931. Available online: http://www.ijabbr.com/article_7851.html (accessed on 2 February 2022).
- Jain, P.; Das, D.; Pandey, N.; Jain, P. Traditional Indian Herb Emblica Officinalis and Its Medicinal Importance. Int. J. Pharm. Pharm. Sci. 2016, 4, 1–15. [Google Scholar]
- Yu, J.Y.; Gupta, B.; Park, H.G.; Son, M.; Jun, J.-H.; Yong, C.S.; Kim, J.A.; Kim, J.O. Preclinical and Clinical Studies Demonstrate That the Proprietary Herbal Extract DA-5512 Effectively Stimulates Hair Growth and Promotes Hair Health. Evid. -Based Complement. Altern. Med. 2017, 2017, 4395638. [Google Scholar] [CrossRef]
- Kumar, K.S.; Bhowmik, D.; Dutta, A.; Yadav, A.P.; Paswan, S.; Srivastava, S.; Deb, L. Recent Trends in Potential Traditional Indian Herbs Emblica officinalis and Its Medicinal Importance. J. Pharmacogn. Phytochem. 2012, 1, 24–32. Available online: https://www.phytojournal.com (accessed on 19 August 2022).
- Park, W.-S.; Kwon, O.; Yoon, T.-J.; Chung, J.H. Anti-graying effect of the extract of Pueraria thunbergiana via upregulation of cAMP/MITF-M signaling pathway. J. Dermatol. Sci. 2014, 75, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, E.K.; Granter, S.R.; Fisher, D.E. Mechanisms of Hair Graying: Incomplete Melanocyte Stem Cell Maintenance in the Niche. Science 2005, 307, 720–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, R.-M.; Tian, Y.-X.; Becker, E.M.; Andersen, M.L.; Zhang, J.-P.; Skibsted, L.H. Puerarin and Conjugate Bases as Radical Scavengers and Antioxidants: Molecular Mechanism and Synergism with β-Carotene. J. Agric. Food Chem. 2007, 55, 2384–2391. [Google Scholar] [CrossRef]
- Rho, S.-S.; Park, S.-J.; Hwang, S.-L.; Lee, M.-H.; Kim, C.D.; Chang, S.-Y.; Rang, M.-J. The hair growth promoting effect of Asiasari radix extract and its molecular regulation. J. Dermatol. Sci. 2005, 38, 89–97. [Google Scholar] [CrossRef]
- Mori-Okamoto, J.; Otawara-Hamamoto, Y.; Yamato, H.; Yoshimura, H. Pomegranate extract improves a depressive state and bone properties in menopausal syndrome model ovariectomized mice. J. Ethnopharmacol. 2004, 92, 93–101. [Google Scholar] [CrossRef]
- Dario, M.F.; Pahl, R.; de Castro, J.R.; de Lima, F.S.; Kaneko, T.M.; Pinto, C.A.; Baby, A.R.; Velasco, M.V.R. Efficacy of Punica granatum L. hydroalcoholic extract on properties of dyed hair exposed to UVA radiation. J. Photochem. Photobiol. B Biol. 2013, 120, 142–147. [Google Scholar] [CrossRef]
- Rao, G.-X.; Xue, Y.-M.; Hui, T.-T.; Wang, W.-J.; Zhang, Q.-L. Studies on the chemical constituents of the leaves of Polygonum multiflorum. J. Chin. Med. Mater. 2009, 32, 891–893. [Google Scholar]
- Thang, N.D.; Diep, P.N.; Lien, P.T.H.; Lien, L.T. Polygonum multiflorum root extract as a potential candidate for treatment of early graying hair. J. Adv. Pharm. Technol. Res. 2017, 8, 8–13. [Google Scholar] [CrossRef]
- Park, H.-J.; Zhang, N.; Park, D.K. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J. Ethnopharmacol. 2011, 135, 369–375. [Google Scholar] [CrossRef]
- Han, M.; Lu, J.; Zhang, M.; Zhao, R. Mechanistic Studies on the Use of Polygonum multiflorum for the Treatment of Hair Graying. Biomed. Res. Int. 2015, 2015, 651048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-C.; Kang, J.-I.; Park, D.-B.; Lee, Y.-K.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S.; Kim, J.A.; Kim, Y.H.; Kang, H.-K. Promotion effect of acankoreoside J, a lupane-triterpene in Acanthopanax koreanum, on hair growth. Arch. Pharmacal Res. 2012, 35, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Perna, S.; Peroni, G.; Guido, D. A bibliometric study of scientific literature in Scopus on botanicals for treatment of androgenetic alopecia. J. Cosmet. Dermatol. 2016, 15, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Campiglia, P.; Ritieni, A.; Novellino, E. In vitro bioaccessibility, bioavailability and plasma protein interaction of polyphenols from Annurca apple (M. pumila Miller cv Annurca). Food Chem. 2013, 141, 3519–3524. [Google Scholar] [CrossRef]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Santamaria, R.; Irace, C.; Piccolo, M.; Maisto, M.; Novellino, E. Annurca Apple Nutraceutical Formulation Enhances Keratin Expression in a Human Model of Skin and Promotes Hair Growth and Tropism in a Randomized Clinical Trial. J. Med. Food 2018, 21, 90–103. [Google Scholar] [CrossRef] [Green Version]
- Kamimura, A.; Takahashi, T. Procyanidin B-2, extracted from apples, promotes hair growth: A laboratory study. Br. J. Dermatol. 2002, 146, 41–51. [Google Scholar] [CrossRef]
- Suleria, H.A.R.; Butt, M.S.; Khalid, N.; Sultan, S.; Raza, A.; Aleem, M.; Abbas, M. Garlic (Allium sativum): Diet based therapy of 21st century–a review. Asian Pac. J. Trop. Dis. 2015, 5, 271–278. [Google Scholar] [CrossRef]
- Ichikawa, M.; Yoshida, J.; Ide, N.; Sasaoka, T.; Yamaguchi, H.; Ono, K.; Ichikawa, M.; Yoshida, J.; Ide, N.; Sasaoka, T.; et al. Tetrahydro-β-Carboline Derivatives in Aged Garlic Extract Show Antioxidant Properties. J. Nutr. 2006, 136, 726S–731S. [Google Scholar] [CrossRef] [Green Version]
- Fischer, T.W.; Hipler, U.C.; Elsner, P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int. J. Dermatol. 2007, 46, 27–35. [Google Scholar] [CrossRef]
- Dhurat, R.; Chitallia, J.; May, T.W.; Jayaraaman, A.M.; Madhukara, J.; Anandan, S.; Vaidya, P.; Klenk, A. An Open-Label Randomized Multicenter Study Assessing the Noninferiority of a Caffeine-Based Topical Liquid 0.2% versus Minoxidil 5% Solution in Male Androgenetic Alopecia. Ski. Pharmacol. Physiol. 2017, 30, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Klančnik, A.; Guzej, B.; Kolar, M.H.; Abramovič, H.; Možina, S.S. In Vitro Antimicrobial and Antioxidant Activity of Commercial Rosemary Extract Formulations. J. Food Prot. 2009, 72, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Hall, C. Overview of the Oilseed Safflower (Carthamus tinctorius L.). In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Junlatat, J.; Sripanidkulchai, B. Hair Growth-Promoting Effect of Carthamus tinctorius Floret Extract. Phytother. Res. 2013, 28, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Mohsen, H.; Bassem, J.; Lamia, H. Chemical composition of Thuja orientalis L. essential oils and study of their allelopathic potential on germination and seedling growth of weeds. Arch. Phytopathol. Plant Prot. 2014, 48, 18–27. [Google Scholar] [CrossRef]
- Zhang, N.-N.; Park, D.K.; Park, H.-J. Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement. Altern. Med. 2013, 13, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.X.; Wang, Y.; Kong, L.D.; Yang, C.; Zhang, X. Effects of Biota orientalis extract and its flavonoid constituents, quercetin and rutin on serum uric acid levels in oxonate-induced mice and xanthine dehydrogenase and xanthine oxidase activities in mouse liver. J. Ethnopharmacol. 2004, 93, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Zakl, R.P.; Jakubczyk, K.; Janda, K.; Watychowicz, K.; Łukasiak, J.; Wolska, J. Garden nasturtium (tropaeolum majus L.)-A source of mineral elements and bioactive compounds. Rocz. Państwowego Zakładu Hig. 2018, 69, 119–126. Available online: http://wydawnictwa.pzh.gov.pl/roczniki_pzh/ (accessed on 19 August 2022).
- Al Bratty, M.; Alhazmi, H.A.; Thangavel, N. GC–MS profiling and in silico prediction of MAPK receptor activation by fatty acids of watercress oil for hair growth marketed in Saudi Arabia. J. Saudi Chem. Soc. 2021, 25, 101196. [Google Scholar] [CrossRef]
- Munkhbayar, S.; Jang, S.; Cho, A.-R.; Choi, S.-J.; Shin, C.Y.; Eun, H.C.; Kim, K.H.; Kwon, O. Role of Arachidonic Acid in Promoting Hair Growth. Ann. Dermatol. 2016, 28, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Hibbert, L.; Taylor, G. Improving phosphate use efficiency in the aquatic crop watercress (Nasturtium officinale). Hortic. Res. 2022, 9, uhac011. [Google Scholar] [CrossRef]
- Amiri, H. Volatile constituents and antioxidant activity of flowers, stems and leaves of Nasturtium officinale R. Br. Nat. Prod. Res. 2011, 26, 109–115. [Google Scholar] [CrossRef]
- Takahashi, T.; Ishino, A.; Arai, T.; Hamada, C.; Nakazawa, Y.; Iwabuchi, T.; Tajima, M. Improvement of androgenetic alopecia with topical Sophora flavescens Aiton extract, and identification of the two active compounds in the extract that stimulate proliferation of human hair keratinocytes. Clin. Exp. Dermatol. 2015, 41, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Ichimura, M.; Ishikawa, N.; Tanaka, T.; Iinuma, M.; Mizuno, M. Localization of Prenylated Flavonoids in Sophora flavescens var. angustifolia Plants. Z. Für Nat. C 1992, 47, 535–539. [Google Scholar] [CrossRef]
- Roh, S.S.; Kim, C.D.; Lee, M.H.; Hwang, S.L.; Rang, M.J.; Yoon, Y.K. The hair growth promoting effect of Sophora flavescens extract and its molecular regulation. J. Dermatol. Sci. 2002, 30, 43–49. [Google Scholar] [CrossRef]
- Kim, J.H. Nanoparticle Composition for Prevention of Hair Loss and Promotion of Hair Growth. U.S. Patent US20100104646A1, 2012. [Google Scholar]
- Choi, E. Composition for Nano Particle Comprising Henna Extract and Manufacturing Method for Nano Particle Using It. Korean Patent KR100675808B1, 19 January 2007. [Google Scholar]
- Choi, E. Composition for Preventing Hair Loss or Promoting Hair Growth, Containing Ginseng-Derived Exosome-Like Vesicles. Korean Patent WO2017057881, 30 December 2020. [Google Scholar]
- Lee. J.H. Hair Growth-Promoting Ingredient-Loaded and Skin Temperature-Responsive Ionic Polymer-Immobilized Lipidic Nanostructures and Method for Preparation of the Same. Korean Patent KR101536996B1, 15 July 2015.
- Jong, E.H. Composition for Transdermal Delivery Comprising Nanoemulsion and Modified Layered Double Hydroxide. Korean Patent KR101883719B1, 1 August 2018. [Google Scholar]
- Taepyeong, Y. Compositions for Hair Cosmetic Containing Nano-Emulsion. Korean Patent KR20040033117A, 24 April 2004. [Google Scholar]



| Sr. No | Factors | Effects | Stimulation/Inhibition for Growth. |
|---|---|---|---|
| 1 | Insulin-like Growth Factor (IGF) | Stimulates cellular proliferation and inhibits DHT. | Stimulation |
| 2 | Vascular endothelial growth factor (VEGF) | Supply the nutrients and increased the blood circulation on the scalp, resulting in stimulation of hair growth. | Stimulation |
| 3 | Fibroblast growth factor-2 (FGF-2) | Stimulation to the hair follicle development. | Stimulation |
| 4 | Fibroblast growth factor-5 (FGF-5) | Inhibition of hair growth in the anagen phase. | Inhibition |
| 5 | Epidermal growth factor (EGF) | Proliferation and formation of hair follicles. | Stimulation |
| 6 | Wingless-related integration site (WNT) | Stimulates, the growth, and development of hair follicles. | Stimulation |
| 8 | Prostaglandin (PGD) | Hair growth inhibition. | Inhibition |
| 9 | Transforming growth factor beta (TGF-β) | Reduce the duration of a cycle. | Inhibition |
| Biological Source | Extract. | Mechanism of Action | Effects | Reference |
|---|---|---|---|---|
| Sweet potato shochu | - | Increases expression of vascular endothelial growth factor | The hair growth-inducing effect promotes hair growth and activates the hair growth cycle. Act as a hair restorer | [73] |
| Ginkgo biloba | Ethanolic | Increase VEGF and HGF levels, and decrease Inflammatory factors such as TNF-α and IL-β. | The hair-growth promoting effect, treat alopecia areata, | [74] |
| Prunus mira | Pet. ether | Induce anagen, by Wnt\β-catenin, protein expression. | Promoting hair growth, induction of anagen phase. | [75] |
| Ginsenoside Rb | - | Increases VEGFA and VEGF-R2, while decreasing the TGF-β expression in hair follicles and DPCs. | Promoted the growth of hair follicle cells. Induce the growth of DPCs. | [76] |
| Cinnamomum osmophloeum | Aqueous | Increases HGF, VEGF, KGF, TGF-β2 (↑) | Promotion of anagen, proliferation of dermal papilla cells, stimulate hair growth, prevent hair loss. | [77] |
| Centella asiatica | Ethanolic | Increases expression VEGF in DPCs | Hair growth stimulating effect, modulating DPCs, antioxidant activity. | [78] |
| Sargassum muticum | Activates the Wnt/β-Catenin, VEGF-R2 | Hair-fiber lengths increase promotes the anagen phase and the proliferation of dermal papilla cells | [79] |
| Biological Source | Extract | Mechanism of Action | Effects | Reference |
|---|---|---|---|---|
| Punica granatum | Alcoholic and aqueous extracts | Stimulate telogen follicle and makes larger anagen follicles size. | Anti-dandruff activity and growth-promoting effects on hair. | [84] |
| Hibiscus rosa-sinensis | Petroleum ether | Transformation from telogen to anagen phase. | Thicker hair and prevents graying of hair | [85] |
| Nardostachys jatamansi | Hexane | Enlargement of follicles prolongs the anagen phase. | Hair growth-promoting activity | [87] |
| Polygonum multiflorum extract | Aqueous | Proliferation by MTT (↑) | Increased in hair fiber length | [89] |
| Crataegus pinnatifida extract | - | Activates protein kinases. | Induction of anagen phase | [90] |
| Origanum vulgare extract | - | Stimulation of insulin growth factor- (IGF-) | The proliferation of hair dermal papilla cells. | [91] |
| Panax ginseng | Ethanolic | Anti-apoptotic activation. | Elongation of the anagen phase. | [92] |
| Aconitum ciliare Tuber extract | Aqueous | Activates the Wnt/β-catenin signaling pathway | Induce anagen hair growth | [93] |
| Schisandra nigra extract | Ethanolic | Transforming growth factor-beta2 (TGF-beta2) | Promote hair growth | [79] |
| Erica multiflora extract | Ethanolic | Proliferation by MTT (↑) G2/M phase in the cell cycle (↑) | Telogen to anagen induction | [94] |
| Asiasari radix extract | Ethanolic | Thymidine incorporation (↑), VEGF (↑) | Telogen to anagen conversion | [95] |
| Biological Source | Extract | Mechanism of Action | Effects | Reference |
|---|---|---|---|---|
| Eclipta alba | Methanolic | 5-reductase inhibition | Hair growth-promoting activity | [98] |
| Cuscuta reflexa | Pet. ether | Inhibition of 5a-reductase activity | Hair growth promote | [99,104] |
| Boehmeria nipononivea | Acetone | 5 reductase Type II Inhibitors | Hair regrowth promotion | [100] |
| Emblica officinalis | Aqueous | Powerful inhibitor of 5α-reductase | Antibacterial and anti-microbial properties nourish hair. | [101] |
| Rosmarinus officinalis | Essential oils | Inhibiting 5-alpha-reductase, improving vascularity of scalp. | Stimulate the growth of hair regeneration of follicles | [102] |
| Scutellaria baicalensis extract | - | Androgen receptor antagonistic effect DHT (↓) | The proliferation of hair dermal papilla cells | [79,103] |
| Biological Source/Phytoconstituents | Mechanism of Action | Effects | Reference |
|---|---|---|---|
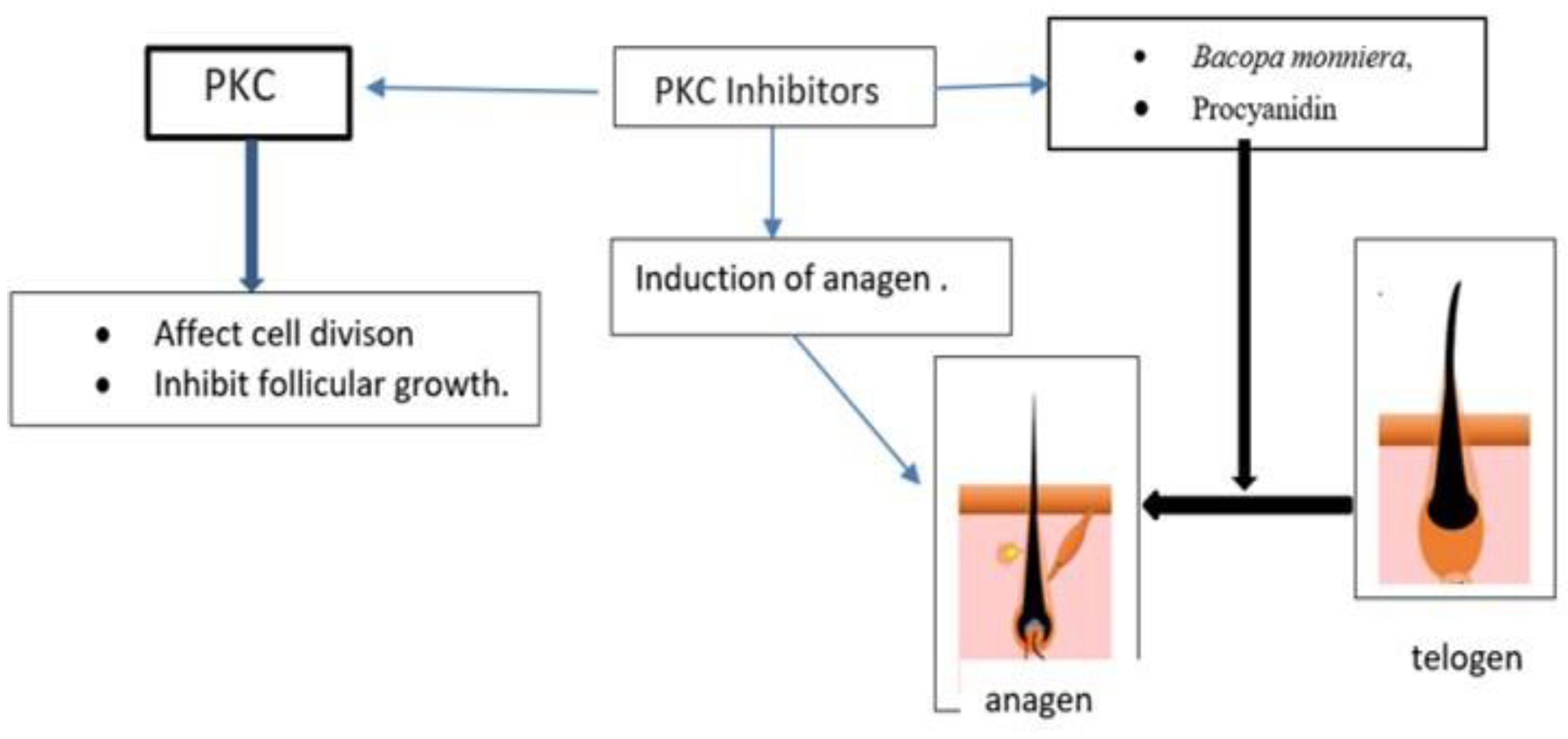
| Cinnamomum verum | Protein kinase C (PKC) inhibitors, | Promotion of epithelial cell growth and induce anagen phase. | [105] |
| Bacopa monniera | Protein kinase C (PKC) inhibitors, | enlargement of follicular size and prolongation of the anagen phase | [106] |
| Biological Source/Phytoconstituents. | Mechanism of Action | Effects | Reference |
|---|---|---|---|
| Capsicum annum | Production of IGF-I | Promotes hair growth. | [107] |
| Stephania cepharanthap | Production of IGF-I | Induction of hair growth | [108,109] |
| Patent Number | Title | Description | Reference |
|---|---|---|---|
| Us 2010/0104646a1 | Nanoparticle composition for prevention of hair loss and promotion of hair growth | This invention formulates the herbal hair care composition-based lecithin-capsuled nanoparticles. Nanoparticles activates hair follicles. It has antioxidant effects. | [165] |
| Kr1020050024694ar100675808b1 | Composition for nano particle comprising henna extract and manufacturing method for nano particle using it | This invention relates to a nano particle comprising henna extract, lecithin, ethanol, triglyceride, anionic surfactant for hair treatments. | [166] |
| Wo2017057881a1 | Composition for preventing hair loss or promoting hair growth, containing ginseng-derived exosome-like vesicles | This invention formulates ginseng-derived exosome-like vesicles for hair loss or promoting hair growth | [167] |
| Kr101536996b1 | Hair growth-promoting ingredient-loaded and skin temperature-responsive ionic polymer-immobilized lipidic nanostructures and method for preparation of the same | This invention formulates ionic polymer-immobilized lipidic nanostructures contains Thuja orientalis, Polygonum multiflorum, and espinosilla. For promoting hair growth | [168] |
| Kr101883719b1 | Composition for transdermal delivery comprising nanoemulsion and modified layered double hydroxide | This invention formulates nanoemulsion and modified layered double hydroxide for hair care | [169] |
| Kr20040033117a | Compositions for hair cosmetic containing nano-emulsion | This invention is concerned with nano-emulsion based natural source polymer for hair care | [170] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padule, K.; Shinde, S.; Chitlange, S.; Giram, P.; Nagore, D. The Advancement of Herbal-Based Nanomedicine for Hair. Cosmetics 2022, 9, 118. https://doi.org/10.3390/cosmetics9060118
Padule K, Shinde S, Chitlange S, Giram P, Nagore D. The Advancement of Herbal-Based Nanomedicine for Hair. Cosmetics. 2022; 9(6):118. https://doi.org/10.3390/cosmetics9060118
Chicago/Turabian StylePadule, Komal, Sonali Shinde, Sohan Chitlange, Prabhanjan Giram, and Dheeraj Nagore. 2022. "The Advancement of Herbal-Based Nanomedicine for Hair" Cosmetics 9, no. 6: 118. https://doi.org/10.3390/cosmetics9060118
APA StylePadule, K., Shinde, S., Chitlange, S., Giram, P., & Nagore, D. (2022). The Advancement of Herbal-Based Nanomedicine for Hair. Cosmetics, 9(6), 118. https://doi.org/10.3390/cosmetics9060118







