Dermatocosmetic Emulsions Based on Resveratrol, Ferulic Acid and Saffron (Crocus sativus) Extract to Combat Skin Oxidative Stress-Trigger Factor of Some Potential Malignant Effects: Stability Studies and Rheological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Active Compounds
2.1.2. Obtaining Emulsions with Dermatocosmetic Applications
2.2. Methods
2.2.1. Stability Evaluation
2.2.2. pH Determination
2.2.3. Phase Separation
2.2.4. Microscopic Images
2.2.5. Conductivity Measurements
2.2.6. Microbiological Control
2.2.7. Antioxidant Activity
2.2.8. Antioxidant Activity Assessment of Dermatocosmetic Emulsions
2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging Assay
2,2′-Azino-bis(3-ethylbenzothiazoline) 6-sulfonic Acid Radical Scavenging Assay
2.2.9. Rheological Tests
2.2.10. Rheological Measurements
3. Results
3.1. Microbiological Control
3.2. Antioxidant Activity
3.3. Rheological Tests
3.3.1. Flow Curves
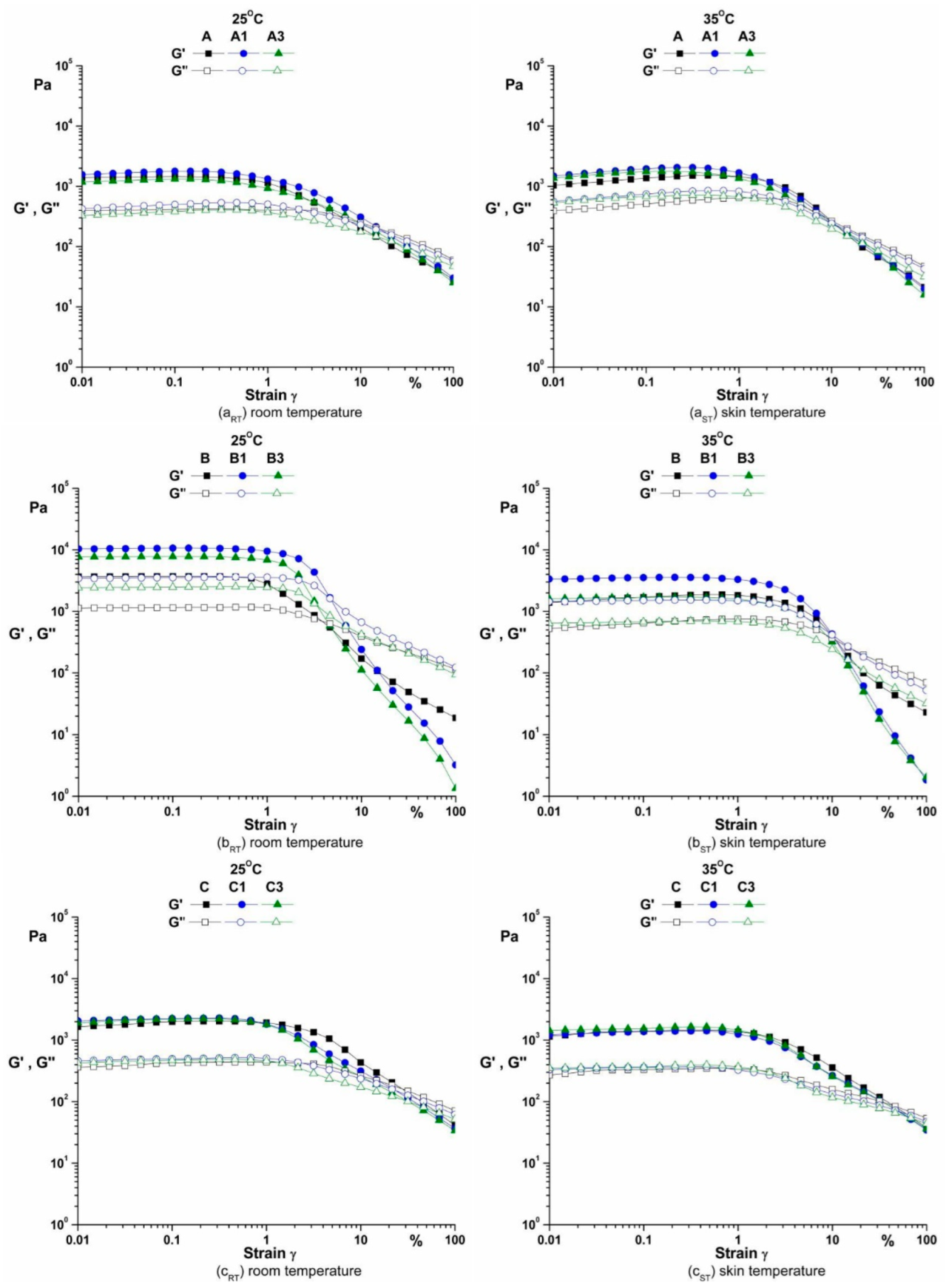
3.3.2. The Amplitude Sweep
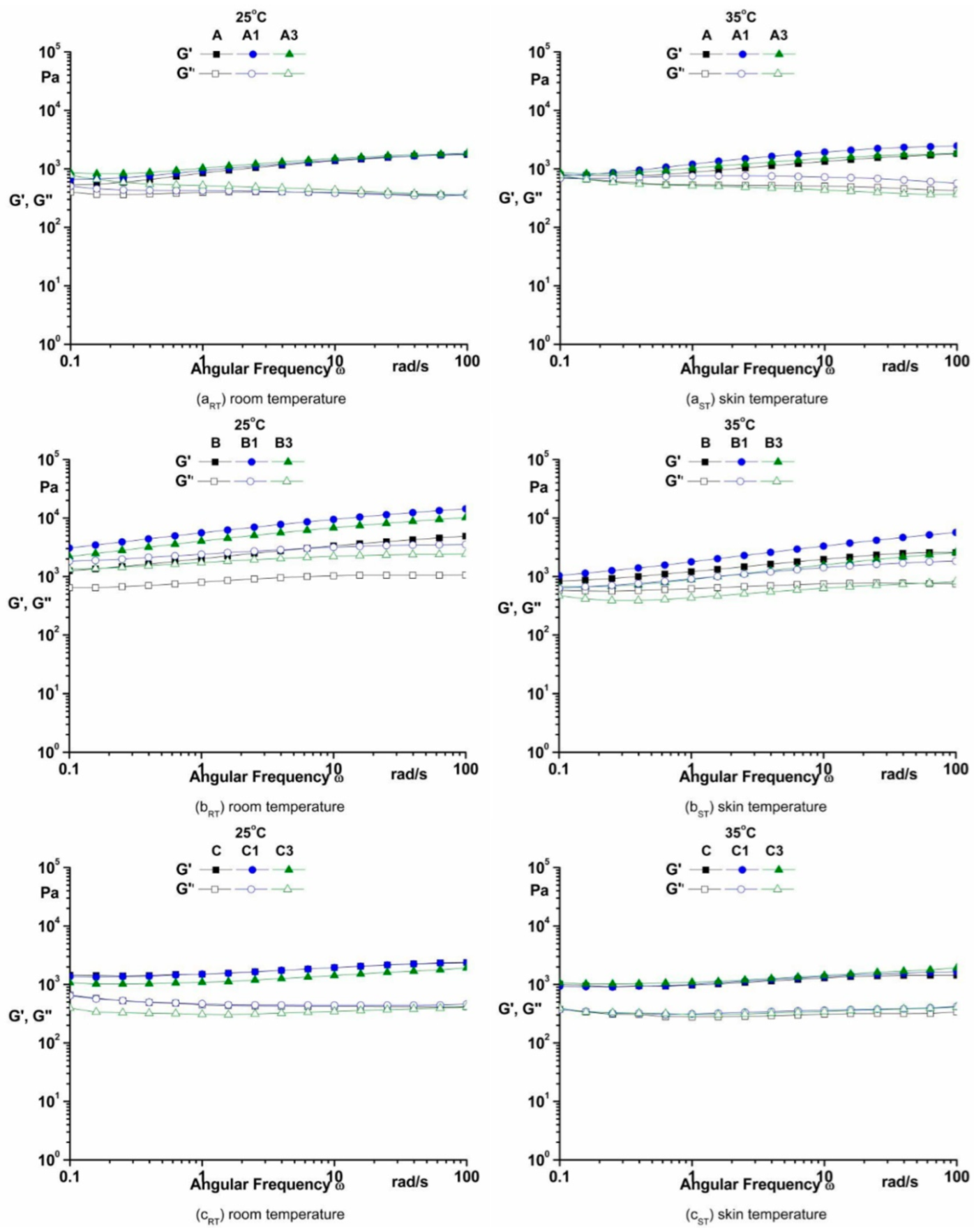
3.3.3. The Frequency Sweep
3.3.4. Dynamic Temperature Sweep Tests
3.3.5. Time Sweep Tests
4. Discussions
4.1. Microbiological Control
4.2. Antioxidant Activity
4.3. Rheological Tests
4.3.1. Flow Curves
4.3.2. The Amplitude Sweep
4.3.3. The Frequency Sweep
4.3.4. Dynamic Temperature Sweep Tests
4.3.5. Time Sweep Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Addor, F.A.S. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro e Silva, S.A.; Michniak-Kohn, B.; Leonardi, G.R. An overview about oxidation in clinical practice of skin aging. Bras. Dermatol. 2017, 92, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Ceglinski, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef]
- Makhafola, T.J.; Elgorashi, E.E.; McGaw, L.J.; Verschaeve, L.; Eloff, J.N. The correlation between antimutagenic activity and total phenolic content of extracts of 31 plant species with high antioxidant activity. BMC Complement. Altern. Med. 2016, 16, 490. [Google Scholar] [CrossRef] [Green Version]
- Baek, J.; Lee, M.G. Oxidative stress and antioxidant strategies in Dermatology. Redox Rep. Commun. Free Radic. Res. 2016, 21, 164–169. [Google Scholar] [CrossRef]
- Oresajo, C.; Pillai, S.; Yatskayer, M.; Puccetti, G.; McDaniel, D.H. Antioxidants and Skin Aging: A Review. Cosmet. Dermatol. 2009, 22, 563–570. [Google Scholar]
- Colucci, G.; Santamaria-Echart, A.; Silva, S.C.; Fernandes, I.P.M.; Sipoli, C.C.; Barreiro, F.M. Development of Water-in-Oil Emulsions as Delivery Vehicles and Testing with a Natural Antimicrobial Extract. Molecules 2020, 25, 2105. [Google Scholar] [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.-C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Sarbu, I.; Musuc, A.M.; Atkinson, I.; et al. Formulation and Development of Bioadhesive Oral Films Containing Usnea barbata (L.) F.H.Wigg Dry Ethanol Extract (F-UBE-HPC) with Antimicrobial and Anticancer Properties for Potential Use in Oral Cancer Complementary Therapy. Pharmaceutics 2022, 14, 1808. [Google Scholar] [CrossRef]
- Zip, C. The Role of Skin Care in Optimizing Treatment of Acne and Rosacea. Ski. Ther. Lett. 2017, 22, 5–7. [Google Scholar]
- Sikora, E. Cosmetic Emulsions: Monograph; Ogonowski, J., Ed.; Politechnika Krakowska: Kraków, Poland, 2019. [Google Scholar]
- Brinke, A.S.T.; Janssens-Böcker, C.; Kerscher, M. Skin Anti-Aging Benefits of a 2% Resveratrol Emulsion. J. Cosmet. Dermatol. Sci. Appl. 2021, 11, 155–168. [Google Scholar] [CrossRef]
- Turcov, D.; Zbranca, A.; Horciu, L.I.; Suteu, D. Resveratrol in the prevention and treatment of oxidative stress. Bull. IPI 2020, 66, 55–65. [Google Scholar]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Turcov, D.; Barna, S.; Profire, L.; Iacob, A.T.; Lisa, G.; Puitel, A.; Zbranca, A.; Suteu, D. Physico-chemical characterization of the antioxidant mixture resveratrol-ferulic acid for applications in dermato-cosmetic products. Farmacia 2022, 70, 410–416. [Google Scholar] [CrossRef]
- Turcov, D.; Barna, A.S.; Apreutesei, O.T.; Puitel, A.C.; Suteu, D. Valorization of Bioactive Compounds from Residual Saffron Biomass (Crocus sativus L.) for Obtaining of High Value Added Dermato-Cosmetic Products. BioResources 2022, 17, 4730–4744. [Google Scholar] [CrossRef]
- Mahmood, T.; Akhtar, N. Stability of a cosmetic multiple emulsion loaded with green tea extract. Sci. World J. 2013, 2013, 153695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.M.; Oh, H.M.; Lee, J.H. Controlling the emulsion stability of cosmetics through shear mixing process. Korea-Aust. Rheol. J. 2020, 32, 243–249. [Google Scholar] [CrossRef]
- Hu, Y.T.; Ting, Y.; Hu, J.Y.; Hsieh, S.C. Techniques and methods to study functional characteristics of emulsion systems. J. Food Drug Anal. 2017, 25, 16–26. [Google Scholar] [CrossRef] [Green Version]
- ISO 18415:2007; Cosmetics—Microbiology—Detection of Specified and Non-Specified Microorganisms. International Organization for Standardization: Geneva, Switzerland, 2007.
- Mapoung, S.; Semmarath, W.; Arjsri, P.; Umsumarng, S.; Srisawad, K.; Thippraphan, P.; Yodkeeree, S.; Limtrakul, P. Determination of Phenolic Content, Antioxidant Activity, and Tyrosinase Inhibitory Effects of Functional Cosmetic Creams Available on the Thailand Market. Plants 2021, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Caccavale, C.; Della Sala, F.; Zeppetelli, S.; Veneziano, R.; Borzacchiello, A. Natural Formulations Provide Antioxidant Complement to Hyaluronic Acid-Based Topical Applications Used in Wound Healing. Polymers 2020, 12, 1847. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Luca, S.V.; Kulinowski, L.; Ciobanu, C.; Zengin, G.; Czerwinska, M.E.; Granica, S.; Xiao, J.; Skalicka-Woźniak, K.; Trifan, A. Phytochemical and multi-biological characterization of two Cynara scolymus L. varieties: A glance into their potential large scale cultivation and valorization as bio-functional ingredients. Ind. Crop. Prod. 2022, 178, 114623. [Google Scholar] [CrossRef]
- Cobzaru, C.; Gherghescu, O.; Aursulesei, A.E.; Ibanescu, C.; Danu, M.; Apostolescu, G.A.; Cernatescu, C. Rheological Behaviour of Cold Creams with Cinnamon and Thuja Alcoholic Extract. Rev. Chim. 2017, 68, 1959–1962. [Google Scholar] [CrossRef]
- Nagarnaik, M.; Sarjoshi, A.; Linge, P.; Bhore, S.; Pandya, G. A microbial study of some cosmetics and raw materials used in personal care products in urban area. Res. J. Top. Cosmet. Sci. 2015, 6, 48–51. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Zyoud, S.H. An Investigation into Incidences of Microbial Contamination in Cosmeceuticals in the UAE: Imbalances between Preservation and Microbial Contamination. Cosmetics 2020, 7, 92. [Google Scholar] [CrossRef]
- Ibegbulam-Njoku, P.; Chijioke-Osuji, C.C. Microbiological evaluation of cosmetics products sourced in Aba city, Nigeria. Int. J. Sci. Rep. 2016, 2, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Aleem, A.; Khan, M.; Abid, U.; Ishfaq, M.F.; Rouf, A.; Jamshaid, T. Microbial Analysis of Selected Brands of Whitening Creams. Saudi J. Med. Pharm. Sci. 2020, 6, 178–182. [Google Scholar] [CrossRef]
- Kwak, M.S.; Ahn, H.J.; Song, K.W. Rheological investigation of body cream and body lotion in actual application conditions. Korea-Aust. Rheol. J. 2015, 27, 241–251. [Google Scholar] [CrossRef]
- Eren, N.M.; Santos, P.H.S.; Campanella, O. Mechanically modified xanthan gum: Rheology and polydispersity aspects. Carbohydr. Polym. 2015, 134, 475–484. [Google Scholar] [CrossRef]
- Adejokun, D.A.; Dodou, K. Quantitative Sensory Interpretation of Rheological Parameters of a Cream Formulation. Cosmetics 2020, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Gohla, S.H.; Müller, R.H. Rheology of nanostructured lipid carriers (NLC®) suspended in a viscoelastic medium. Die Pharm. Int. J. Pharm. Sci. 2005, 60, 671–673. [Google Scholar]
- Mason, T.G. New fundamental concepts in emulsion rheology. Curr. Opin. Colloid Interface Sci. 1999, 4, 231–238. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Barbosa-Cánovas, G.V. Rheology for the food industry. J. Food Eng. 2005, 67, 147–156. [Google Scholar] [CrossRef]
- Dimitrova, T.D.; Leal-Calderon, F. Rheological properties of highly concentrated protein-stabilized emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Colo, S.M.; Herh, P.K.W.; Roye, N.; Larsson, M. Rheology and the texture of pharmaceutical and cosmetic semisolids. Am. Lab. 2004, 36, 26–30. [Google Scholar]
- Herh, P.; Tkachuk, J.; Wu, S.; Bernzen, M.; Rudolph, B. The rheology of pharmaceutical and cosmetic semisolids. Am. Lab. 1998, 30, 12–14. [Google Scholar]
- Cheng, D.C.H. Yield stress: A time-dependent property and how to measure it. Rheol. Acta 1986, 25, 542–554. [Google Scholar] [CrossRef]
- Barnes, H.A. The yield stress—A review or ‘παντα ρεί’—Everything flows? J. Non-Newtonian Fluid Mech. 1999, 81, 133–178. [Google Scholar] [CrossRef]
- Moravkova, T.; Stern, P. Rheological and textural properties of cosmetic emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar]
- Bekker, M.; Webber, G.V.; Louw, N.R. Relating rheological measurements to primary and secondary skin feeling when mineral-based and Fischer-Tropsch wax-based cosmetic emulsions and jellies are applied to the skin. Int. J. Cosmet. Sci. 2013, 35, 354–361. [Google Scholar] [CrossRef]
- Park, E.K.; Song, K.W. Rheological evaluation of petroleum jelly as a base material in ointment and cream formulations: Steady shear flow behavior. Arch. Pharm. Res. 2010, 33, 141–150. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodriguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, K.Y.; Dodou, K. Rheological studies on pressure-sensitive silicone adhesives and drug-in-adhesive layers as a means to characterise adhesive performance. Int. J. Pharm. 2007, 333, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, M.; Niskanen, H.; Kiesvaara, J.; Yliruusi, J. Determination of optimal combination of surfactants in creams using rheology measurements. Int. J. Pharm. 2000, 197, 143–151. [Google Scholar] [CrossRef]
- Datta, A.; Tanmay, V.S.; Tan, G.X.; Reynolds, G.W.; Jamadagni, S.N.; Larson, R.G. Characterizing the rheology, slip, and velocity profiles of lamellar gel networks. J. Rheol. 2020, 64, 851–862. [Google Scholar] [CrossRef]
- Miller, D.; Wiener, E.M.; Turowski, A.; Thunig, C.; Hoffmann, H. O/W emulsions for cosmetic products stabilized by alkyl phosphates-rheology and storage tests. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 155–160. [Google Scholar] [CrossRef]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 227–258. [Google Scholar] [CrossRef]
- Masmoudi, H.; Piccerelle, P.; Dreau, Y.L.; Kister, J. A rheological method to evaluate the physical stability of highly viscous pharmaceutical oil-in-water emulsions. Pharm. Res. 2006, 23, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Park, E.K.; Song, K.W. Rheological evaluation of petroleum jelly as a base material in ointment and cream formulations with respect to rubbing onto the human body. Korea-Aust. Rheol. J. 2010, 22, 279–289. [Google Scholar]
- Lupi, F.R.; Shakeel, A.; Greco, V.; Baldino, N.; Calabro, V.; Gabriele, D. Organogelation of extra virgin olive oil with fatty alcohols, glyceryl stearate and their mixture. LWT Food Sci. Technol. 2017, 77, 422–429. [Google Scholar] [CrossRef]
- Ciriminna, R.; Katryniok, B.; Paul, S.; Dumeignil, F.; Pagliaro, M. Glycerol-Derived Renewable Polyglycerols: A Class of Versatile Chemicals of Wide Potential Application. Org. Process Res. Dev. 2015, 19, 748–754. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, C.S.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narendhirakannan, R.T.; Hannah, M.A.C. Oxidative Stress and Skin Cancer: An Overview. Indian J. Clin. Biochem. 2013, 28, 110–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godic, A.; Poljšak, B.; Adamic, M.; Dahmane, R. The Role of Antioxidants in Skin Cancer Prevention and Treatment. Oxidative Med. Cell. Longev. 2014, 2014, 860479. [Google Scholar] [CrossRef]
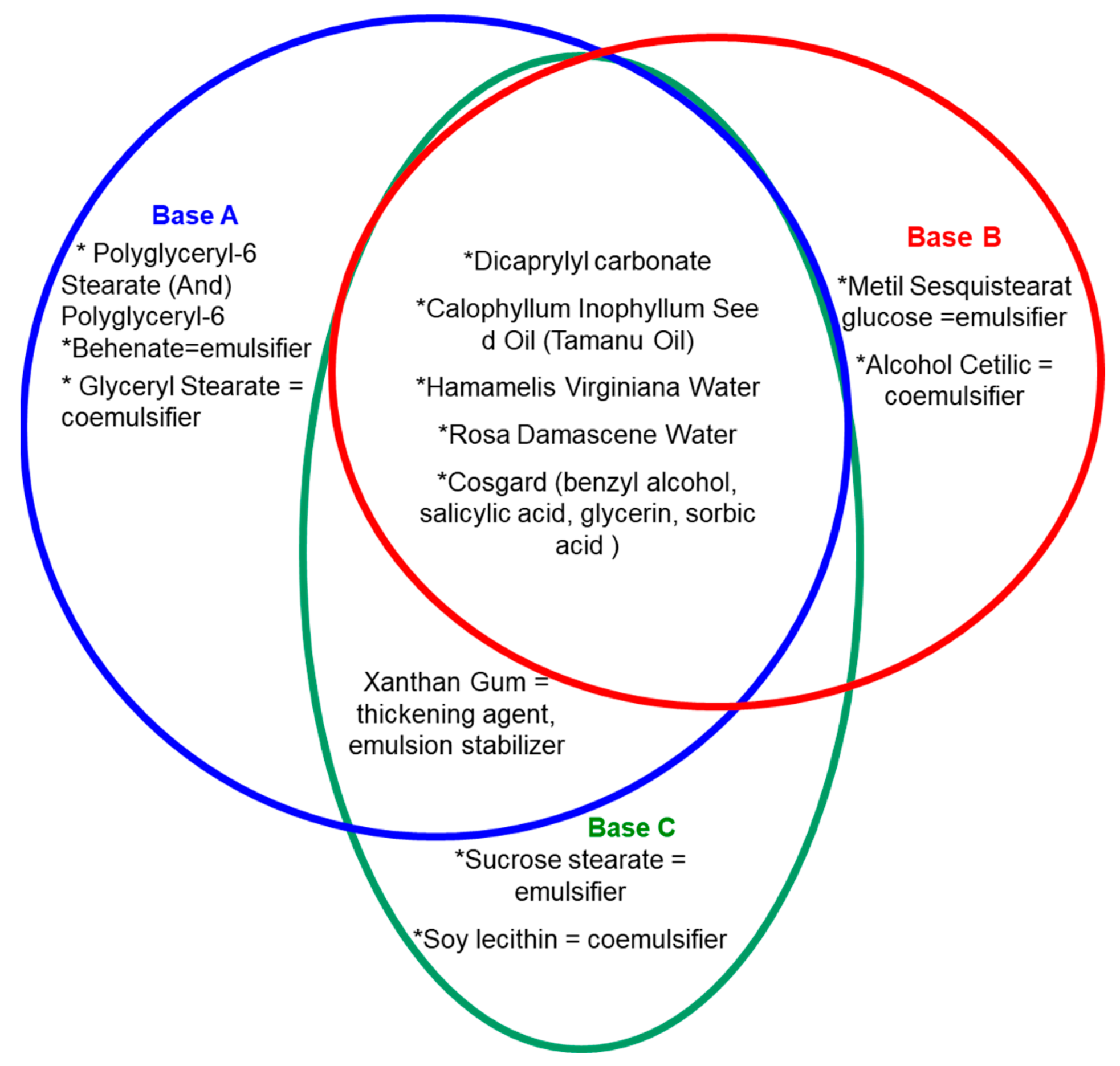



| Base | Phase, % (w/w) | Active Substances, % (w/w) | |
|---|---|---|---|
| Dispersed (O) | Continuous (W) | ||
| A | 28 | 67.3 | 4.7 |
| B | 29 | 67 | 4 |
| C | 37 | 58.3 | 4.7 |
| Emulsion Sample | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | A1 | A3 | B | B1 | B3 | C | C1 | C3 |
 |  |  |  |  |  |  |  |  |
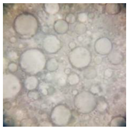 | 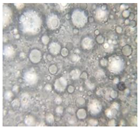 | 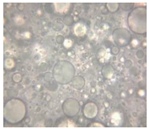 |
| Base A | A1 emulsion | A3 emulsion |
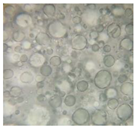 | 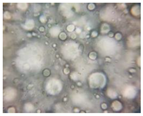 |  |
| Base B | B1 emulsion | B3 emulsion |
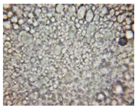 |  |  |
| Base C | C1 emulsion | C3 emulsion |
| Dermatocosmetic Emulsion | Total Viable Microbial Count, UFC/g | Total Viable Bacterial Count, UFC/g | Total Yeast and Fungi Count, UFC/g |
|---|---|---|---|
| Base A | 0 | 0 | 0 |
| A1 | 0 | 0 | 0 |
| A3 | 10 | 0 | 10 |
| Base B | 0 | 0 | 0 |
| B1 | 10 | 10 | 0 |
| B3 | 10 | 0 | 10 |
| Base C | 0 | 0 | 0 |
| C1 | 0 | 0 | 0 |
| C3 | 0 | 0 | 0 |
| Sample | TPC (mg GAE/g Emulsion) | TFC (mg RE/g Emulsion) | DPPH (mg TE/g Emulsion) | ABTS (mg TE/g Emulsion) |
|---|---|---|---|---|
| A1 | 21.32 ± 0.40 | 0.17 ± 0.01 | 4.38 ± 0.02 | 10.85 ± 0.08 |
| A3 | 6.25 ± 0.38 | 0.10 ± 0.01 | 5.63 ± 0.09 | 12.77 ± 0.26 |
| B1 | 20.68 ± 1.35 | 0.29 ± 0.02 | 9.41 ± 0.05 | 13.14 ± 0.14 |
| B3 | 8.24 ± 0.81 | 0.65 ± 0.05 | 7.90 ± 0.01 | 11.65 ± 0.03 |
| C1 | 23.31 ± 0.95 | 0.06 ± 0.00 | 7.74 ± 0.06 | 12.72 ± 0.22 |
| C3 | 21.96 ± 0.88 | 0.27 ± 0.02 | 8.57 ± 0.11 | 13.48 ± 0.15 |
| Samples | Strain (ɣ = 0.1%) | |||
|---|---|---|---|---|
| G’ (Pa) | G” (Pa) | |||
| T = 25 °C | T = 35 °C | T = 25 °C | T = 35 °C | |
| A | 1460 | 1370 | 415 | 512 |
| B | 3710 | 1700 | 1150 | 647 |
| C | 2000 | 1400 | 428 | 333 |
| A1 | 1780 | 1960 | 500 | 745 |
| B1 | 10700 | 3550 | 3540 | 1510 |
| C1 | 2250 | 1380 | 498 | 362 |
| A3 | 1320 | 1760 | 383 | 679 |
| B3 | 7790 | 1690 | 2480 | 676 |
| C3 | 2210 | 1540 | 472 | 366 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turcov, D.; Barna, A.S.; Blaga, A.C.; Ibanescu, C.; Danu, M.; Trifan, A.; Zbranca, A.; Suteu, D. Dermatocosmetic Emulsions Based on Resveratrol, Ferulic Acid and Saffron (Crocus sativus) Extract to Combat Skin Oxidative Stress-Trigger Factor of Some Potential Malignant Effects: Stability Studies and Rheological Properties. Pharmaceutics 2022, 14, 2376. https://doi.org/10.3390/pharmaceutics14112376
Turcov D, Barna AS, Blaga AC, Ibanescu C, Danu M, Trifan A, Zbranca A, Suteu D. Dermatocosmetic Emulsions Based on Resveratrol, Ferulic Acid and Saffron (Crocus sativus) Extract to Combat Skin Oxidative Stress-Trigger Factor of Some Potential Malignant Effects: Stability Studies and Rheological Properties. Pharmaceutics. 2022; 14(11):2376. https://doi.org/10.3390/pharmaceutics14112376
Chicago/Turabian StyleTurcov, Delia, Ana Simona Barna, Alexandra Cristina Blaga, Constanta Ibanescu, Maricel Danu, Adriana Trifan, Anca Zbranca, and Daniela Suteu. 2022. "Dermatocosmetic Emulsions Based on Resveratrol, Ferulic Acid and Saffron (Crocus sativus) Extract to Combat Skin Oxidative Stress-Trigger Factor of Some Potential Malignant Effects: Stability Studies and Rheological Properties" Pharmaceutics 14, no. 11: 2376. https://doi.org/10.3390/pharmaceutics14112376
APA StyleTurcov, D., Barna, A. S., Blaga, A. C., Ibanescu, C., Danu, M., Trifan, A., Zbranca, A., & Suteu, D. (2022). Dermatocosmetic Emulsions Based on Resveratrol, Ferulic Acid and Saffron (Crocus sativus) Extract to Combat Skin Oxidative Stress-Trigger Factor of Some Potential Malignant Effects: Stability Studies and Rheological Properties. Pharmaceutics, 14(11), 2376. https://doi.org/10.3390/pharmaceutics14112376









