The Use of Medicinal Plant-Derived Metallic Nanoparticles in Theranostics
Abstract
1. Introduction
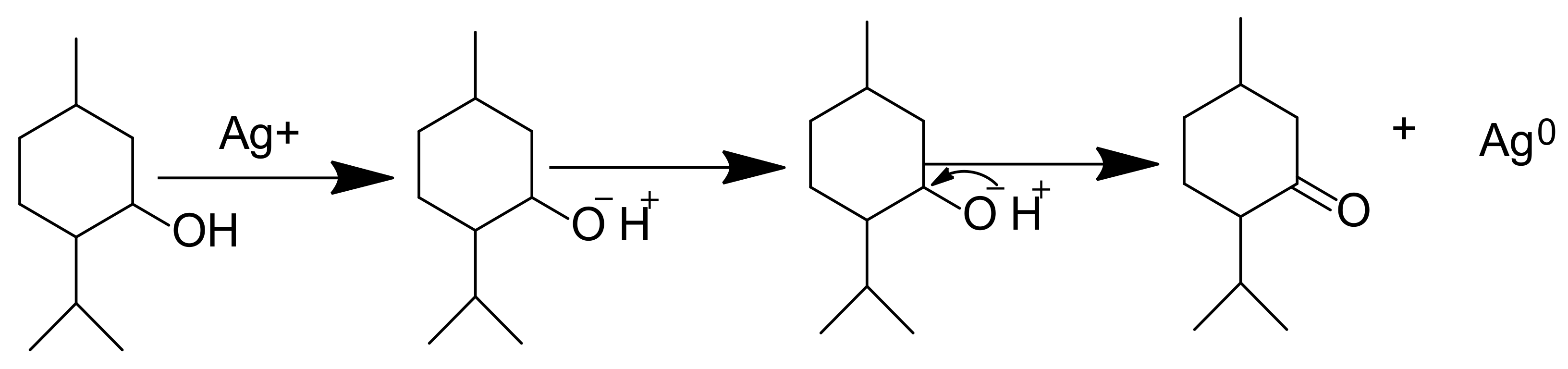
2. Medicinal Plant-Derived Phytochemicals Used in the Green Synthesis of MNPs
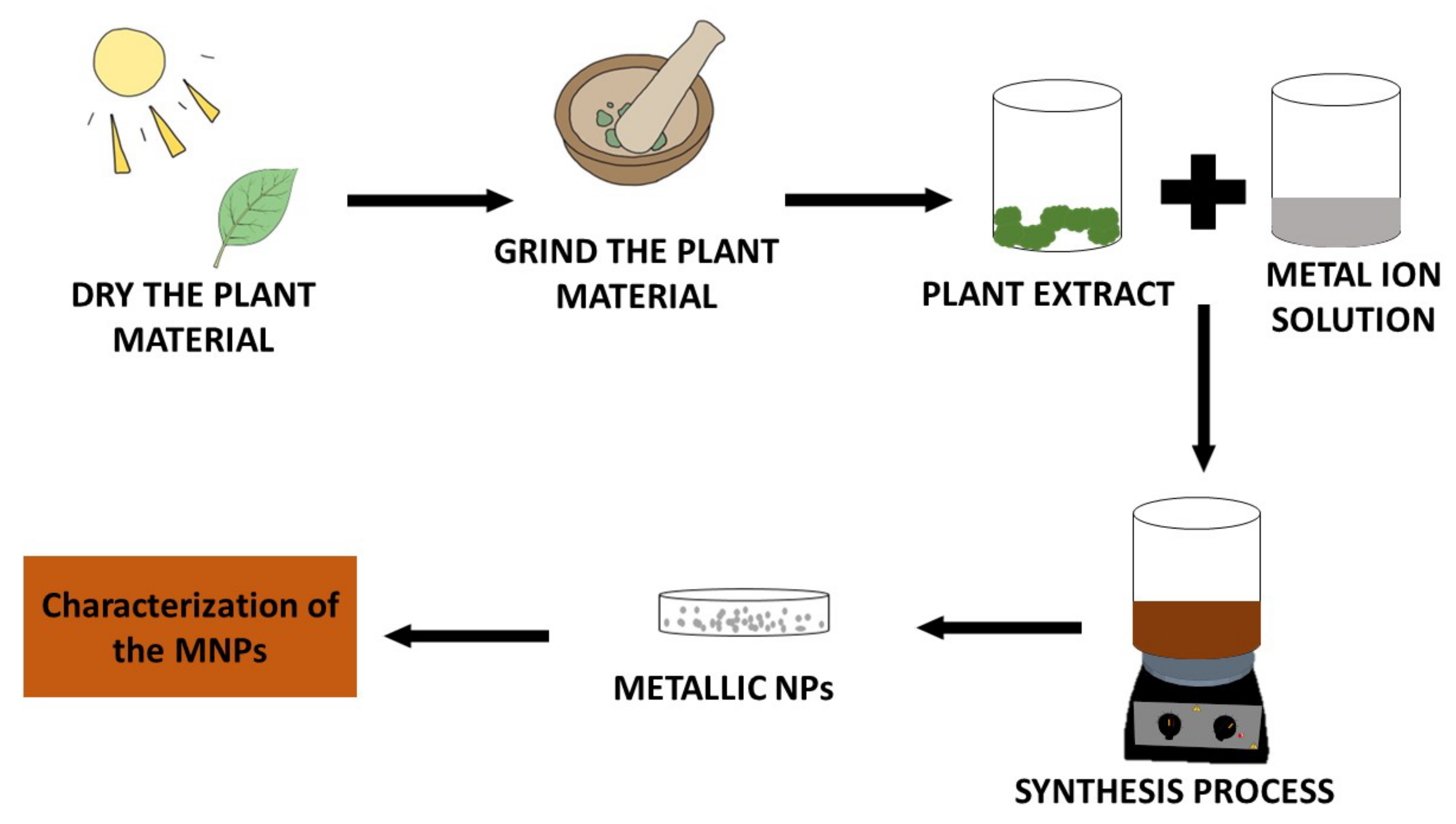
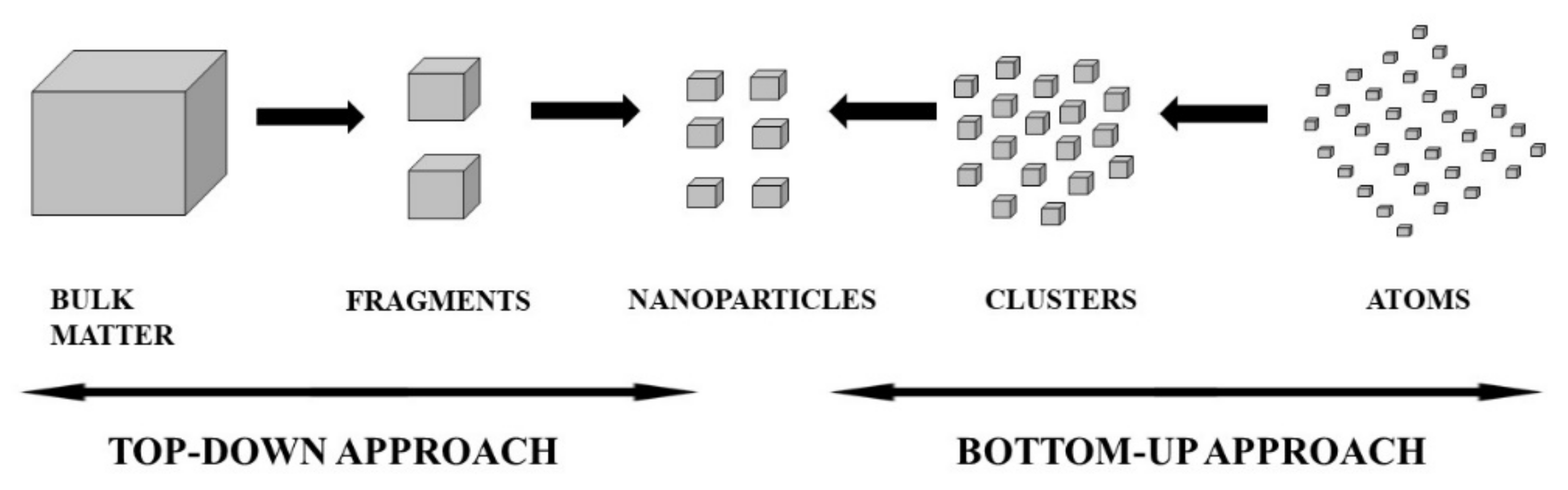
3. Synthesis of Metallic Nanoparticles (MNPs)
4. Characterisation of MNPs
5. Therapeutic and Diagnostic Applications of MNPs
5.1. MNPs in Cancer
5.2. MNPs in Microbial Diseases
5.3. MNPs in Cardiovascular Diseases (CVDs)
5.4. MNPs in Malaria
6. MNPs for Diagnosis
7. Biological Safety
7.1. Organ Damage
7.2. DNA Damage and Genotoxicity
7.3. Fetotoxicity
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafael, F.; Coutinho, A.M.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G. Theranostics in Nuclear Medicine: Emerging and Re-Emerging Inte- Grated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef]
- Filippov, A.; Bonjoc, K.C.; Chea, J.; Bowles, N.; Poku, E. Role of Theranostics in Thoracic Oncology. J. Thorac. Dis. 2020, 12, 5140–5146. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Alkharfy, K.M.; Janardhanan, R.; Mukhopadhyay, D. Nanomedicine: Pharmacological Perspectives. Nanotechnol. Rev. 2012, 1, 235–253. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kshirsagar, P.G.; Gautam, S.K.; Gulati, M.; Wafa, E.I.; Christiansen, J.C.; White, B.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Kumar, S.; et al. Nanocarriers for Pancreatic Cancer Imaging, Treatments, and Immunotherapies. Theranostics 2022, 12, 1030–1060. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The Growing Role of Precision and Personalized Medicine for Cancer Treatment. Technology 2018, 6, 79–100. [Google Scholar] [CrossRef]
- WHO Catalysing Ancient Wisdom and Modern Science for the Health of People and the Planet. Available online: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (accessed on 3 August 2022).
- Woodley, C.M.; Amado, P.S.M.; Cristiano, M.L.S.; O’Neill, P.M. Artemisinin Inspired Synthetic Endoperoxide Drug Candidates: Design, Synthesis, and Mechanism of Action Studies. Med. Res. Rev. 2021, 41, 3062–3095. [Google Scholar] [CrossRef]
- Whayne, T.F. Clinical Use of Digitalis: A State of the Art Review. Am. J. Cardiovasc. Drugs 2018, 18, 427–440. [Google Scholar] [CrossRef]
- Sharma, A.; Kontodimas, K.; Bosmann, M. Nanomedicine: A Diagnostic and Therapeutic Approach to COVID-19. Front. Med. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green Synthesis of Metallic Nanoparticles via Biological Entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Gandhi, P.R.; Jayaseelan, C.; Kamaraj, C.; Rajasree, S.R.R.; Regina Mary, R. In Vitro Antimalarial Activity of Synthesized TiO2 Nanoparticles Using Momordica Charantia Leaf Extract against Plasmodium Falciparum. J. Appl. Biomed. 2018, 16, 378–386. [Google Scholar] [CrossRef]
- Bharadwaj, K.K.; Rabha, B.; Pati, S.; Sarkar, T.; Choudhury, B.K.; Barman, A.; Bhattacharjya, D.; Srivastava, A.; Baishya, D.; Edinur, H.A.; et al. Green Synthesis of Gold Nanoparticles Using Plant Extracts as Beneficial Prospect for Cancer Theranostics. Molecules 2021, 26, 6389. [Google Scholar] [CrossRef]
- Yuan, P.; Ding, X.; Yang, Y.Y.; Xu, Q.H. Metal Nanoparticles for Diagnosis and Therapy of Bacterial Infection. Adv. Healthc. Mater. 2018, 7, 1701392. [Google Scholar] [CrossRef]
- Oshadie, G.; Silva, D.; Abeysundara, A.T.; Minoli, M.; Aponso, W. Extraction Methods, Qualitative and Quantitative Techniques for Screening of Phytochemicals from Plants. Am. J. Essent. Oils Nat. Prod. 2017, 5, 29–32. [Google Scholar]
- Aboyewa, J.A.; Sibuyi, N.R.S.; Meyer, M.; Oguntibeju, O.O. Green Synthesis of Metallic Nanoparticles Using Some Selected Medicinal Plants from Southern Africa and Their Biological Applications. Plants 2021, 10, 1929. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Kharissova, O.V.; Kharisov, B.I.; González, C.M.O.; Méndez, Y.P.; López, I. Greener Synthesis of Chemical Compounds and Materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef]
- Govindarajan, M.; Rajeswary, M.; Veerakumar, K.; Muthukumaran, U.; Hoti, S.L.; Benelli, G. Green Synthesis and Characterization of Silver Nanoparticles Fabricated Using Anisomeles Indica: Mosquitocidal Potential against Malaria, Dengue and Japanese Encephalitis Vectors. Exp. Parasitol. 2016, 161, 40–47. [Google Scholar] [CrossRef]
- Verma, A.; Gautam, S.; Bansal, K.; Prabhakar, N.; Rosenholm, J. Green Nanotechnology: Advancement in Phytoformulation Research. Medicines 2019, 6, 39. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Islam, N.U.; Ayaz, M.; Saravanan, M.; Ali, M.; Ahmad, I.; Shahid, M.; Shinwari, Z.K. Multifunctional Theranostic Applications of Biocompatible Green-Synthesized Colloidal Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4393–4408. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of Metallic Nanoparticles Using Plant Derivatives and Their New Avenues in Pharmacological Applications—An Updated Report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef] [PubMed]
- Parveen, K.; Banse, V.; Ledwani, L. Green Synthesis of Nanoparticles: Their Advantages and Disadvantages. AIP Conf. Proc. 2016, 1724, 020048. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Islam, N.U.; Ahmad, I.; Ayaz, M.; Saravanan, M.; Shinwari, Z.K.; Mukherjee, S. Role of Plant Phytochemicals and Microbial Enzymes in Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 6799–6814. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Nazli, A.; Baig, M.W.; Zia, M.; Ali, M.; Shinwari, Z.K.; Ul Haq, I. Plant-Based Metallic Nanoparticles as Potential Theranostics Agents: Bioinspired Tool for Imaging and Treatment. IET Nanobiotechnology 2018, 12, 869–878. [Google Scholar] [CrossRef]
- Johnson, R.; De Beer, D.; Dludla, P.V.; Ferreira, D.; Muller, C.J.F.; Joubert, E. Aspalathin from Rooibos (Aspalathus Linearis): A Bioactive C -Glucosyl Dihydrochalcone with Potential to Target the Metabolic Syndrome. Planta Med. 2018, 84, 568–583. [Google Scholar] [CrossRef]
- Rai, M.; Yadav, A. Plants as Potential Synthesiser of Precious Metal Nanoparticles: Progress and Prospects. IET Nanobiotechnology 2013, 7, 117–124. [Google Scholar] [CrossRef]
- Ponnuchamy, K.; Jacob, J.A. Metal Nanoparticles from Marine Seaweeds—A Review. Nanotechnol. Rev. 2016, 5, 589–600. [Google Scholar] [CrossRef]
- Romero, N.; Fernández, A.; Robert, P. A Polyphenol Extract of Tara Pods (Caesalpinia Spinosa) as a Potential Antioxidant in Oils. Eur. J. Lipid Sci. Technol. 2012, 114, 951–957. [Google Scholar] [CrossRef]
- Alshammari, A.; Köckritz, A.; Narayana Kalevaru, V.; Bagabas, A.; Martin, A. Influence of Single Use and Combination of Reductants on the Size, Morphology and Growth Steps of Gold Nanoparticles in Colloidal Mixture. Open J. Phys. Chem. 2012, 2, 252–261. [Google Scholar] [CrossRef]
- Jeganathan, B.; Punyasiri, P.A.N.; Kottawa-Arachchi, J.D.; Ranatunga, M.A.B.; Abeysinghe, I.S.B.; Gunasekare, M.T.K.; Bandara, B.M.R. Genetic Variation of Flavonols Quercetin, Myricetin, and Kaempferol in the Sri Lankan Tea (Camellia Sinensis L.) and Their Health-Promoting Aspects. Int. J. Food Sci. 2016, 2016, 6057434. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Das, S.; Bera, T.; Mukherjee, A. Rapid Synthesis for Monodispersed Gold Nanoparticles in Kaempferol and Anti-Leishmanial Efficacy against Wild and Drug Resistant Strains. RSC Adv. 2017, 7, 14159–14167. [Google Scholar] [CrossRef]
- Joshi, C.; Savai, J.; Varghese, A.; Pandita, N. Development and Validation of HPTLC Method for Simultaneous Determination of Quercetin and Kaempferol in Leaves of Two Chemotypes of Centella asiatica. J. Planar Chromatogr.—Mod. TLC 2012, 25, 433–438. [Google Scholar] [CrossRef]
- Mittal, A.K.; Kumar, S.; Banerjee, U.C. Quercetin and Gallic Acid Mediated Synthesis of Bimetallic (Silver and Selenium) Nanoparticles and Their Antitumor and Antimicrobial Potential. J. Colloid Interface Sci. 2014, 431, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.S.; Sujatha, S.; Anand, S.; Sangeetha, K.N.; Narayanan, R.B.; Katiyar, C.; Kanaujia, A.; Duggar, R.; Singh, Y.; Srinivas, K.; et al. Cinnamic Acid, from the Bark of Cinnamomum Cassia, Regulates Glucose Transport via Activation of GLUT4 on L6 Myotubes in a Phosphatidylinositol 3-Kinase-Independent Manner. J. Diabetes 2009, 1, 99–106. [Google Scholar] [CrossRef]
- Anwar, A.; Siddiqui, R.; Shah, M.R.; Khan, N.A. Gold nanoparticle-conjugated cinnamic acid exhibits antiacanthamoebic and antibacterial properties. Antimicrob. Agents Chemother. 2018, 62, e00630-18. [Google Scholar] [CrossRef]
- Al-Bayati, F.A.; Mohammed, M.J. Isolation, Identification, and Purification of Cinnamaldehyde from Cinnamomum Zeylanicum Bark Oil. An Antibacterial Study. Pharm. Biol. 2009, 47, 61–66. [Google Scholar] [CrossRef]
- Ramasamy, M.; Lee, J.; Lee, J. IJN-132784-Development-of-Gold-Nanoparticles-Coated-with-Silica-Contain. Int. J. Nanomedicine 2017, 12, 2813–2828. [Google Scholar] [CrossRef]
- Ranasinghe, L.; Jayawardena, B.; Abeywickrama, K. Fungicidal Activity of Essential Oils of Cinnamomum Zeylanicum (L.) and Syzygium Aromaticum (L.) Merr et L.M.Perry against Crown Rot and Anthracnose Pathogens Isolated from Banana. Lett. Appl. Microbiol. 2002, 35, 208–211. [Google Scholar] [CrossRef]
- Abed, M.S.; Abed, A.S.; Othman, F.M. Green Synthesis of Silver Nanoparticles from Natural Compounds: Glucose, Eugenol and Thymol. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 60, 95–111. [Google Scholar]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of Silver Nanoparticles Using Flavonoids: Hesperidin, Naringin and Diosmin, and Their Antibacterial Effects and Cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Giannuzzo, A.N.; Boggetti, H.J.; Nazareno, M.A.; Mishima, H.T. Supercritical Fluid Extraction of Naringin from the Peel of Citrus paradisi. Phytochem. Anal. 2003, 14, 221–223. [Google Scholar] [CrossRef]
- Yuan, C.G.; Huo, C.; Gui, B.; Cao, W.P. Green Synthesis of Gold Nanoparticles Using Citrus Maxima Peel Extract and Their Catalytic/Antibacterial Activities. IET Nanobiotechnol. 2017, 11, 523–530. [Google Scholar] [CrossRef]
- Park, H.Y.; Ha, S.K.; Eom, H.; Choi, I. Narirutin Fraction from Citrus Peels Attenuates Alcoholic Liver Disease in Mice. Food Chem. Toxicol. 2013, 55, 637–644. [Google Scholar] [CrossRef]
- Noh, H.J.; Kim, H.S.; Jun, S.H.; Kang, Y.H.; Cho, S.; Park, Y. Biogenic Silver Nanoparticles with Chlorogenic Acid as a Bioreducing Agent. J. Nanosci. Nanotechnol. 2013, 13, 5787–5793. [Google Scholar] [CrossRef]
- Mahesh, V.; Million-Rousseau, R.; Ullmann, P.; Chabrillange, N.; Bustamante, J.; Mondolot, L.; Morant, M.; Noirot, M.; Hamon, S.; De Kochko, A.; et al. Functional Characterization of Two P-Coumaroyl Ester 3′-Hydroxylase Genes from Coffee Tree: Evidence of a Candidate for Chlorogenic Acid Biosynthesis. Plant Mol. Biol. 2007, 64, 145–159. [Google Scholar] [CrossRef]
- Revathy, S.; Elumalai, S.; Benny, M.; Antony, B. Isolation, Purification and Identification of Curcuminoids from Turmeric (Curcuma Longa L.) by Column Chromatography. J. Exp. Sci. 2011, 2, 21–25. [Google Scholar]
- Jaiswal, S.; Mishra, P. Antimicrobial and Antibiofilm Activity of Curcumin-Silver Nanoparticles with Improved Stability and Selective Toxicity to Bacteria over Mammalian Cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef]
- Bartoszewski, R.; Hering, A.; Marszałł, M.; Hajduk, J.S.; Bartoszewska, S.; Kapoor, N.; Kochan, K.; Ochocka, R. Mangiferin Has an Additive Effect on the Apoptotic Properties of Hesperidin in Cyclopia Sp. Tea Extracts. PLoS ONE 2014, 9, e92128. [Google Scholar] [CrossRef]
- Zucca, P.; Rosa, A.; Tuberoso, C.I.G.; Piras, A.; Rinaldi, A.C.; Sanjust, E.; Dessì, M.A.; Rescigno, A. Evaluation of Antioxidant Potential of “Maltese Mushroom” (Cynomorium coccineum) by Means of Multiple Chemical and Biological Assays. Nutrients 2013, 5, 149–161. [Google Scholar] [CrossRef]
- Li, D.; Liu, Z.; Yuan, Y.; Liu, Y.; Niu, F. Green Synthesis of Gallic Acid-Coated Silver Nanoparticles with High Antimicrobial Activity and Low Cytotoxicity to Normal Cells. Process Biochem. 2015, 50, 357–366. [Google Scholar] [CrossRef]
- Pan, M.; Lei, Q.; Zang, N.; Zhang, H. A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves. Int. J. Mol. Sci. 2019, 20, 3875. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Dou, D.; Ge, L.; Huang, Z.; Wang, L.; Gu, N. A Caffeic Acid Mediated Facile Synthesis of Silver Nanoparticles with Powerful Anti-Cancer Activity. Colloids Surf. B Biointerfaces 2015, 134, 229–234. [Google Scholar] [CrossRef] [PubMed]
- AlSalhi, M.S.; Elangovan, K.; Ranjitsingh, A.J.A.; Murali, P.; Devanesan, S. Synthesis of Silver Nanoparticles Using Plant Derived 4-N-Methyl Benzoic Acid and Evaluation of Antimicrobial, Antioxidant and Antitumor Activity. Saudi J. Biol. Sci. 2019, 26, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Marrinhas, E.; Carvalho, R.; Dias, C.; Saavedra, M.J. Phytochemical Composition and Antibacterial Activity of Hydroalcoholic Extracts of Pterospartum Tridentatum and Mentha Pulegium against Staphylococcus aureus Isolates. Biomed Res. Int. 2016, 2016, 5201879. [Google Scholar] [CrossRef]
- Mohan, U.P.; Sriram, B.; Panneerselvam, T.; Devaraj, S.; MubarakAli, D.; Parasuraman, P.; Palanisamy, P.; Premanand, A.; Arunachalam, S.; Kunjiappan, S. Utilization of Plant-Derived Myricetin Molecule Coupled with Ultrasound for the Synthesis of Gold Nanoparticles against Breast Cancer. Naunyn. Schmiedebergs. Arch. Pharmacol. 2020, 393, 1963–1976. [Google Scholar] [CrossRef]
- Patel, K.G.; Patel, V.G.; Patel, K.V.; Gandhi, T.R. Validated HPTLC Method for Quantification of Myricetin in the Stem Bark of Myrica Esculenta Buch. Ham. Ex D. Don, Myricaceae. J. Planar Chromatogr.—Mod. TLC 2010, 23, 326–331. [Google Scholar] [CrossRef]
- Abouaitah, K.; Allayh, A.K.; Wojnarowicz, J.; Shaker, Y.M.; Swiderska-Sroda, A.; Lojkowski, W. Nanoformulation Composed of Ellagic Acid and Functionalized Zinc Oxide Nanoparticles Inactivates Dna and Rna Viruses. Pharmaceutics 2021, 13, 2174. [Google Scholar] [CrossRef]
- Mullen, W.; Yokota, T.; Lean, M.E.J.; Crozier, A. Analysis of Ellagitannins and Conjugates of Ellagic Acid and Quercetin in Raspberry Fruits by LC-MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Raju, D.; Muchintala, D.; Reddy, M.; Rao, B. Synthesis and Characterization of Oligosaccharide Based Silver Nanoparticles and Its Assessment as an Antimicrobial Agent. Adv. Sci. Eng. Med. 2018, 10, 14–21. [Google Scholar] [CrossRef]
- Wild, G.M.; French, D. The galactan series of oligosaccharides. Proc. Iowa Acad. Sci. Galactan Ser. Oligosacch. 1952, 59, 226–230. [Google Scholar]
- Zeng, Q.; Che, Y.; Zhang, Y.; Chen, M.; Guo, Q.; Zhang, W. Thymol Isolated from Thymus Vulgaris L. Inhibits Colorectal Cancer Cell Growth and Metastasis by Suppressing the Wnt/β-Catenin Pathway. Drug Des. Devel. Ther. 2020, 14, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Cha, S.H.; Cho, I.; Park, S.; Park, Y.; Cho, S.; Park, Y. Antibacterial Nanocarriers of Resveratrol with Gold and Silver Nanoparticles. Mater. Sci. Eng. C 2016, 58, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Mattivi, F.; Vrhovsek, U.; Malacarne, G.; Masuero, D.; Zulini, L.; Stefanini, M.; Mose, C.; Velasco, R.; Guella, G. Profiling of Resveratrol Oligomers, Important Stress Metabolites, Accumulating in the Leaves of Hybrid Vitis vinifera (Merzling × Teroldego) Genotypes Infected with Plasmopara viticola. J. Agric. Food Chem. 2011, 59, 5364–5375. [Google Scholar] [CrossRef] [PubMed]
- Chinnappan, R.S.; Kandasamy, K.; Sekar, A. A Review on Marine Based Nanoparticles and Their Potential Applications. Afr. J. Biotechnol. 2015, 14, 1525–1532. [Google Scholar] [CrossRef]
- Ghosh, V. Marine Bioresources as Potential Source for Synthesis of Nanoparticles. Encycl. Mar. Biotechnol. 2020, 1521–1534. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Asghar, H.M.A.; Bhattacharjee, S. Mechanistic Investigation of Phytochemicals Involved in Green Synthesis of Gold Nanoparticles Using Aqueous Elaeis Guineensis Leaves Extract: Role of Phenolic Compounds and Flavonoids. Biotechnol. Appl. Biochem. 2019, 66, 698–708. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Cupido, C.N.; Meyer, M.; Hussein, A.A. Large Scale Screening of Southern African Plant Extracts for the Green Synthesis of Gold Nanoparticles Using Microtitre-Plate Method. Molecules 2016, 21, 1498. [Google Scholar] [CrossRef]
- Khairunnisa, S.; Wonoputri, V.; Samadhi, T.W. Effective Deagglomeration in Biosynthesized Nanoparticles: A Mini Review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1143, 012006. [Google Scholar] [CrossRef]
- Dumur, F.; Guerlin, A.; Dumas, E.; Bertin, D.; Gigmes, D.; Mayer, C.R. Controlled Spontaneous Generation of Gold Nanoparticles Assisted by Dual Reducing and Capping Agents. Gold Bull. 2011, 44, 119–137. [Google Scholar] [CrossRef]
- Niu, Z.; Li, Y. Removal and Utilization of Capping Agents in Nanocatalysis. Chem. Mater. 2014, 26, 72–83. [Google Scholar] [CrossRef]
- Ali, S.; Sharma, A.S.; Ahmad, W.; Zareef, M.; Hassan, M.M.; Viswadevarayalu, A.; Jiao, T.; Li, H.; Chen, Q. Noble Metals Based Bimetallic and Trimetallic Nanoparticles: Controlled Synthesis, Antimicrobial and Anticancer Applications. Crit. Rev. Anal. Chem. 2021, 51, 454–481. [Google Scholar] [CrossRef]
- Ribeiro, A.I.; Dias, A.M.; Zille, A. Synergistic Effects between Metal Nanoparticles and Commercial Antimicrobial Agents: A Review. ACS Appl. Nano Mater. 2022, 5, 3030–3064. [Google Scholar] [CrossRef]
- Melkamu, W.W.; Bitew, L.T. Green Synthesis of Silver Nanoparticles Using Hagenia abyssinica (Bruce) J.F. Gmel Plant Leaf Extract and Their Antibacterial and Anti-Oxidant Activities. Heliyon 2021, 7, e08459. [Google Scholar] [CrossRef]
- Ray, P.C.; Yu, H.; Fu, P.P. Toxicity and environmental risks of nanomaterials: Challenges and future needs. J. Environ. Sci. Health Part C 2009, 27, 1–35. [Google Scholar] [CrossRef]
- Adeyemi, J.O.; Oriola, A.O.; Onwudiwe, D.C.; Oyedeji, A.O. Plant Extracts Mediated Metal-Based Nanoparticles: Synthesis and Biological Applications. Biomolecules 2022, 12, 627. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.-Y.; Huang, J.; Chen, C.-Y.; Wang, Z.-X.; Xie, H. Silver Nanoparticles: Synthesis, Medical Applications and Biosafety. Theranostics 2020, 10, 8996–9031. [Google Scholar] [CrossRef]
- Habeeb Rahuman, H.B.; Dhandapani, R.; Narayanan, S.; Palanivel, V.; Paramasivam, R.; Subbarayalu, R.; Thangavelu, S.; Muthupandian, S. Medicinal Plants Mediated the Green Synthesis of Silver Nanoparticles and Their Biomedical Applications. IET Nanobiotechnol. 2022, 16, 115–144. [Google Scholar] [CrossRef]
- El Shafey, A.M. Green Synthesis of Metal and Metal Oxide Nanoparticles from Plant Leaf Extracts and Their Applications: A Review. Green Process. Synth. 2020, 9, 304–339. [Google Scholar] [CrossRef]
- Raj, S.; Trivedi, R.; Soni, V. Biogenic Synthesis of Silver Nanoparticles, Characterization and Their Applications—A Review. Surfaces 2021, 5, 67–90. [Google Scholar] [CrossRef]
- Iqbal, P.; Preece, J.A.; Mendes, P.M. Nanotechnology: The “Top-Down” and “Bottom-Up” Approaches. Supramol. Chem. 2012. [Google Scholar] [CrossRef]
- Tsuzuki, T. Commercial Scale Production of Inorganic Nanoparticles. Int. J. Nanotechnol. 2009, 6, 567–578. [Google Scholar] [CrossRef]
- Aliofkhazraei, M. Synthesis, Processing and Application of Nanostructured Coatings. Eng. Mater. 2011, 1–28. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.; Cai, Z.; Feng, Y.; Wang, Y.; Zhang, D.; Pan, X. Detection of Engineered Nanoparticles in Aquatic Environments: Current Status and Challenges in Enrichment, Separation, and Analysis. Environ. Sci. Nano 2019, 6, 709–735. [Google Scholar] [CrossRef]
- Jabir, M.S.; Taha, A.A.; Sahib, U.I. Linalool Loaded on Glutathione-Modified Gold Nanoparticles: A Drug Delivery System for a Successful Antimicrobial Therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 345–355. [Google Scholar] [CrossRef]
- Kumar, A.; Chaudhary, R.K.; Singh, R.; Singh, S.P.; Wang, S.Y.; Hoe, Z.Y.; Pan, C.T.; Shiue, Y.L.; Wei, D.Q.; Kaushik, A.C.; et al. Nanotheranostic Applications for Detection and Targeting Neurodegenerative Diseases. Front. Neurosci. 2020, 14, 305. [Google Scholar] [CrossRef]
- Witika, B.A.; Aucamp, M.; Mweetwa, L.L.; Makoni, P.A. Application of Fundamental Techniques for Physicochemical Characterizations to Understand Post-Formulation Performance of Pharmaceutical Nanocrystalline Materials. Crystals 2021, 11, 310. [Google Scholar] [CrossRef]
- Brar, S.K.; Verma, M. Measurement of Nanoparticles by Light-Scattering Techniques. TrAC Trends Anal. Chem. 2011, 30, 4–17. [Google Scholar] [CrossRef]
- Lin, P.-C.; Lin, S.; Wang, P.C.; Sridhar, R. Techniques for Physicochemical Characterization of Nanomaterials. Biotechnol. Adv. 2014, 32, 711–726. [Google Scholar] [CrossRef]
- Avitabile, E.; Senes, N.; D’Avino, C.; Tsamesidis, I.; Pinna, A.; Medici, S.; Pantaleo, A. The Potential Antimalarial Efficacy of Hemocompatible Silver Nanoparticles from Artemisia Species against P. Falciparum Parasite. PLoS ONE 2020, 15, e0238532. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Patra, B.; Gautam, R.; Priyadarsini, E.; Rajamani, P.; Pradhan, S.N.; Saravanan, M.; Meena, R. Piper Betle: Augmented Synthesis of Gold Nanoparticles and Its In-Vitro Cytotoxicity Assessment on HeLa and HEK293 Cells. J. Clust. Sci. 2020, 31, 133–145. [Google Scholar] [CrossRef]
- Baalousha, M.; Ju-Nam, Y.; Cole, P.A.; Gaiser, B.; Fernandes, T.F.; Hriljac, J.A.; Jepson, M.A.; Stone, V.; Tyler, C.R.; Lead, J.R. Characterization of Cerium Oxide Nanoparticles-Part 1: Size Measurements. Environ. Toxicol. Chem. 2012, 31, 983–993. [Google Scholar] [CrossRef]
- Knox, S.L.; Steinauer, A.; Alpha-Cobb, G.; Trexler, A.; Rhoades, E.; Schepartz, A. Chapter Twenty-One—Quantification of Protein Delivery in Live Cells Using Fluorescence Correlation Spectroscopy. In Chemical Tools for Imaging, Manipulating, and Tracking Biological Systems: Diverse Chemical, Optical and Bioorthogonal Methods; Academic Press: Cambridge, MA, USA, 2020; Volume 641, pp. 477–505. ISBN 0076-6879. [Google Scholar]
- Patil, R.B.; Chougale, A.D. Analytical Methods for the Identification and Characterization of Silver Nanoparticles: A Brief Review. Mater. Today Proc. 2021, 47, 5520–5532. [Google Scholar] [CrossRef]
- Hartschuh, A. Scanning Near-Field Optical Microscopy BT—Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2016; pp. 3508–3521. ISBN 978-94-017-9780-1. [Google Scholar]
- Kaszuba, M.; McKnight, D.; Connah, M.T.; McNeil-Watson, F.K.; Nobbmann, U. Measuring Sub Nanometre Sizes Using Dynamic Light Scattering. J. Nanopart. Res. 2008, 10, 823–829. [Google Scholar] [CrossRef]
- Li, Y.; Lee, J.S. Insights into Characterization Methods and Biomedical Applications of Nanoparticle-Protein Corona. Materials 2020, 13, 3093. [Google Scholar] [CrossRef]
- Sapsford, K.E.; Tyner, K.M.; Dair, B.J.; Deschamps, R.; Medintz, I.L. Analyzing Nanomaterial Bioconjugates: A Review of Current and Emerging Purification and Characterization Techniques. Anal. Chem. 2011, 83, 4453–4488. [Google Scholar] [CrossRef]
- Mernie, E.G.; Chen, Y. Nanoprobe-Based Mass Spectrometry and Fourier Transform Infrared Spectroscopy for Rapid Phospholipid Profiling. J. Chin. Chem. Soc. 2022, 69, 94–106. [Google Scholar] [CrossRef]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef]
- Sarma, D.D.; Santra, P.K.; Mukherjee, S.; Nag, A. X-Ray Photoelectron Spectroscopy: A Unique Tool to Determine the Internal Heterostructure of Nanoparticles. Chem. Mater. 2013, 25, 1222–1232. [Google Scholar] [CrossRef]
- Perera, Y.R.; Hill, R.A.; Fitzkee, N.C. Protein Interactions with Nanoparticle Surfaces: Highlighting Solution NMR Techniques. Isr. J. Chem. 2019, 59, 962–979. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.C.; Schmidt, S.J. Thermal Analysis BT—Food Analysis; Nielsen, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 529–544. ISBN 978-3-319-45776-5. [Google Scholar]
- Witika, B.A.; Smith, V.J.; Walker, R.B. A Comparative Study of the Effect of Different Stabilizers on the Critical Quality Attributes of Self-Assembling Nano Co-Crystals. Pharmaceutics 2020, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Ladisa, M.; Altamura, D.; Siliqi, D.; Sibillano, T.; De Caro, L. X-Ray Diffraction: A Powerful Technique for the Multiple-Length-Scale Structural Analysis of Nanomaterials. Crystals 2016, 6, 87. [Google Scholar] [CrossRef]
- Jain, A.; Jain, P.; Soni, P.; Tiwari, A.; Prasad, S. Design and Characterization of Silver Nanoparticles of Different Species of Curcuma in the Treatment of Cancer Using Human Colon Cancer Cell Line (HT—29). J. Gastrointest. Cancer 2021, 1–6. [Google Scholar] [CrossRef]
- Kristanc, L.; Kreft, S. European Medicinal and Edible Plants Associated with Subacute and Chronic Toxicity Part I: Plants with Carcinogenic, Teratogenic and Endocrine-Disrupting Effects. Food Chem. Toxicol. 2016, 92, 150–164. [Google Scholar] [CrossRef]
- Novak, M.; Žegura, B.; Modic, B.; Heath, E.; Filipič, M. Cytotoxicity and Genotoxicity of Anticancer Drug Residues and Their Mixtures in Experimental Model with Zebrafish Liver Cells. Sci. Total Environ. 2017, 601, 293–300. [Google Scholar] [CrossRef]
- Weber, G.F. DNA Damaging Drugs. Molecular Therapies of Cancer; Springer: Cham, Switzerland, 2014; pp. 9–112. [Google Scholar] [CrossRef]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef]
- Gao, Y.; Shang, Q.; Li, W.; Guo, W.; Stojadinovic, A.; Mannion, C.; Man, Y.-G.; Chen, T. Antibiotics for Cancer Treatment: A Double-Edged Sword. J. Cancer 2020, 11, 5135–5149. [Google Scholar] [CrossRef]
- Hailan, W.A.; Al-Anazi, K.M.; Farah, M.A.; Ali, M.A.; Al-Kawmani, A.A.; Abou-Tarboush, F.M. Reactive Oxygen Species-Mediated Cytotoxicity in Liver Carcinoma Cells Induced by Silver Nanoparticles Biosynthesized Using Schinus Molle Extract. Nanomaterials 2022, 12, 161. [Google Scholar] [CrossRef]
- Tailor, G.; Lawal, A.M. Phytochemical Screening; Green Synthesis, Characterization and Biological Significance of Lead Oxide Nanoparticles from Eucalyptus globulus Labill. (Leaves). Nanotechnol. Environ. Eng. 2021, 6, 48. [Google Scholar] [CrossRef]
- Ramamurthy, C.H.; Padma, M.; Mareeswaran, R.; Suyavaran, A.; Kumar, M.S.; Premkumar, K.; Thirunavukkarasu, C. The Extra Cellular Synthesis of Gold and Silver Nanoparticles and Their Free Radical Scavenging and Antibacterial Properties. Colloids Surf. B Biointerfaces 2013, 102, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal Nanoparticles: Understanding the Mechanisms behind Antibacterial Activity. J. Nanobiotechnol. 2017, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 12 June 2022).
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler Jr, V.G. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Celebioglu, A.; Topuz, F.; Yildiz, Z.I.; Uyar, T. One-Step Green Synthesis of Antibacterial Silver Nanoparticles Embedded in Electrospun Cyclodextrin Nanofibers. Carbohydr. Polym. 2019, 207, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.G.M.; Elekhnawy, E.; Negm, W.A.; Hussein, I.A.; Mokhtar, F.A.; Al-Fakhrany, O.M. In Vivo and in Vitro Antimicrobial Activity of Biogenic Silver Nanoparticles against Staphylococcus aureus Clinical Isolates. Pharmaceuticals 2022, 15, 194. [Google Scholar] [CrossRef]
- World Health Organization. Cardiovascular Diseases (cvds). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 20 June 2022).
- Aronson, D.; Edelman, E.R. Revascularization for Coronary Artery Disease in Diabetes Mellitus: Angioplasty, Stents and Coronary Artery Bypass Grafting. Rev. Endocr. Metab. Disord. 2010, 11, 75–86. [Google Scholar] [CrossRef]
- Bardage, C.; Isacson, D.G.L. Self-Reported Side-Effects of Antihypertensive Drugs: An Epidemiological Study on Prevalence and Impact on Health-State Utility. Blood Press. 2000, 9, 328–334. [Google Scholar] [CrossRef]
- Olivier, T.T.; Moïse, F.; Jackson, S.A.; Francis, N.T. A Review on Traditional Uses, Phytochemical and Pharmacological Profiles, Spiritual and Economic Values, and Toxicity of Dacryodes Edulis (G. Don) H.J. Lam. J. Drug Deliv. Ther. 2016, 6, 84–90. [Google Scholar] [CrossRef]
- Stendahl, J.C.; Sinusas, A.J. Nanoparticles for Cardiovascular Imaging and Therapeutic Delivery, Part 1: Compositions and Features. J. Nucl. Med. 2015, 56, 1469–1475. [Google Scholar] [CrossRef]
- Liu, Y.; Welch, M.J. Nanoparticles Labeled with Positron Emitting Nuclides: Advantages, Methods, and Applications. Bioconjug. Chem. 2012, 23, 671–682. [Google Scholar] [CrossRef]
- Ojha, N.; Dhamoon, A.S. Myocardial Infarction. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Huang, S.; Frangogiannis, N.G. Anti-Inflammatory Therapies in Myocardial Infarction: Failures, Hopes and Challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, A.; Attar, F.; Sharifi, M.; Siddique, R.; Mraiche, F.; Falahati, M. Gold Nanoparticle-Based Platforms for Diagnosis and Treatment of Myocardial Infarction. ACS Biomater. Sci. Eng. 2020, 6, 6460–6477. [Google Scholar] [CrossRef]
- Mir, M.; Ahmed, N.; ur Rehman, A. Recent Applications of PLGA Based Nanostructures in Drug Delivery. Colloids Surf. B Biointerfaces 2017, 159, 217–231. [Google Scholar] [CrossRef]
- Dong, F.; Cui, Z.; Teng, G.; Shangguan, K.; Zhang, Q.; Zhang, G. Green Synthesis of Gold Nanoparticles (AuNPs) As Potential Drug Carrier for Treatment and Care of Cardiac Hypertrophy Agents. J. Clust. Sci. 2022, 33, 1129–1137. [Google Scholar] [CrossRef]
- Sui, Y.; Xie, L.; Meng, D.; Ruan, Y.; Zhong, Z.; Huang, L. Cardiovascular Protective Properties of Green Synthesised Iron Nanoparticles from Calendula Officinalis Leaf Aqueous Extract on Mitoxantrone-Induced DNA Fragmentation and Apoptosis in HDMVECn, HUVEC, HAEC, HCAEC, HCASMC and HPAEC Cells. J. Exp. Nanosci. 2022, 17, 126–137. [Google Scholar] [CrossRef]
- Utzinger, J.; Becker, S.L.; Knopp, S.; Blum, J.; Neumayr, A.L.; Keiser, J.; Hatz, C.F. Neglected Tropical Diseases: Diagnosis, Clinical Management, Treatment and Control. Swiss Med. Wkly. Off. J. Swiss Soc. Infect. Dis. Swiss Soc. Intern. Med. Swiss Soc. Pneumol. 2012, 142, 1–24. [Google Scholar] [CrossRef]
- Reuling, I.J. The Use of Controlled Human Malaria Infection As Fit-For-Purpose Model In The Fight Against Malaria. 2021. Available online: https://repository.ubn.ru.nl/handle/2066/217375 (accessed on 18 September 2022).
- Salem, S.S.; Hammad, E.N.; Mohamed, A.A.; El-Dougdoug, W. A Comprehensive Review of Nanomaterials: Types, Synthesis, Characterization, and Applications. Biointerface Res. Appl. Chem. 2022, 13, 41. [Google Scholar] [CrossRef]
- Obisesan, O.R.; Adekunle, A.S.; Oyekunle, J.A.O.; Ogunfowokan, A.O.; Olaniran, O.; Thomas, S.; Nkambule, T.T.I.; Mamba, B.B. Catalytic Degradation of β-Hematin (Malaria Biomaker) Using Some Selected Metal Oxide Nanoparticles. Mater. Res. Express 2020, 7, 015044. [Google Scholar] [CrossRef]
- Almatroudi, A. Silver Nanoparticles: Synthesis, Characterisation and Biomedical Applications. Open Life Sci. 2020, 15, 819–839. [Google Scholar] [CrossRef]
- Rahman, K.; Khan, S.U.; Fahad, S.; Chang, M.X.; Abbas, A.; Khan, W.U.; Rahman, L.; Haq, Z.U.; Nabi, G.; Khan, D. Nano-Biotechnology: A New Approach to Treat and Prevent Malaria. Int. J. Nanomed. 2019, 14, 1401. [Google Scholar] [CrossRef] [PubMed]
- Goodman, A.L.; Forbes, E.K.; Williams, A.R.; Douglas, A.D.; De Cassan, S.C.; Bauza, K.; Biswas, S.; Dicks, M.D.J.; Llewellyn, D.; Moore, A.C.; et al. The Utility of Plasmodium Berghei as a Rodent Model for Anti-Merozoite Malaria Vaccine Assessment. Sci. Rep. 2013, 3, 1706. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Ingle, A.P.; Paralikar, P.; Gupta, I.; Medici, S.; Santos, C.A. Recent Advances in Use of Silver Nanoparticles as Antimalarial Agents. Int. J. Pharm. 2017, 526, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Okaiyeto, K.; Hoppe, H.; Okoh, A.I. Plant-Based Synthesis of Silver Nanoparticles Using Aqueous Leaf Extract of Salvia officinalis: Characterization and Its Antiplasmodial Activity. J. Clust. Sci. 2021, 32, 101–109. [Google Scholar] [CrossRef]
- Ojemaye, M.O.; Okoh, S.O.; Okoh, A.I. Silver Nanoparticles (AgNPs) Facilitated by Plant Parts of Crataegus Ambigua Becker AK Extracts and Their Antibacterial, Antioxidant and Antimalarial Activities. Green Chem. Lett. Rev. 2021, 14, 49–59. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of Silver Nanoparticles Using Acalypha Indica Leaf Extracts and Its Antibacterial Activity against Water Borne Pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Muthukumaran, P.; Ramachandran, R.; Balakumaran, M.D.; Kalaichelvan, P.T. Acalypha indica Linn: Biogenic Synthesis of Silver and Gold Nanoparticles and Their Cytotoxic Effects against MDA-MB-231, Human Breast Cancer Cells. Biotechnol. Rep. 2014, 4, 42–49. [Google Scholar] [CrossRef]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of Silver Nanoparticles Using Alternanthera sessilis (Linn.) Extract and Their Antimicrobial, Antioxidant Activities. Colloids Surf. B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef]
- Wang, D.; Cui, L.; Chang, X.; Guan, D. Biosynthesis and Characterization of Zinc Oxide Nanoparticles from Artemisia Annua and Investigate Their Effect on Proliferation, Osteogenic Differentiation and Mineralization in Human Osteoblast-like MG-63 Cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111652. [Google Scholar] [CrossRef]
- Singh, S.P.; Mishra, A.; Shyanti, R.K.; Singh, R.P.; Acharya, A. Silver Nanoparticles Synthesized Using Carica papaya Leaf Extract (AgNPs-PLE) Causes Cell Cycle Arrest and Apoptosis in Human Prostate (DU145) Cancer Cells. Biol. Trace Elem. Res. 2021, 199, 1316–1331. [Google Scholar] [CrossRef]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S.; Sheba Rani, N.D.; Lakshmi, T.; Deepak Nallaswamy, V. Phyto-Assisted Synthesis of Zinc Oxide Nanoparticles Using Cassia Alata and Its Antibacterial Activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef]
- Al-Shmgani, H.S.A.; Mohammed, W.H.; Sulaiman, G.M.; Saadoon, A.H. Biosynthesis of Silver Nanoparticles from Catharanthus Roseus Leaf Extract and Assessing Their Antioxidant, Antimicrobial, and Wound-Healing Activities. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1234–1240. [Google Scholar] [CrossRef]
- Velsankar, K.; Sudhahar, S.; Parvathy, G.; Kaliammal, R. Effect of Cytotoxicity and AAntibacterial Activity of Biosynthesis of ZnO Hexagonal Shaped Nanoparticles by Echinochloa Frumentacea Grains Extract as a Reducing Agent. Mater. Chem. Phys. 2020, 239, 121976. [Google Scholar] [CrossRef]
- Ahmad, W.; Kalra, D. Green Synthesis, Characterization and Anti Microbial Activities of ZnO Nanoparticles Using Euphorbia Hirta Leaf Extract. J. King Saud Univ.—Sci. 2020, 32, 2358–2364. [Google Scholar] [CrossRef]
- Elumalai, D.; Hemavathi, M.; Deepaa, C.V.; Kaleena, P.K. Evaluation of Phytosynthesised Silver Nanoparticles from Leaf Extracts of Leucas Aspera and Hyptis Suaveolens and Their Larvicidal Activity against Malaria, Dengue and Filariasis Vectors. Parasite Epidemiol. Control 2017, 2, 15–26. [Google Scholar] [CrossRef]
- Muralikrishna, T.; Malothu, R.; Pattanayak, M.; Nayak, P.L. Green Synthesis of Gold Nanoparticles Using Mangifera indica (Mango Leaves) Aqueous Extract. World J. Nano Sci. Technol. 2014, 3, 66–73. [Google Scholar] [CrossRef]
- Dhandapani, K.V.; Anbumani, D.; Gandhi, A.D.; Annamalai, P.; Muthuvenkatachalam, B.S.; Kavitha, P.; Ranganathan, B. Green Route for the Synthesis of Zinc Oxide Nanoparticles from Melia azedarach Leaf Extract and Evaluation of Their Antioxidant and Antibacterial Activities. Biocatal. Agric. Biotechnol. 2020, 24, 101517. [Google Scholar] [CrossRef]
- Vankar, P.S.; Bajpai, D. Preparation of Gold Nanoparticles from Mirabilis Jalapa Flowers. Indian J. Biochem. Biophys. 2010, 47, 157–160. [Google Scholar] [PubMed]
- Tang, Q.; Xia, H.; Liang, W.; Huo, X.; Wei, X. Synthesis and Characterization of Zinc Oxide Nanoparticles from Morus Nigra and Its Anticancer Activity of AGS Gastric Cancer Cells. J. Photochem. Photobiol. B Biol. 2020, 202, 111698. [Google Scholar] [CrossRef]
- Bhau, B.S.; Ghosh, S.; Puri, S.; Borah, B.; Sarmah, D.K.; Khan, R. Green Synthesis of Gold Nanoparticles from the Leaf Extract of Nepenthes Khasiana and Antimicrobial Assay. Adv. Mater. Lett. 2015, 6, 55–58. [Google Scholar] [CrossRef]
- Velmurugan, P.; Lee, S.-M.; Iydroose, M.; Lee, K.-J.; Oh, B.-T. Pine Cone-Mediated Green Synthesis of Silver Nanoparticles and Their Antibacterial Activity against Agricultural Pathogens. Appl. Microbiol. Biotechnol. 2013, 97, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Naidoo, Y.; Mocktar, C.; Baijnath, H. Biosynthesis of Silver Nanoparticles Using Plumbago auriculata Leaf and Calyx Extracts and Evaluation of Their Antimicrobial Activities. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035004. [Google Scholar] [CrossRef]
- Panneerselvam, C.; Murugan, K.; Roni, M.; Aziz, A.T.; Suresh, U.; Rajaganesh, R.; Madhiyazhagan, P.; Subramaniam, J.; Dinesh, D.; Nicoletti, M.; et al. Fern-Synthesized Nanoparticles in the Fight against Malaria: LC/MS Analysis of Pteridium aquilinum Leaf Extract and Biosynthesis of Silver Nanoparticles with High Mosquitocidal and Antiplasmodial Activity. Parasitol. Res. 2016, 115, 997–1013. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wu, Y.; Song, L.; Chinnathambi, A.; Ali Alharbi, S.; Fang, L. Anticarcinogenic Effect of Zinc Oxide Nanoparticles Synthesized from Rhizoma Paridis Saponins on Molt-4 Leukemia Cells. J. King Saud Univ.—Sci. 2020, 32, 1865–1871. [Google Scholar] [CrossRef]
- Dube, P.; Meyer, S.; Madiehe, A.; Meyer, M. Antibacterial Activity of Biogenic Silver and Gold Nanoparticles Synthesized from Salvia Africana-Lutea and Sutherlandia frutescens. Nanotechnology 2020, 31, 505607. [Google Scholar] [CrossRef] [PubMed]
- Bayrami, A.; Alioghli, S.; Rahim Pouran, S.; Habibi-Yangjeh, A.; Khataee, A.; Ramesh, S. A Facile Ultrasonic-Aided Biosynthesis of ZnO Nanoparticles Using Vaccinium Arctostaphylos L. Leaf Extract and Its Antidiabetic, Antibacterial, and Oxidative Activity Evaluation. Ultrason. Sonochem. 2019, 55, 57–66. [Google Scholar] [CrossRef]
- Haider, A.; Ijaz, M.; Ali, S.; Haider, J.; Imran, M.; Majeed, H.; Shahzadi, I.; Ali, M.M.; Khan, J.A.; Ikram, M. Green Synthesized Phytochemically (Zingiber Officinale and Allium sativum) Reduced Nickel Oxide Nanoparticles Confirmed Bactericidal and Catalytic Potential. Nanoscale Res. Lett. 2020, 15, 50. [Google Scholar] [CrossRef]
- Mujeeb, A.A.; Khan, N.A.; Jamal, F.; Badre Alam, K.F.; Saeed, H.; Kazmi, S.; Alshameri, A.W.F.; Kashif, M.; Ghazi, I.; Owais, M. Olax Scandens Mediated Biogenic Synthesis of Ag-Cu Nanocomposites: Potential Against Inhibition of Drug-Resistant Microbes. Front. Chem. 2020, 8, 1–12. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chowdhury, D.; Kotcherlakota, R.; Patra, S.; Vinothkumar, B.; Bhadra, M.P.; Sreedhar, B.; Patra, C.R. Potential Theranostics Application of Bio-Synthesized Silver Nanoparticles (4-in-1 System). Theranostics 2014, 4, 316–335. [Google Scholar] [CrossRef]
- Păduraru, D.N.; Ion, D.; Niculescu, A.G.; Mușat, F.; Andronic, O.; Grumezescu, A.M.; Bolocan, A. Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications. Pharmaceutics 2022, 14, 435. [Google Scholar] [CrossRef]
- Nilghaz, A.; Mousavi, S.M.; Tian, J.; Cao, R.; Guijt, R.M.; Wang, X. Noble-Metal Nanoparticle-Based Colorimetric Diagnostic Assays for Point-of-Need Applications. ACS Appl. Nano Mater. 2021, 4, 12808–12824. [Google Scholar] [CrossRef]
- Sibuyi, N.R.S.; Moabelo, K.L.; Fadaka, A.O.; Meyer, S.; Onani, M.O.; Madiehe, A.M.; Meyer, M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Dhanjal, D.S.; Sharma, A.; Nepovimova, E.; Kalia, A.; Thakur, S.; Bhardwaj, S.; Chopra, C.; Singh, R.; Verma, R.; et al. Conifer-Derived Metallic Nanoparticles: Green Synthesis and Biological Applications. Int. J. Mol. Sci. 2020, 21, 9028. [Google Scholar] [CrossRef]
- Govindaraju, K.; Krishnamoorthy, K.; Alsagaby, S.A.; Singaravelu, G.; Premanathan, M. Green Synthesis of Silver Nanoparticles for Selective Toxicity towards Cancer Cells. IET Nanobiotechnol. 2015, 9, 325–330. [Google Scholar] [CrossRef]
- Sabella, S.; Carney, R.P.; Brunetti, V.; Malvindi, M.A.; Al-Juffali, N.; Vecchio, G.; Janes, S.M.; Bakr, O.M.; Cingolani, R.; Stellacci, F.; et al. A General Mechanism for Intracellular Toxicity of Metal-Containing Nanoparticles. Nanoscale 2014, 6, 7052–7061. [Google Scholar] [CrossRef]
- Sengul, A.; Asmatulu, E. Toxicity of Metal and Metal Oxide Nanoparticles: A Review. Environ. Chem. Lett. 2020, 18, 1659–1683. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A Review on the Toxicity of Silver Nanoparticles on Human Health. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Enrico, C.; Enrico, C. Biophysical Interaction, Nanotoxicology Evaluation, and Biocompatibility and Biosafety of Metal Nanoparticles. arXiv 2021. [Google Scholar] [CrossRef]
- Mukherjee, S.; Patra, C.R. Biologically Synthesized Metal Nanoparticles: Recent Advancement and Future Perspectives in Cancer Theranostics. Futur. Sci. OA 2017, 3, FSO203. [Google Scholar] [CrossRef]
- Escudero-Francos, M.A.; Cepas, V.; González-Menéndez, P.; Badía-Laíño, R.; Díaz-García, M.E.; Sainz, R.M.; Mayo, J.C.; Hevia, D. Cellular Uptake and Tissue Biodistribution of Functionalized Gold Nanoparticles and Nanoclusters. J. Biomed. Nanotechnol. 2017, 13, 167–179. [Google Scholar] [CrossRef]
- Khan, S.A. Metal Nanoparticles Toxicity: Role of Physicochemical Aspects; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128169605. [Google Scholar]
- Kumar, V.; Sharma, N.; Maitra, S.S. In Vitro and in Vivo Toxicity Assessment of Nanoparticles. Int. Nano Lett. 2017, 7, 243–256. [Google Scholar] [CrossRef]
- Suker, D.K.; Jasim, F.A. Liver Histopathological Alteration after Repeated Intra-Tracheal Instillation of Titanium Dioxide in Male Rats. Gastroenterol. Hepatol. Bed Bench 2018, 11, 159–168. [Google Scholar] [PubMed]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity Of Metallic Nanoparticles On Liver: The Subcellular Damages, Mechanisms, And Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Pelucelli, A.; Zoroddu, M.A. An Updated Overview on Metal Nanoparticles Toxicity. Semin. Cancer Biol. 2021, 76, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Yadav, K.; Jagadevan, S. A Comprehensive Review on Green Synthesis of Nature-Inspired Metal Nanoparticles: Mechanism, Application and Toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Sharma, A.; Muresanu, D.F.; Patnaik, R.; Sharma, H.S. Size- and Age-Dependent Neurotoxicity of Engineered Metal Nanoparticles in Rats. Mol. Neurobiol. 2013, 48, 386–396. [Google Scholar] [CrossRef]
- Mukherjee, S.; Sau, S.; Madhuri, D.; Bollu, V.S.; Madhusudana, K.; Sreedhar, B.; Banerjee, R.; Patra, C.R. Green Synthesis and Characterization of Monodispersed Gold Nanoparticles: Toxicity Study, Delivery of Doxorubicin and Its Bio-Distribution in Mouse Model. J. Biomed. Nanotechnol. 2016, 12, 165–181. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.L.; Gu, Y.; Huang, H.; Zhang, G.W. Green Synthesis of Metallic Nanoparticles and Their Potential Applications to Treat Cancer. Front. Chem. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Niska, K.; Pyszka, K.; Tukaj, C.; Wozniak, M.; Radomski, M.W.; Inkielewicz-Stepniak, I. Titanium Dioxide Nanoparticles Enhance Production of Superoxide Anion and Alter the Antioxidant System in Human Osteoblast Cells. Int. J. Nanomed. 2015, 10, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Chibber, S.; Ansari, S.A.; Satar, R. New Vision to CuO, ZnO, and TiO2 Nanoparticles: Their Outcome and Effects. J. Nanopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Song, M.F.; Li, Y.S.; Kasai, H.; Kawai, K. Metal Nanoparticle-Induced Micronuclei and Oxidative DNA Damage in Mice. J. Clin. Biochem. Nutr. 2012, 50, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Santonastaso, M.; Mottola, F.; Colacurci, N.; Iovine, C.; Pacifico, S.; Cammarota, M.; Cesaroni, F.; Rocco, L. In Vitro Genotoxic Effects of Titanium Dioxide Nanoparticles (n-TiO2) in Human Sperm Cells. Mol. Reprod. Dev. 2019, 86, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Liu, J.; Li, N.; Wang, J.; Duan, Y.; Yan, J.; Liu, H.; Wang, H.; Hong, F. Oxidative Stress in the Brain of Mice Caused by Translocated Nanoparticulate TiO2 Delivered to the Abdominal Cavity. Biomaterials 2010, 31, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z. Nanoparticles Induced Embryo–Fetal Toxicity. Toxicol. Ind. Health 2020, 36, 181–213. [Google Scholar] [CrossRef] [PubMed]
- Campagnolo, L.; Massimiani, M.; Vecchione, L.; Piccirilli, D.; Toschi, N.; Magrini, A.; Bonanno, E.; Scimeca, M.; Castagnozzi, L.; Buonanno, G.; et al. Silver Nanoparticles Inhaled during Pregnancy Reach and Affect the Placenta and the Foetus. Nanotoxicology 2017, 11, 687–698. [Google Scholar] [CrossRef]
- Asharani, P.V.; Lian Wu, Y.; Gong, Z.; Valiyaveettil, S. Toxicity of Silver Nanoparticles in Zebrafish Models. Nanotechnology 2008, 19, 255102. [Google Scholar] [CrossRef]
- Teng, C.; Jia, J.; Wang, Z.; Sharma, V.K.; Yan, B. Size-Dependent Maternal-Fetal Transfer and Fetal Developmental Toxicity of ZnO Nanoparticles after Oral Exposures in Pregnant Mice. Ecotoxicol. Environ. Saf. 2019, 182, 109439. [Google Scholar] [CrossRef]
- Yang, H.; Du, L.; Tian, X.; Fan, Z.; Sun, C.; Liu, Y.; Keelan, J.A.; Nie, G. Effects of Nanoparticle Size and Gestational Age on Maternal Biodistribution and Toxicity of Gold Nanoparticles in Pregnant Mice. Toxicol. Lett. 2014, 230, 10–18. [Google Scholar] [CrossRef]
- Jing, X.; Park, J.H.; Peters, T.M.; Thorne, P.S. Toxicity of Copper Oxide Nanoparticles in Lung Epithelial Cells Exposed at the Air-Liquid Interface Compared with in Vivo Assessment. Toxicol. Vitr. 2015, 29, 502–511. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Z.; Zhai, S.; Cheng, Y.; Liu, J.; Liu, B. In Vitro Cytotoxicity of Silver Nanoparticles in Primary Rat Hepatic Stellate Cells. Mol. Med. Rep. 2013, 8, 1365–1372. [Google Scholar] [CrossRef]
- Roshan Rezaee Ranjbar Sardari Toxicological Effects of Silver Nanoparticles in Rats. African J. Microbiol. Res. 2012, 6, 5587–5593. [CrossRef]
- Hussain, S.M.; Hess, K.L.; Gearhart, J.M.; Geiss, K.T.; Schlager, J.J. In Vitro Toxicity of Nanoparticles in BRL 3A Rat Liver Cells. Toxicol. Vitr. 2005, 19, 975–983. [Google Scholar] [CrossRef]
- Mohammed Salih, N.F.; Al-Nakeeb, G.D. Histological Comparative of Kidney of Neonatal Mice Exposed to Silver Nanoparticles during Fetal Development. Int. J. Pharm. Qual. Assur. 2019, 10, 145–150. [Google Scholar] [CrossRef]
- Fatemi, M.; Moshtaghian, J.; Ghaedi, K.; Dinani, N.J. Effects of Silver Nanoparticle on the Developing Liver of Rat Pups after Maternal Exposure. Iran. J. Pharm. Res. 2017, 16, 685–693. [Google Scholar] [PubMed]
- Recordati, C.; De Maglie, M.; Bianchessi, S.; Argentiere, S.; Cella, C.; Mattiello, S.; Cubadda, F.; Aureli, F.; D’Amato, M.; Raggi, A.; et al. Tissue Distribution and Acute Toxicity of Silver after Single Intravenous Administration in Mice: Nano-Specific and Size-Dependent Effects. Part. Fibre Toxicol. 2016, 13, 1–17. [Google Scholar] [CrossRef]
- Balansky, R.; Longobardi, M.; Ganchev, G.; Iltcheva, M.; Nedyalkov, N.; Atanasov, P.; Toshkova, R.; De Flora, S.; Izzotti, A. Transplacental Clastogenic and Epigenetic Effects of Gold Nanoparticles in Mice. Mutat. Res.—Fundam. Mol. Mech. Mutagen. 2013, 751, 42–48. [Google Scholar] [CrossRef]
- Zhang, X.D.; Wu, D.; Shen, X.; Liu, P.X.; Yang, N.; Zhao, B.; Zhang, H.; Sun, Y.M.; Zhang, L.A.; Fan, F.Y. Size-Dependent in Vivo Toxicity of PEG-Coated Gold Nanoparticles. Int. J. Nanomed. 2011, 6, 2071–2081. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeong, S.; Jang, S.H.; Park, J.; Park, J.H.; Ock, K.S.; Lee, S.Y.; Joo, S.W. In Vitro Toxicity of Serum Protein-Adsorbed Citrate-Reduced Gold Nanoparticles in Human Lung Adenocarcinoma Cells. Toxicol. Vitr. 2012, 26, 229–237. [Google Scholar] [CrossRef]
- Chen, J.; Wang, H.; Long, W.; Shen, X.; Wu, D.; Song, S.S.; Sun, Y.M.; Liu, P.X.; Fan, S.; Fan, F.; et al. Sex Differences in the Toxicity of Polyethylene Glycol-Coated Gold Nanoparticles in Mice. Int. J. Nanomed. 2013, 8, 2409–2419. [Google Scholar] [CrossRef]
- Fraga, S.; Brandão, A.; Soares, M.E.; Morais, T.; Duarte, J.A.; Pereira, L.; Soares, L.; Neves, C.; Pereira, E.; de Lourdes Bastos, M.; et al. Short- and Long-Term Distribution and Toxicity of Gold Nanoparticles in the Rat after a Single-Dose Intravenous Administration. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1757–1766. [Google Scholar] [CrossRef]
- El Ghareeb, A.E.W.; Hamdi, H.; El Bakry, A.; Hmela, H.A. Teratogenic Effects of the Titanium Dioxide Nanoparticles on the Pregnant Female Rats And Their Off Springs. Res. J. Pharm. Biol. Chem. Sci. 2015, 6, 510. [Google Scholar]
- Ezealisiji, K.M.; Siwe-Noundou, X.; Maduelosi, B.; Nwachukwu, N.; Krause, R.W.M. Green Synthesis of Zinc Oxide Nanoparticles Using Solanum torvum (L) Leaf Extract and Evaluation of the Toxicological Profile of the ZnO Nanoparticles–Hydrogel Composite in Wistar Albino Rats. Int. Nano Lett. 2019, 9, 99–107. [Google Scholar] [CrossRef]
- Wang, C.; Cheng, K.; Zhou, L.; He, J.; Zheng, X.; Zhang, L.; Zhong, X.; Wang, T. Evaluation of Long-Term Toxicity of Oral Zinc Oxide Nanoparticles and Zinc Sulfate in Mice. Biol. Trace Elem. Res. 2017, 178, 276–282. [Google Scholar] [CrossRef] [PubMed]
| Plant | Compound | MW (g/mol) | MNP | Bioactivity | Reference |
|---|---|---|---|---|---|
| Aspalathus linearis | 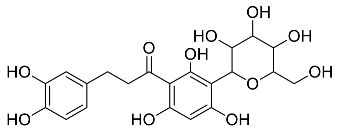 Aspalathin | 452.13 | AuNPs and RhNPs | Antimicrobial | [16,27] |
| Caesalpinia spinosa | 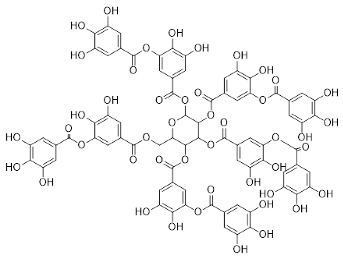 Tannic acid | 1701.19 | AuNPs | Antibacterial | [16,30,31] |
| Centella asiatic | 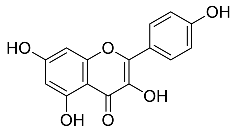 Kaempferol | 2686.23 | AuNPs | Anti-leishmanial | [16,32,33,34] |
| Centella asiatic | 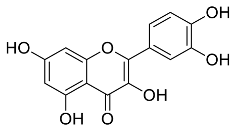 Quercetin | 302.24 | AgNPs | Antitumor, Antimicrobial | [34,35] |
| Cinnamomum cassia | 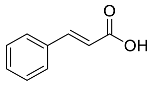 Cinnamic acid | 148.15 | AuNPs | Antimicrobial | [36,37] |
| Cinnamomum zeylanicum | 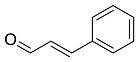 Cinnamaldehyde | 132.16 | AgNPs | Antimicrobial | [38,39] |
| Cinnamomum zeylanicumverum | 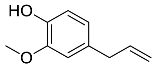 Eugenol | 164.20 | AgNPs | Antioxidant | [40,41] |
| Citrus paradisi | 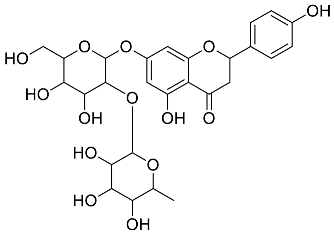 Naringin | 580.54 | AgNP | Antibacterial, Cytotoxic | [42,43] |
| Citrus unshiu | 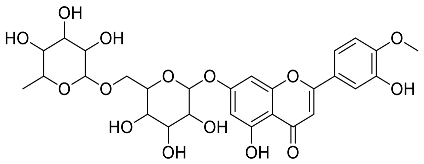 Narirutin | 179.13 | AuNPs | Antibacterial | [44,45] |
| Coffea canephora | 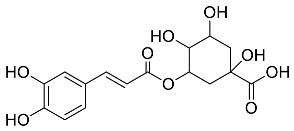 Chlorogenic acid | 354.31 | AgNPs | Antibacterial | [46,47] |
| Curcuma longa | 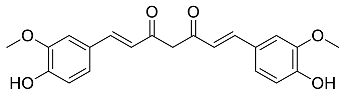 Curcumin | 368.38 | AgNPs | Antimicrobial | [48,49] |
| Cyclopia intermedia | 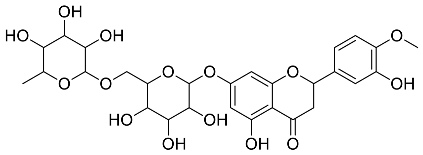 Hesperidin | 610.19 | AgNPs | Antibacterial, Cytotoxic | [42,50] |
| Cynomorium coccineum | 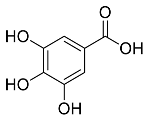 Gallic acid | 170.12 | AgNPs | Antimicrobial | [51,52] |
| Eucalyptus globus | 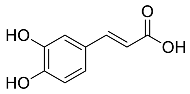 Caffeic acid | 180.16 | AgNPs | Anticancer | [53,54] |
| Memecylon umbellatum | 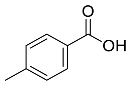 4-N-methylbenzoic acid | 136.15 | AgNPs | Antimicrobial, antioxidant, anticancertumor | [55] |
| Mentha pulegium | 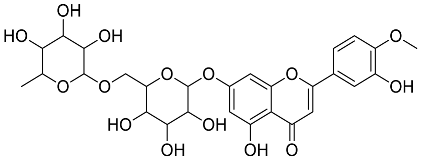 Diosmin | 608.55 | AgNPs | Antibacterial, Cytotoxic | [42,56] |
| Myrica Esculenta | 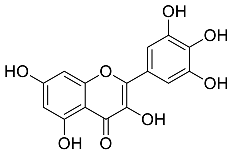 Myricetin | 318.23 | AuNPs | Anticancer | [57,58] |
| Rubus idaeus | 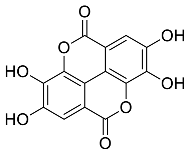 Ellagic acid | 302.20 | ZnNPs | Antiviral | [59,60] |
| Stachys tuberifera | 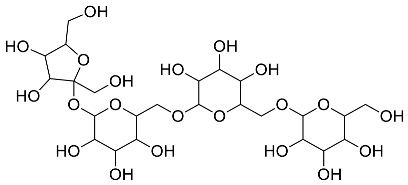 Stachyose | 666.60 | AgNPs | Antimicrobial | [61,62] |
| Thymus vulgaris | 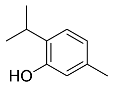 Thymol | 150.22 | AgNPs | Antimicrobial | [41,63] |
| Vitis vinifera | 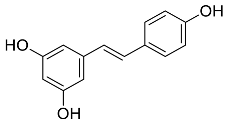 Resveratrol | 228.25 | AgNPs and AuNPs | Antibacterial | [64,65] |
| Characterization Technique | Physiochemical Parameter | Ref. |
|---|---|---|
| Particle Size and Polydispersity Index | ||
| Atomic force microscopy (AFM) | This technique is used to determine the size and size distribution, shape, structure, dispersion and aggregation of the NPs. | [90,91] |
| Dynamic light scattering (DLS) | Essential in measuring crystallite size and for the distinction between the amorphous and the crystalline NPs. The dynamic light-scattering determines the size and quantification, while the transmission on the electron microscope is crucial in measuring the morphology and size of NPs. | [31,52,92,93] |
| Transmission scanning microscopy (TEM) | Images can be used to visualize the morphology of biosynthesized metallic NPs. | [64,94] |
| Scanning electron microscopy (SEM) | To determine the size as well as the morphology of NPs. | [49,95] |
| Fluorescence correlation spectroscopy (FCS) | A quantitative single-molecule technique that assesses the concentration and rate of diffusion of fluorophore-tagged molecules of all sizes in living cells and in vitro, as well as inside specific cellular compartments. | [91,96] |
| Scanning tunnelling microscopy (STM) | An analytical technique used to determine the surface composition through size and size distribution, shape, structure, dispersion and aggregation of the NPs. | [91,97] |
| Near-field scanning optical microscopy | A technique of microscopy for studying nanostructures that overcomes the far-field resolution barrier by taking advantage of evanescent wave properties. | [91,98] |
| Zeta Potential | ||
| Electrophoretic Mobility (EM) | Used to determine the zeta potential, which is a measure or estimation of the colloidal stability. | [31,99,100] |
| Chemical Composition and Surface Chemistry | ||
| Infrared spectroscopy (IR) Attenuated total reflection Fourier transform infrared (ATR–FTIR) | Provides data on the chemical composition (functional groups) of the structure of nanomaterials and conformation of the bioconjugates. | [91,97,101] |
| Mass spectroscopy (MS) | Used to determine the mass-to-charge ratio of molecules in a sample. | [91,102] |
| X-ray Photoelectron Spectroscopy (XPS) | A powerful quantitative technique often used to elucidate the electronic structure, elemental composition and oxidation states of elements in a nanomaterial. | [103,104] |
| Nuclear magnetic resonance (NMR) | Used to determine the size through indirect analysis, structure, composition, purity and conformational change. | [91,105] |
| Raman scattering (RS) Surface-enhanced Raman (SERS), Tip-enhanced Raman spectroscopy (TERS) | Primarily, it identifies the NPs’ structural, chemical and electrical properties. It can also be used to calculate the protein-metallic nanoparticle conjugate’s hydrodynamic size and size distribution. | [91,100] |
| Crystal Habit | ||
| Thermal gravimetric analysis (TGA) | Used to evaluate the weight shift that takes place as a sample, it is heated at a constant rate in order to measure the percentage of volatile components and the thermal stability of a material. It can also be used to determine the changes in polymorph by noting whether a sample is a hydrate or solvate. | [89,106] |
| Differential Scanning Calorimetry | Utilises the difference in the amount of heat required to increase the temperature of a sample and a reference. It can determine whether the sample is amorphous or crystalline as well as determining if a polymorphic change has occurred. | [89,107] |
| X-ray diffraction (XRD) | A technique to determine the size, shape and structure for nano materials or crystals. | [91,108] |
| Optical Properties | ||
| Ultraviolet, visible, near infrared (UV–vis–NIR) spectroscopy | Predominantly used in determining the surface plasmon resonance (SPR) of MNPs. Reported studies have determined metals such as gold to be identified at wavelengths of 520–560 nm. | [46,87,94] |
| Plant | MNPs | Morphology | Application | Reference |
|---|---|---|---|---|
| Acalypha indica | AgNPs | Spherical | Antimicrobial | [145] |
| Acalypha indica | AgNPs AuNPs | Spherical | Anticancer | [146] |
| Alternanthera sessilis | AgNPs | Spherical | Antimicrobial | [147] |
| Anisomeles indica | AgNPs | Spherical | Antimalarial | [19] |
| Artemisia annua | ZnONPs | Spherical | Anticancer | [148] |
| Carica papaya | AgNPs | Spherical | Anticancer | [149] |
| Cassia alata | ZnONPs | Spherical | Antimicrobial | [150] |
| Catharanthus roseus | AgNPs | Spherical | Antimicrobial | [151] |
| Crataegus ambigua | AgNPs | Spherical | Antimalarial, antimicrobial | [144] |
| Cyclopia intermedia | AuNPs | Spherical and triangular | Anticancer | [144] |
| Echinochloa frumentacea | ZnONPs | Hexagonal | Antimicrobial | [152] |
| Euphorbia hirta | ZnONPs | Spherical | Antimicrobial | [153] |
| Leucas aspera and Hyptis suaveolens | AgNPs | Spherical, hexagonal, triangular, and polyhedral | Antimalarial | [154] |
| Mangifera indica | AuNPs | Spherical | Anticancer | [155] |
| Melia azedarach | ZnONPs | Spherical and hexagonal | Antimicrobial | [156] |
| Mirabilis jalapa | AuNPs | Spherical | Antimicrobial | [4,157] |
| Morus nigra | ZnONPs | Spherical | Anticancer | [158] |
| Nepenthes khasiana | AuNPs | Triangular and spherical | Antimicrobial | [159] |
| Pinus thunbergia | AgNPs | Triangular and hexagonal | Antimicrobial | [160] |
| Plumbago auriculata | AgNPs | Spherical and oblong | Antimicrobial | [161] |
| Pteridium aquilinum | AgNPs | Spherical | Antimalarial | [162] |
| Rhizoma paridis | ZnONPs | Spherical | Anticancer | [163] |
| Salvia africana-lutea and Sutherlandia frutescens | AgNPs AuNPs | Spherical and Polygon | Antimicrobial, anticancer | [164] |
| Salvia officinalis | AgNPs | Spherical | Antimalarial | [143] |
| Vaccinium arctostaphylos | ZnONPs | Spindle | Antidiabetic, antimicrobial | [165] |
| Zingiber officinale and Allium sativum | NiONPs | Spherical | Antimicrobial | [166] |
| MNPs | Size (nm) | Model | Toxic Effect(s) | Ref. |
|---|---|---|---|---|
| AgNPs | 30–50 | Rat hepatic stellate cells | Proliferative and apoptotic effect | [200] |
| AgNPs | 70 | Rat | Tissue damages, bloodshed, cell necrosis | [201] |
| AgNPs | 15, 100 | Rat liver cells BRL 3A (ATCC, CRL-1442) | Decreased mitochondrial function | [202] |
| AgNPs | 35–100 | Mice | Alteration of neonatal kidney | [203] |
| AgNPs | 20 | Rat | Induce oxidative stress and apoptosis in the liver | [204] |
| AgNPs | 10, 40, 100 | Rat | Hepatobiliary toxicity | [205] |
| AuNPs | 40, 100 | Mice | Changes miRNA expression in foetus | [206] |
| AuNPs | 5, 10, 30, 60 | Mice | Elevation of liver enzymes, accumulation in the liver and spleen | [207] |
| AuNPs | 20 | Human lung adenocarcinoma cells (A549 cells) | Causes cell damage | [208] |
| AuNPs | 4.4–36.1 | Mice | Causes liver and kidney damage | [209] |
| AuNPs | 20 | Rat | Spleen atrophy | [210] |
| TiO2NPs | <25 | Rat | Teratogenic (impairs foetal skeletal formation, causes weight loss, liver and kidney degeneration) | [211] |
| ZnONPs | 34–40 | Rat | Affects hepatic and renal performance, cumulative toxicity | [212] |
| ZnONPs | 20,120 | Mice | Weight loss, liver damage, accumulation of Zn in the liver and kidney | [213] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xulu, J.H.; Ndongwe, T.; Ezealisiji, K.M.; Tembu, V.J.; Mncwangi, N.P.; Witika, B.A.; Siwe-Noundou, X. The Use of Medicinal Plant-Derived Metallic Nanoparticles in Theranostics. Pharmaceutics 2022, 14, 2437. https://doi.org/10.3390/pharmaceutics14112437
Xulu JH, Ndongwe T, Ezealisiji KM, Tembu VJ, Mncwangi NP, Witika BA, Siwe-Noundou X. The Use of Medicinal Plant-Derived Metallic Nanoparticles in Theranostics. Pharmaceutics. 2022; 14(11):2437. https://doi.org/10.3390/pharmaceutics14112437
Chicago/Turabian StyleXulu, Jabulile Happiness, Tanaka Ndongwe, Kenneth M. Ezealisiji, Vuyelwa J. Tembu, Nontobeko P. Mncwangi, Bwalya A. Witika, and Xavier Siwe-Noundou. 2022. "The Use of Medicinal Plant-Derived Metallic Nanoparticles in Theranostics" Pharmaceutics 14, no. 11: 2437. https://doi.org/10.3390/pharmaceutics14112437
APA StyleXulu, J. H., Ndongwe, T., Ezealisiji, K. M., Tembu, V. J., Mncwangi, N. P., Witika, B. A., & Siwe-Noundou, X. (2022). The Use of Medicinal Plant-Derived Metallic Nanoparticles in Theranostics. Pharmaceutics, 14(11), 2437. https://doi.org/10.3390/pharmaceutics14112437









