Encapsulated Phytomedicines against Cancer: Overcoming the “Valley of Death”
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Optimization of P2Et Encapsulation
2.3. Physico-Chemical Characterization of Nanoparticles
2.3.1. Size, Zeta Potential and Yield
2.3.2. P2Et Quantification
2.4. In Vitro Release Study
2.5. Microbiology Evaluation of the Nanocapsules
2.6. In Vivo Evaluation of P2Et Encapsulation
2.6.1. Tumor Cell Line and Culture Conditions
2.6.2. Animals and Housing Conditions
2.6.3. In Vivo Tumor Model and P2Et Treatment
2.7. Stability Study
2.8. Statistical Analysis
3. Results
3.1. Preparation of P2Et Nanoparticles
3.2. In Vitro Release Studies
3.3. Microbiological Evaluation of the Nanoparticles
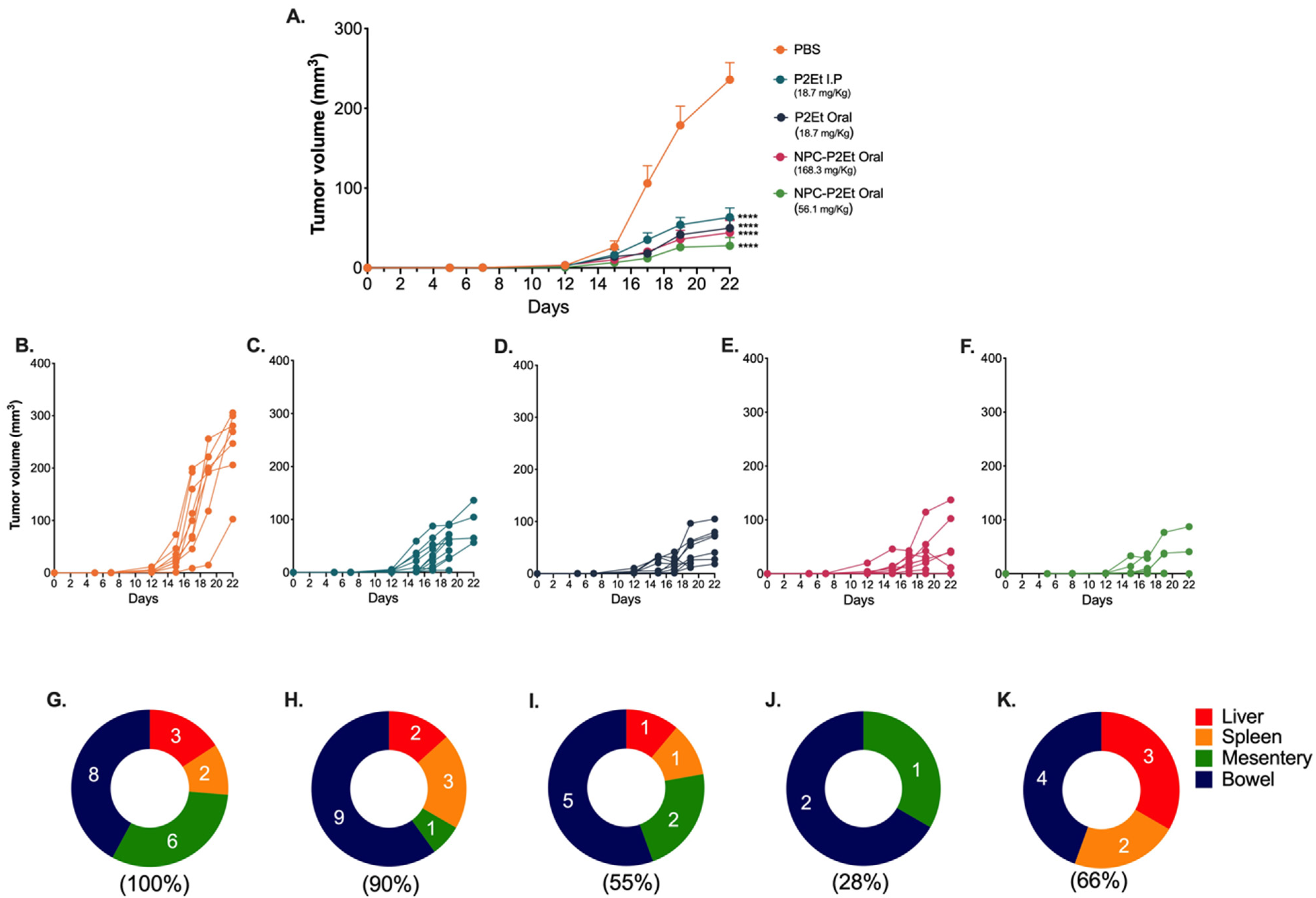
3.4. In Vivo NPC-P2Et Treatment Delays Tumor Growth and Metastasis in Breast Cancer Model
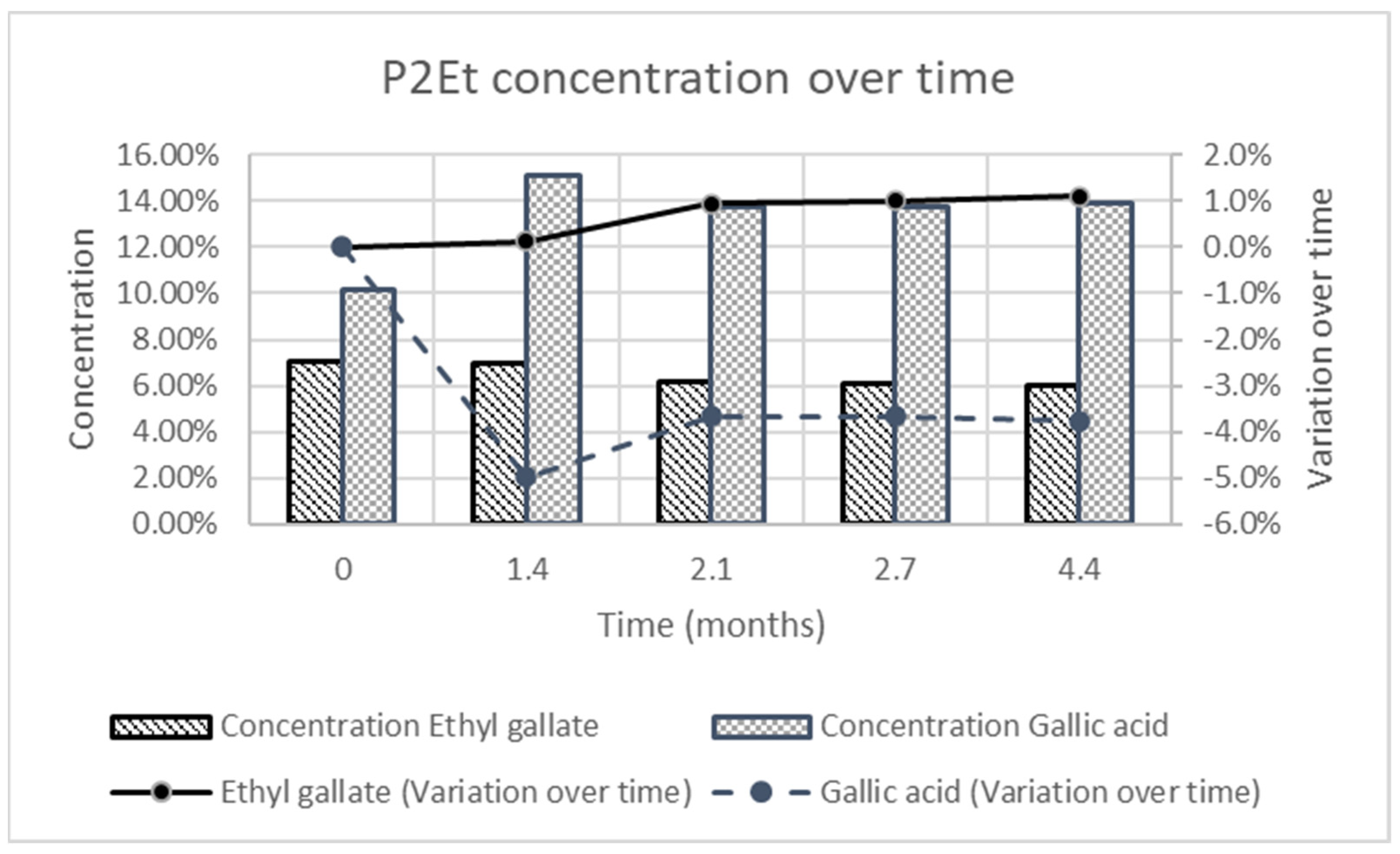
3.5. Stability Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lasso, P.; Gomez-Cadena, A.; Urueña, C.; Donda, A.; Martinez-Usatorre, A.; Barreto, A.; Romero, P.; Fiorentino, S. Prophylactic vs. Therapeutic Treatment With P2Et Polyphenol-Rich Extract Has Opposite Effects on Tumor Growth. Front. Oncol. 2018, 8, 356. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.; Torre, L.; Jemal, A. Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2020, 70, 313. [Google Scholar]
- Haddad, P.S.; Azar, G.A.; Groom, S.; Boivin, M. Natural health products, modulation of immune function and prevention of chronic diseases. Evid.-Based Complement. Altern. Med. 2005, 2, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Underwood, J.R. Plant-derived medicines: A novel class of immunological adjuvants. Int. Immunopharmacol. 2011, 11, 390–398. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, S.; Urueña, C.; Lasso, P.; Prieto, K.; Barreto, A. Phyto-Immunotherapy, a Complementary Therapeutic Option to Decrease Metastasis and Attack Breast Cancer Stem Cells. Front. Oncol. 2020, 10, 1334. [Google Scholar] [CrossRef]
- Xiang, Y.; Guo, Z.; Zhu, P.; Chen, J.; Huang, Y. Traditional Chinese medicine as a cancer treatment: Modern perspectives of ancient but advanced science. Cancer Med. 2019, 8, 1958–1975. [Google Scholar] [CrossRef]
- Kapinova, A.; Kubatka, P.; Golubnitschaja, O.; Kello, M.; Zubor, P.; Solar, P.; Pec, M. Dietary phytochemicals in breast cancer research: Anticancer effects and potential utility for effective chemoprevention. Environ. Health Prev. Med. 2018, 23, 36. [Google Scholar] [CrossRef]
- Mouhid, L.; Corzo-Martínez, M.; Torres, C.; Vázquez, L.; Reglero, G.; Fornari, T.; Ramírez de Molina, A. Improving. J. Oncol. 2017, 2017, 7351976. [Google Scholar] [CrossRef]
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of Polyphenols and Flavonoids in the Era of Precision Medicine. Mol. Pharm. 2017, 14, 2861–2863. [Google Scholar] [CrossRef]
- Ruskovska, T.; Maksimova, V.; Milenkovic, D. Polyphenols in human nutrition: From the. Br. J. Nutr. 2020, 123, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, M.; Ghidoni, R. Dietary Curcumin: Correlation between Bioavailability and Health Potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef] [PubMed]
- Selby-Pham, S.N.B.; Miller, R.B.; Howell, K.; Dunshea, F.; Bennett, L.E. Physicochemical properties of dietary phytochemicals can predict their passive absorption in the human small intestine. Sci. Rep. 2017, 7, 1931. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Munagala, R.; Jeyabalan, J.; Vadhanam, M.V. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013, 334, 133–141. [Google Scholar] [CrossRef]
- Johnson, J.J.; Nihal, M.; Siddiqui, I.A.; Scarlett, C.O.; Bailey, H.H.; Mukhtar, H.; Ahmad, N. Enhancing the bioavailability of resveratrol by combining it with piperine. Mol. Nutr. Food Res. 2011, 55, 1169–1176. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Majumdar, A.P.; Banerjee, S.; Nautiyal, J.; Patel, B.B.; Patel, V.; Du, J.; Yu, Y.; Elliott, A.A.; Levi, E.; Sarkar, F.H. Curcumin synergizes with resveratrol to inhibit colon cancer. Nutr. Cancer 2009, 61, 544–553. [Google Scholar] [CrossRef]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein Based Nanostructures for Drug Delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef]
- Lepak, A.; Gutmann, A.; Kulmer, S.T.; Nidetzky, B. Creating a Water-Soluble Resveratrol-Based Antioxidant by Site-Selective Enzymatic Glucosylation. Chembiochem 2015, 16, 1870–1874. [Google Scholar] [CrossRef]
- Rushdi, M. Modification of Chemical Structures of Certain Natural Antioxidants into Cytotoxic Compounds; Noor Publishing: London, UK, 2019. [Google Scholar]
- Chimento, A.; De Amicis, F.; Sirianni, R.; Sinicropi, M.S.; Puoci, F.; Casaburi, I.; Saturnino, C.; Pezzi, V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20, 1381. [Google Scholar] [CrossRef]
- Stohs, S.J.; Chen, O.; Ray, S.D.; Ji, J.; Bucci, L.R.; Preuss, H.G. Highly Bioavailable Forms of Curcumin and Promising Avenues for Curcumin-Based Research and Application: A Review. Molecules 2020, 25, 1397. [Google Scholar] [CrossRef]
- Brotons-Canto, A.; González-Navarro, C.J.; Gil, A.G.; Asin-Prieto, E.; Saiz, M.J.; Llabrés, J.M. Zein Nanoparticles Improve the Oral Bioavailability of Curcumin in Wistar Rats. Pharmaceutics 2021, 13, 361. [Google Scholar] [CrossRef]
- Duran, M.I.; Ballesteros-Ramírez, R.; Tellez, A.; Torregrosa, L.; Olejua, P.A.; Galvis, S.; Urueña, C.; Fiorentino, S. Safety Evaluation in Healthy Colombian Volunteers of P2Et Extract Obtained from. Evid.-Based Complement. Altern. Med. 2022, 2022, 7943001. [Google Scholar] [CrossRef]
- Gilani, S.M.U.; Ahmed, S.; Baig, S.G.; Hasan, M.M. Ethnopharmacognosy, phytochemistry and pharmacology of genus Caesalpinia: A review. J. Pharmacogn. Phytochem. 2019, 8, 2222–2229. [Google Scholar]
- Pizzi, A. Tannins medical/pharmacological and related applications: A critical review. Sustain. Chem. Pharm. 2021, 22, 100481. [Google Scholar] [CrossRef]
- Bussmann, R.W.; Sharon, D. Medicinal plants of the Andes and the Amazon-The magic and medicinal flora of Northern Peru. Ethnobot. Res. Appl. 2016, 15, 1–295. [Google Scholar] [CrossRef]
- Urueña, C.; Ballesteros-Ramírez, R.; Gomez-Cadena, A.; Barreto, A.; Prieto, K.; Quijano, S.; Aschner, P.; Martínez, C.; Zapata-Cardona, M.I.; El-Ahanidi, H.; et al. Randomized double-blind clinical study in patients with COVID-19 to evaluate the safety and efficacy of a phytomedicine (P2Et). Front. Med. 2022, 9, 991873. [Google Scholar] [CrossRef]
- Prieto, K.; Cao, Y.; Mohamed, E.; Trillo-Tinoco, J.; Sierra, R.A.; Urueña, C.; Sandoval, T.A.; Fiorentino, S.; Rodriguez, P.C.; Barreto, A. Polyphenol-rich extract induces apoptosis with immunogenic markers in melanoma cells through the ER stress-associated kinase PERK. Cell Death Discov. 2019, 5, 134. [Google Scholar] [CrossRef]
- Sandoval, T.A.; Urueña, C.P.; Llano, M.; Gómez-Cadena, A.; Hernández, J.F.; Sequeda, L.G.; Loaiza, A.E.; Barreto, A.; Li, S.; Fiorentino, S. Standardized Extract from Caesalpinia spinosa is Cytotoxic Over Cancer Stem Cells and Enhance Anticancer Activity of Doxorubicin. Am. J. Chin. Med. 2016, 44, 1693–1717. [Google Scholar] [CrossRef]
- Urueña, C.; Sandoval, T.A.; Lasso, P.; Tawil, M.; Barreto, A.; Torregrosa, L.; Fiorentino, S. Evaluation of chemotherapy and P2Et extract combination in ex-vivo derived tumor mammospheres from breast cancer patients. Sci. Rep. 2020, 10, 19639. [Google Scholar] [CrossRef]
- Gomez-Cadena, A.; Urueña, C.; Prieto, K.; Martinez-Usatorre, A.; Donda, A.; Barreto, A.; Romero, P.; Fiorentino, S. Immune-system-dependent anti-tumor activity of a plant-derived polyphenol rich fraction in a melanoma mouse model. Cell Death Dis. 2016, 7, e2243. [Google Scholar] [CrossRef] [PubMed]
- Urueña, C.; Gomez, A.; Sandoval, T.; Hernandez, J.; Li, S.; Barreto, A.; Fiorentino, S. Multifunctional T Lymphocytes Generated After Therapy With an Antitumor Gallotanin-Rich Normalized Fraction Are Related to Primary Tumor Size Reduction in a Breast Cancer Model. Integr. Cancer Ther. 2015, 14, 468–483. [Google Scholar] [CrossRef]
- FDA, U. Botanical drug development guidance for industry. Pharm. Qual. CMC Revis. 2016, 1, 1–34. [Google Scholar]
- Arbós, P.; Wirth, M.; Arangoa, M.A.; Gabor, F.; Irache, J.M. Gantrez AN as a new polymer for the preparation of ligand-nanoparticle conjugates. J. Control. Release 2002, 83, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Regulation, C. No. 1441/2007 amending Regulations (EC) No. 2073/2005 on microbial criteria for foodstuffs. Off. J. Eur. Union L 2007, 322, 12. [Google Scholar]
- Urueña, C.; Mancipe, J.; Hernandez, J.; Castañeda, D.; Pombo, L.; Gomez, A.; Asea, A.; Fiorentino, S. Gallotannin-rich Caesalpinia spinosa fraction decreases the primary tumor and factors associated with poor prognosis in a murine breast cancer model. BMC Complement. Altern. Med. 2013, 13, 74. [Google Scholar] [CrossRef]
- Lasso, P.; Gomez-Cadena, A.; Urueña, C.; Donda, A.; Martinez-Usatorre, A.; Romero, P.; Barreto, A.; Fiorentino, S. An Immunomodulatory Gallotanin-Rich Fraction from Caesalpinia Spinosa Enhances the Therapeutic Effect of Anti-PD-L1 in Melanoma. Front. Immunol. 2020, 11, 584959. [Google Scholar] [CrossRef]
- Penalva, R.; Esparza, I.; Agüeros, M.; Gonzalez-Navarro, C.J.; Gonzalez-Ferrero, C.; Irache, J.M. Casein nanoparticles as carriers for the oral delivery of folic acid. Food Hydrocoll. 2015, 44, 399–406. [Google Scholar] [CrossRef]
- Lasso, P.; Llano Murcia, M.; Sandoval, T.A.; Urueña, C.; Barreto, A.; Fiorentino, S. Breast Tumor Cells Highly Resistant to Drugs Are Controlled Only by the Immune Response Induced in an Immunocompetent Mouse Model. Integr. Cancer Ther. 2019, 18, 1534735419848047. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Wu, S.; Bin, W.; Tu, B.; Li, X.; Wang, W.; Liao, S.; Sun, C. A Delivery System for Oral Administration of Proteins/Peptides Through Bile Acid Transport Channels. J. Pharm. Sci. 2019, 108, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.G.; Irache, J.M.; Peñuelas, I.; González Navarro, C.J.; López de Cerain, A. Toxicity and biodistribution of orally administered casein nanoparticles. Food Chem. Toxicol. 2017, 106, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Recent Developments and Potential for Clinical Use of Casein as a Drug Carrier. Curr. Drug Deliv. 2023, 20, 250–260. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]


| Time (Minutes) | Formic Acid 0.1% in Water (%) | ACN (%) |
|---|---|---|
| 0 | 99 | 1 |
| 6 | 99 | 1 |
| 11 | 40 | 60 |
| 16 | 40 | 60 |
| 26 | 99 | 1 |
| Sample | Mean Size (nm) | PDI | Zeta Potential (mV) | Theoretical [P2Et] (%) |
|---|---|---|---|---|
| NPC (control) | 226 ± 6 | 0.189 ± 0.013 | −17.6 ± 0.3 | - |
| NPC-P2Et-3% | 145 ± 2 | 0.122 ± 0.014 | −13.6 ± 6.4 | 3.00 |
| NPC-P2Et-5% | 146 ± 4 | 0.133 ± 0.031 | −9.5 ± 3.4 | 4.79 |
| NPC-P2Et-9% | 260 ± 13 | 0.116 ± 0.011 | −9.4 ± 0.9 | 9.14 |
| Sample | Gallic Acid Payload (mg g.a./mg NP) | Gallic Acid Encapsulation Efficiency (%) | Ethyl Gallate Payload (mg e.g.,/mg NP) | Ethyl Gallate Efficiency (%) |
|---|---|---|---|---|
| NPC-P2Et-5% | 31.77 | 66 | 35.08 | 100 |
| NPC-P2Et-9% | 92.56 | 100 | 64.73 | 100 |
| Microorganism | Specifications | Result | Methods |
|---|---|---|---|
| Aerobic mesophilic bacteria (CFU/g) | Max 100.000 | 4.000 | ISO 4833:2013 |
| Total coliform (CFU/g) | Max 10 | <10 | ISO 4832:2006 |
| Fecal coliform (CFU/g) | Max 10 | <10 | ISO 7251:2005 |
| Escherichia coli (CFU/10 g) | Max 10 | <10 | ISO 16654:2001 |
| Staphylococcus aureus (CFU/g) | Max 10 | <10 | ISO 6888 |
| Pseudomona aeruginosa (CFU/10 g) | Max 10 | <10 | ISO 22717:2015; |
| Salmonella spp. | Max 10 | <10 | ISO 6579:2017 |
| Time (Months) | Mean Size (nm) | PDI |
|---|---|---|
| 0 | 202 ± 13 | 0.208 ± 0.073 |
| 1.4 | 193 ± 3 | 0.248 ± 0.024 |
| 2.1 | 215 ± 28 | 0.272 ± 0.004 |
| 2.7 | 224 ± 16 | 0.287 ± 0.019 |
| 4.4 | 208 ± 16 | 0.250 ± 0.080 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brotons-Canto, A.; Urueña, C.P.; Imbuluzqueta, I.; Luque-Michel, E.; Martinez-López, A.L.; Ballesteros-Ramírez, R.; Rojas, L.; Fiorentino, S. Encapsulated Phytomedicines against Cancer: Overcoming the “Valley of Death”. Pharmaceutics 2023, 15, 1038. https://doi.org/10.3390/pharmaceutics15041038
Brotons-Canto A, Urueña CP, Imbuluzqueta I, Luque-Michel E, Martinez-López AL, Ballesteros-Ramírez R, Rojas L, Fiorentino S. Encapsulated Phytomedicines against Cancer: Overcoming the “Valley of Death”. Pharmaceutics. 2023; 15(4):1038. https://doi.org/10.3390/pharmaceutics15041038
Chicago/Turabian StyleBrotons-Canto, Ana, Claudia P. Urueña, Izaskun Imbuluzqueta, Edurne Luque-Michel, Ana Luisa Martinez-López, Ricardo Ballesteros-Ramírez, Laura Rojas, and Susana Fiorentino. 2023. "Encapsulated Phytomedicines against Cancer: Overcoming the “Valley of Death”" Pharmaceutics 15, no. 4: 1038. https://doi.org/10.3390/pharmaceutics15041038
APA StyleBrotons-Canto, A., Urueña, C. P., Imbuluzqueta, I., Luque-Michel, E., Martinez-López, A. L., Ballesteros-Ramírez, R., Rojas, L., & Fiorentino, S. (2023). Encapsulated Phytomedicines against Cancer: Overcoming the “Valley of Death”. Pharmaceutics, 15(4), 1038. https://doi.org/10.3390/pharmaceutics15041038






