Increased Expression and Activity of Brain Cortical cPLA2 Due to Chronic Lipopolysaccharide Administration in Mouse Model of Familial Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Study Design and Animals
2.3. Quantitative Targeted Absolute Proteomic (QTAP) Analysis
2.4. Measurement of cPLA2 Activity
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. NFκB p65 Phosphorylation Analysis
2.7. Measurement of Reactive Oxygen Species (ROS) Production
2.8. Statistical Analysis
3. Results
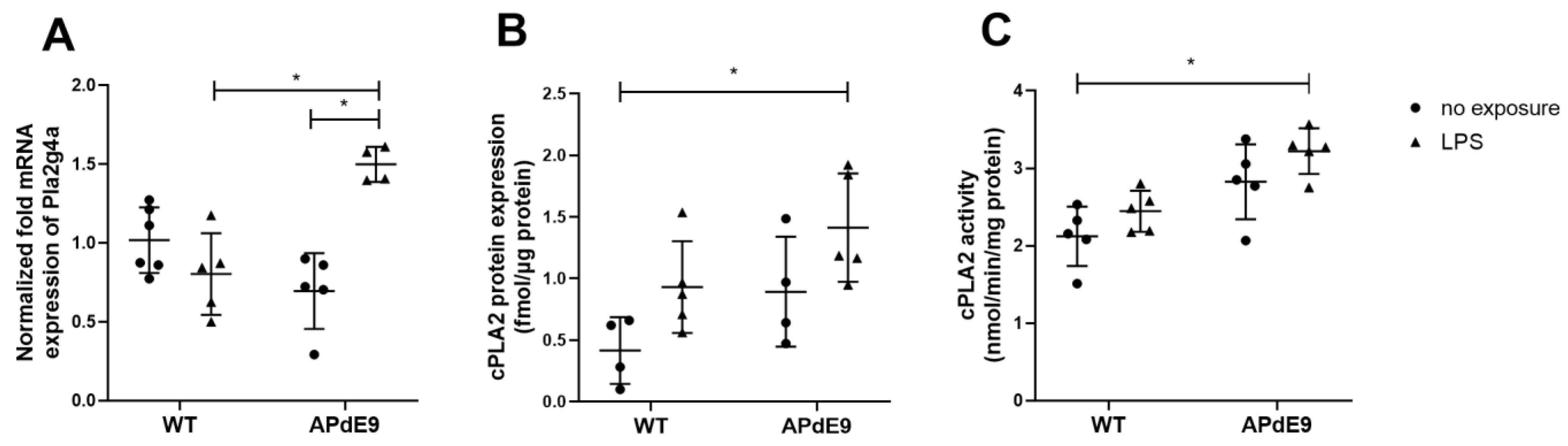
3.1. Expression and Activity of cPLA2α
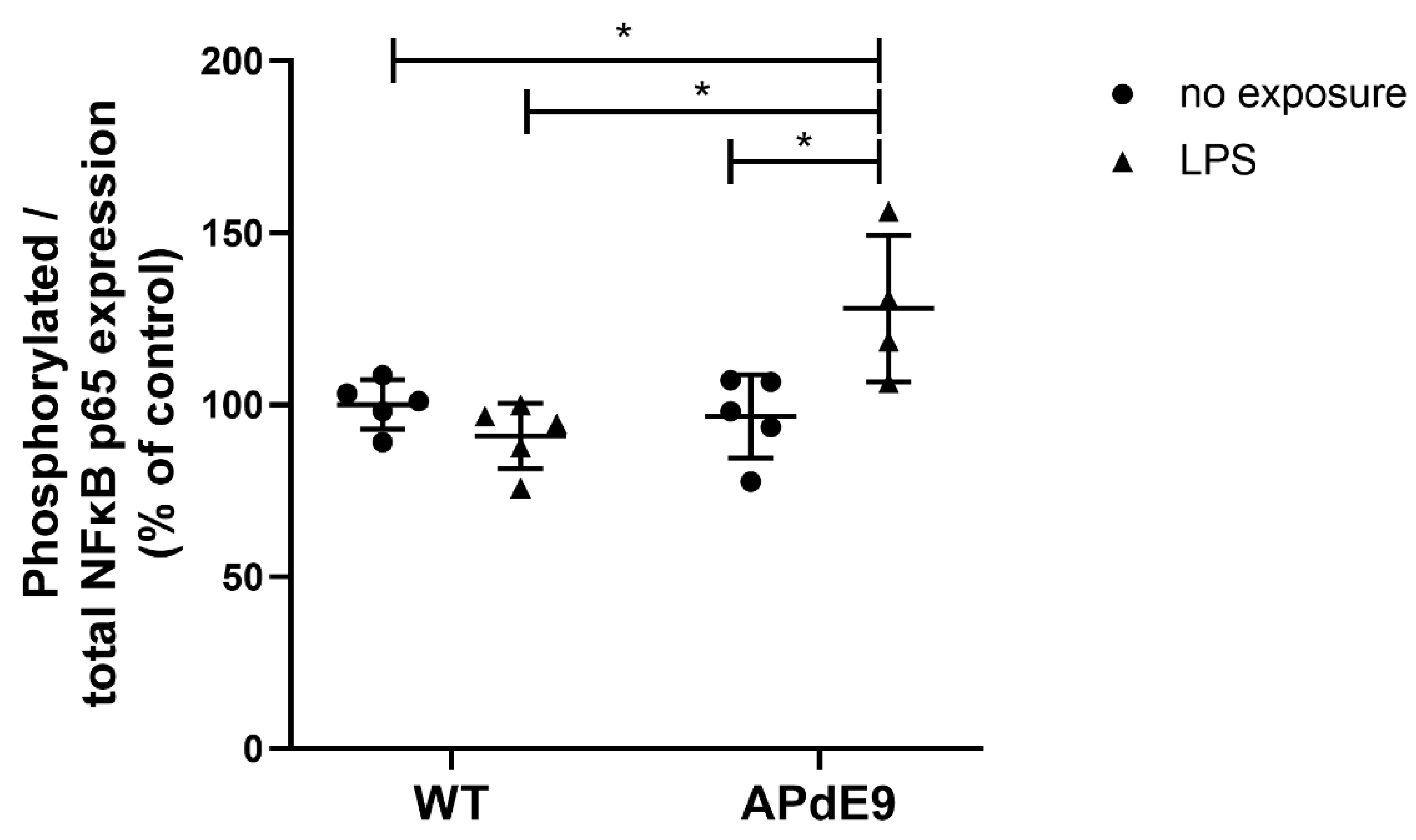
3.2. NFκB Signaling
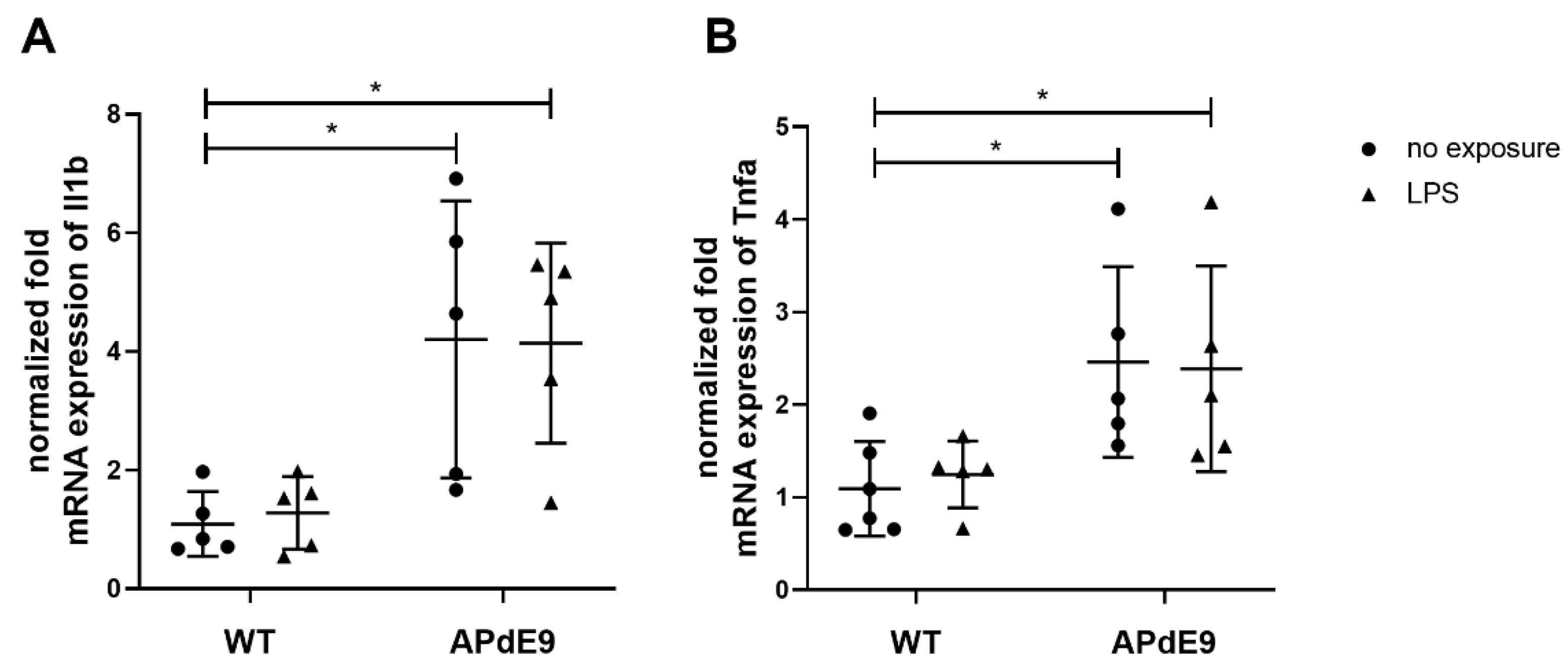
3.3. Inflammatory Responses and Oxidative State in the Brain Cortices of LPS-Treated Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Six, D.A.; Dennis, E.A. The expanding superfamily of phospholipase A2 enzymes: Classification and characterization. Biochim. Biophys. Acta 2000, 1488, 1–19. [Google Scholar] [CrossRef]
- Schaloske, R.H.; Dennis, E.A. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 2006, 1761, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Geng, X.; Teng, T.; Yang, B.; Appenteng, M.K.; Greenlief, C.M.; Lee, J.C. Dynamic Role of Phospholipases A2 in Health and Diseases in the Central Nervous System. Cells 2021, 10, 2963. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; Chuang, D.Y.; Zong, Y.; Jiang, J.; Lee, J.C.; Gu, Z.; Simonyi, A. Role of cytosolic phospholipase A2 in oxidative and inflammatory signaling pathways in different cell types in the central nervous system. Mol. Neurobiol. 2014, 50, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.D.; Schievella, A.R.; Nalefski, E.A.; Lin, L.L. Cytosolic phospholipase A2. J. Lipid Mediat. Cell Signal. 1995, 12, 83–117. [Google Scholar] [CrossRef]
- Czapski, G.A.; Czubowicz, K.; Strosznajder, J.B.; Strosznajder, R.P. The Lipoxygenases: Their Regulation and Implication in Alzheimer’s Disease. Neurochem. Res. 2016, 41, 243–257. [Google Scholar] [CrossRef]
- Figueiredo-Pereira, M.E.; Rockwell, P.; Schmidt-Glenewinkel, T.; Serrano, P. Neuroinflammation and J2 prostaglandins: Linking impairment of the ubiquitin-proteasome pathway and mitochondria to neurodegeneration. Front. Mol. Neurosci. 2014, 7, 104. [Google Scholar] [CrossRef]
- Strokin, M.; Sergeeva, M.; Reiser, G. Docosahexaenoic acid and arachidonic acid release in rat brain astrocytes is mediated by two separate isoforms of phospholipase A2 and is differently regulated by cyclic AMP and Ca2+. Br. J. Pharmacol. 2003, 139, 1014–1022. [Google Scholar] [CrossRef]
- Chao, C.C.; Gutierrez-Vazquez, C.; Rothhammer, V.; Mayo, L.; Wheeler, M.A.; Tjon, E.C.; Zandee, S.E.J.; Blain, M.; de Lima, K.A.; Takenaka, M.C.; et al. Metabolic Control of Astrocyte Pathogenic Activity via cPLA2-MAVS. Cell 2019, 179, 1483–1498.E22. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Simonyi, A.; Kotzbauer, P.T.; Gu, Z.; Sun, G.Y. Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J. Neuroinflamm. 2015, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Simonyi, A.; Sun, A.Y.; Sun, G.Y. Phospholipases A2 and neural membrane dynamics: Implications for Alzheimer’s disease. J. Neurochem. 2011, 116, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, D.T.; Lemere, C.A.; Selkoe, D.J.; Clemens, J.A. Cytosolic phospholipase A2(cPLA2) immunoreactivity is elevated in Alzheimer’s disease brain. Neurobiol. Dis. 1996, 3, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.B.; Lai, Y.; Sheng, W.; Yang, X.; Zhu, D.; Sun, G.Y.; Lee, J.C. Amyloid-beta peptide induces temporal membrane biphasic changes in astrocytes through cytosolic phospholipase A2. Biochim. Biophys. Acta 2008, 1778, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Szaingurten-Solodkin, I.; Hadad, N.; Levy, R. Regulatory role of cytosolic phospholipase A2α in NADPH oxidase activity and in inducible nitric oxide synthase induction by aggregated Aβ1-42 in microglia. Glia 2009, 57, 1727–1740. [Google Scholar] [CrossRef]
- Palavicini, J.P.; Wang, C.; Chen, L.; Hosang, K.; Wang, J.; Tomiyama, T.; Mori, H.; Han, X. Oligomeric amyloid-beta induces MAPK-mediated activation of brain cytosolic and calcium-independent phospholipase A2 in a spatial-specific manner. Acta Neuropathol. Commun. 2017, 5, 56. [Google Scholar] [CrossRef]
- Sanchez-Mejia, R.O.; Newman, J.W.; Toh, S.; Yu, G.Q.; Zhou, Y.; Halabisky, B.; Cisse, M.; Scearce-Levie, K.; Cheng, I.H.; Gan, L.; et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2008, 11, 1311–1318. [Google Scholar] [CrossRef]
- Walker, K.A.; Ficek, B.N.; Westbrook, R. Understanding the Role of Systemic Inflammation in Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 3340–3342. [Google Scholar] [CrossRef]
- Giridharan, V.V.; Masud, F.; Petronilho, F.; Dal-Pizzol, F.; Barichello, T. Infection-Induced Systemic Inflammation Is a Potential Driver of Alzheimer’s Disease Progression. Front. Aging Neurosci. 2019, 11, 122. [Google Scholar] [CrossRef]
- Sheng, J.G.; Bora, S.H.; Xu, G.; Borchelt, D.R.; Price, D.L.; Koliatsos, V.E. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid beta peptide in APPswe transgenic mice. Neurobiol. Dis. 2003, 14, 133–145. [Google Scholar] [CrossRef]
- Catorce, M.N.; Gevorkian, G. LPS-induced Murine Neuroinflammation Model: Main Features and Suitability for Pre-clinical Assessment of Nutraceuticals. Curr. Neuropharmacol. 2016, 14, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Lin, W.N.; Cho, R.L.; Wang, C.Y.; Hsiao, L.D.; Yang, C.M. TNF-α-Induced cPLA2 Expression via NADPH Oxidase/Reactive Oxygen Species-Dependent NF-κB Cascade on Human Pulmonary Alveolar Epithelial Cells. Front. Pharmacol. 2016, 7, 447. [Google Scholar] [CrossRef] [PubMed]
- Hulkower, K.I.; Wertheimer, S.J.; Levin, W.; Coffey, J.W.; Anderson, C.M.; Chen, T.; DeWitt, D.L.; Crowl, R.M.; Hope, W.C.; Morgan, D.W. Interleukin-1β induces cytosolic phospholipase A2 and prostaglandin H synthase in rheumatoid synovial fibroblasts. Evidence for their roles in the production of prostaglandin E2. Arthritis Rheum 1994, 37, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Kouril, S.; Najdekr, L.; Loppi, S.; Korhonen, P.; Kanninen, K.M.; Malm, T.; Koistinaho, J.; Friedecky, D.; Gynther, M. Metabolomic and lipidomic changes triggered by lipopolysaccharide-induced systemic inflammation in transgenic APdE9 mice. Sci. Rep. 2021, 11, 13076. [Google Scholar] [CrossRef] [PubMed]
- Jankowsky, J.L.; Fadale, D.J.; Anderson, J.; Xu, G.M.; Gonzales, V.; Jenkins, N.A.; Copeland, N.G.; Lee, M.K.; Younkin, L.H.; Wagner, S.L.; et al. Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: Evidence for augmentation of a 42-specific γ secretase. Hum. Mol. Genet. 2004, 13, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kaya, I.; Jennische, E.; Lange, S.; Tarik Baykal, A.; Malmberg, P.; Fletcher, J.S. Brain region-specific amyloid plaque-associated myelin lipid loss, APOE deposition and disruption of the myelin sheath in familial Alzheimer’s disease mice. J. Neurochem. 2020, 154, 84–98. [Google Scholar] [CrossRef]
- Malm, T.M.; Iivonen, H.; Goldsteins, G.; Keksa-Goldsteine, V.; Ahtoniemi, T.; Kanninen, K.; Salminen, A.; Auriola, S.; Van Groen, T.; Tanila, H.; et al. Pyrrolidine dithiocarbamate activates Akt and improves spatial learning in APP/PS1 mice without affecting beta-amyloid burden. J. Neurosci. 2007, 27, 3712–3721. [Google Scholar] [CrossRef]
- Little, C.S.; Hammond, C.J.; MacIntyre, A.; Balin, B.J.; Appelt, D.M. Chlamydia pneumoniae induces Alzheimer-like amyloid plaques in brains of BALB/c mice. Neurobiol. Aging 2004, 25, 419–429. [Google Scholar] [CrossRef]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef]
- Puris, E.; Auriola, S.; Korhonen, P.; Loppi, S.; Kanninen, K.M.; Malm, T.; Koistinaho, J.; Gynther, M. Systemic Inflammation Induced Changes in Protein Expression of ABC Transporters and Ionotropic Glutamate Receptor Subunit 1 in the Cerebral Cortex of Familial Alzheimer‘s Disease Mouse Model. J. Pharm. Sci. 2021. [CrossRef]
- Puris, E.; Auriola, S.; Petralla, S.; Hartman, R.; Gynther, M.; de Lange, E.C.M.; Fricker, G. Altered protein expression of membrane transporters in isolated cerebral microvessels and brain cortex of a rat Alzheimer’s disease model. Neurobiol. Dis. 2022, 169, 105741. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Tachikawa, M.; Obuchi, W.; Hoshi, Y.; Tomioka, Y.; Ohtsuki, S.; Terasaki, T. A study protocol for quantitative targeted absolute proteomics (QTAP) by LC-MS/MS: Application for inter-strain differences in protein expression levels of transporters, receptors, claudin-5, and marker proteins at the blood-brain barrier in ddY, FVB, and C57BL/6J mice. Fluids Barriers CNS 2013, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.C.; Nadeau, K.; Abbasi, M.; Lachance, C.; Nguyen, M.; Fenrich, J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019, 37, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Omori, K.; Ali, I.; Tachikawa, M.; Terasaki, T.; Brouwer, K.L.R.; Nicolazzo, J.A. Altered Expression of Small Intestinal Drug Transporters and Hepatic Metabolic Enzymes in a Mouse Model of Familial Alzheimer’s Disease. Mol. Pharm. 2018, 15, 4073–4083. [Google Scholar] [CrossRef]
- Pan, Y.; Omori, K.; Ali, I.; Tachikawa, M.; Terasaki, T.; Brouwer, K.L.R.; Nicolazzo, J.A. Increased Expression of Renal Drug Transporters in a Mouse Model of Familial Alzheimer’s Disease. J. Pharm. Sci. 2019, 108, 2484–2489. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.Y.; He, Y.; Chuang, D.Y.; Lee, J.C.; Gu, Z.; Simonyi, A.; Sun, A.Y. Integrating cytosolic phospholipase A2 with oxidative/nitrosative signaling pathways in neurons: A novel therapeutic strategy for AD. Mol. Neurobiol. 2012, 46, 85–95. [Google Scholar] [CrossRef]
- Colangelo, V.; Schurr, J.; Ball, M.J.; Pelaez, R.P.; Bazan, N.G.; Lukiw, W.J. Gene expression profiling of 12633 genes in Alzheimer hippocampal CA1: Transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J. Neurosci. Res. 2002, 70, 462–473. [Google Scholar] [CrossRef]
- Kang, M.J.; Fujino, T.; Sasano, H.; Minekura, H.; Yabuki, N.; Nagura, H.; Iijima, H.; Yamamoto, T.T. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc. Natl. Acad. Sci. USA 1997, 94, 2880–2884. [Google Scholar] [CrossRef]
- Hashidate-Yoshida, T.; Harayama, T.; Hishikawa, D.; Morimoto, R.; Hamano, F.; Tokuoka, S.M.; Eto, M.; Tamura-Nakano, M.; Yanobu-Takanashi, R.; Mukumoto, Y.; et al. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. eLife 2015, 4, e06328. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, C.C.; Lee, I.T.; Lee, H.C.; Yang, C.M. Activation and induction of cytosolic phospholipase A2 by TNF-α mediated through Nox2, MAPKs, NF-κB, and p300 in human tracheal smooth muscle cells. J. Cell Physiol. 2011, 226, 2103–2114. [Google Scholar] [CrossRef]
- Lee, C.W.; Lee, I.T.; Lin, C.C.; Lee, H.C.; Lin, W.N.; Yang, C.M. Activation and induction of cytosolic phospholipase A2 by IL-1β in human tracheal smooth muscle cells: Role of MAPKs/p300 and NF-κB. J. Cell Biochem. 2010, 109, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Oddo, S.; Yamasaki, T.R.; Green, K.N.; LaFerla, F.M. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J. Neurosci. 2005, 25, 8843–8853. [Google Scholar] [CrossRef] [PubMed]
- Shelat, P.B.; Chalimoniuk, M.; Wang, J.H.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A2 in cortical neurons. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Pla2g4a | 5′-AGAAGACCTGGGAAGTGTGAGA-3′ | 5′-TCTGGAGTGTCCAGCATATCG-3′ |
| Sirt3 | 5′-GCTACATGCACGGTCTGTCGAA-3′ | 5′-CAATGTCGGGTTTCACAACGCC-3′ |
| Oxsr1 | 5′-ACGAGCCAACCATTGCTACA-3′ | 5′-ACTGACGCCGAAATCTGCAA-3′ |
| Tnfa | 5′-GTACCTTGTCTACTCCCAGGTTCTCT-3 | 5′-GTGTGGGTGAGGAGCACGTA-3′ |
| Il1b | 5′-GCAACTGTTCCTGAACTCAACT-3′ | 5′-ATCTTTTGGGGTCCGTCAACT-3′ |
| Actb | 5′-AAGTCCCTCACCCTCCCAAAAG-3′ | 5′-ACACAGAAGCAATGCTGTCACC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gynther, M.; Estrada, M.L.; Loppi, S.; Korhonen, P.; Kanninen, K.M.; Malm, T.; Koistinaho, J.; Auriola, S.; Fricker, G.; Puris, E. Increased Expression and Activity of Brain Cortical cPLA2 Due to Chronic Lipopolysaccharide Administration in Mouse Model of Familial Alzheimer’s Disease. Pharmaceutics 2022, 14, 2438. https://doi.org/10.3390/pharmaceutics14112438
Gynther M, Estrada ML, Loppi S, Korhonen P, Kanninen KM, Malm T, Koistinaho J, Auriola S, Fricker G, Puris E. Increased Expression and Activity of Brain Cortical cPLA2 Due to Chronic Lipopolysaccharide Administration in Mouse Model of Familial Alzheimer’s Disease. Pharmaceutics. 2022; 14(11):2438. https://doi.org/10.3390/pharmaceutics14112438
Chicago/Turabian StyleGynther, Mikko, Mariana Leal Estrada, Sanna Loppi, Paula Korhonen, Katja M. Kanninen, Tarja Malm, Jari Koistinaho, Seppo Auriola, Gert Fricker, and Elena Puris. 2022. "Increased Expression and Activity of Brain Cortical cPLA2 Due to Chronic Lipopolysaccharide Administration in Mouse Model of Familial Alzheimer’s Disease" Pharmaceutics 14, no. 11: 2438. https://doi.org/10.3390/pharmaceutics14112438
APA StyleGynther, M., Estrada, M. L., Loppi, S., Korhonen, P., Kanninen, K. M., Malm, T., Koistinaho, J., Auriola, S., Fricker, G., & Puris, E. (2022). Increased Expression and Activity of Brain Cortical cPLA2 Due to Chronic Lipopolysaccharide Administration in Mouse Model of Familial Alzheimer’s Disease. Pharmaceutics, 14(11), 2438. https://doi.org/10.3390/pharmaceutics14112438







