The Effects of Graphene Oxide and Reduced Graphene Oxide Conductive Additives on Activated Carbon Supercapacitors
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals List
2.2. Graphene Oxide and Reduced Graphene Oxide Synthesis
2.3. Electrode Preparation
2.4. Coin Cell Preparation
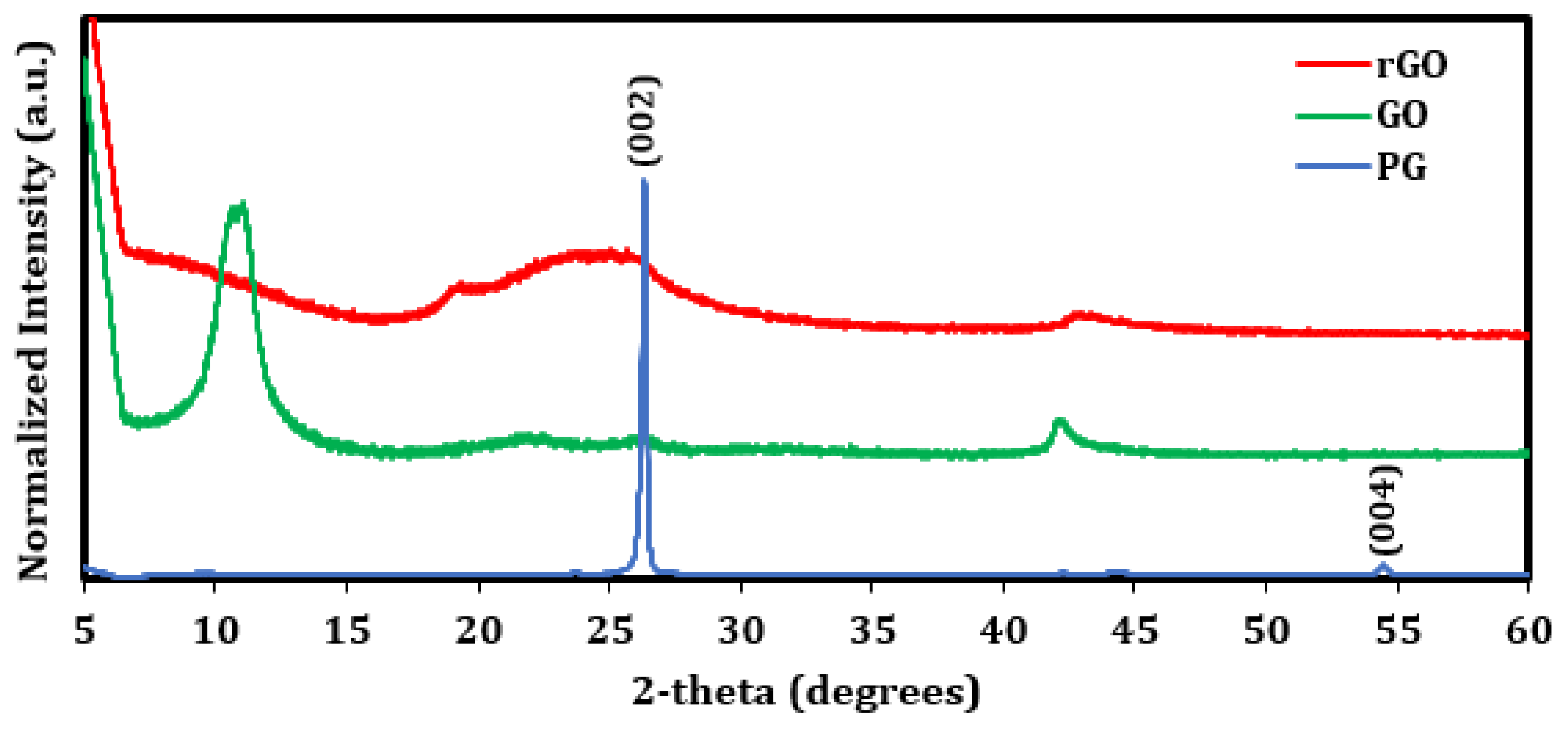
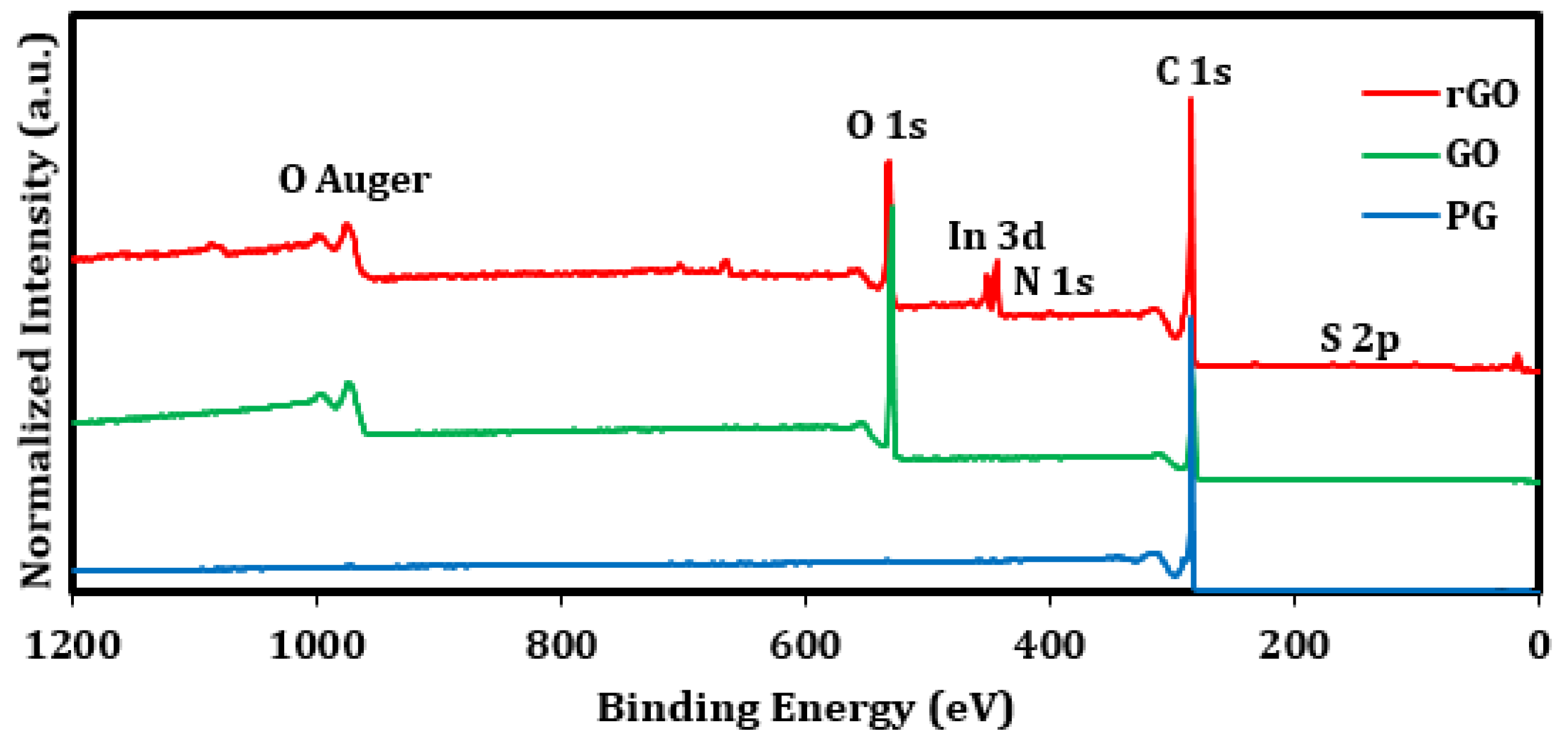
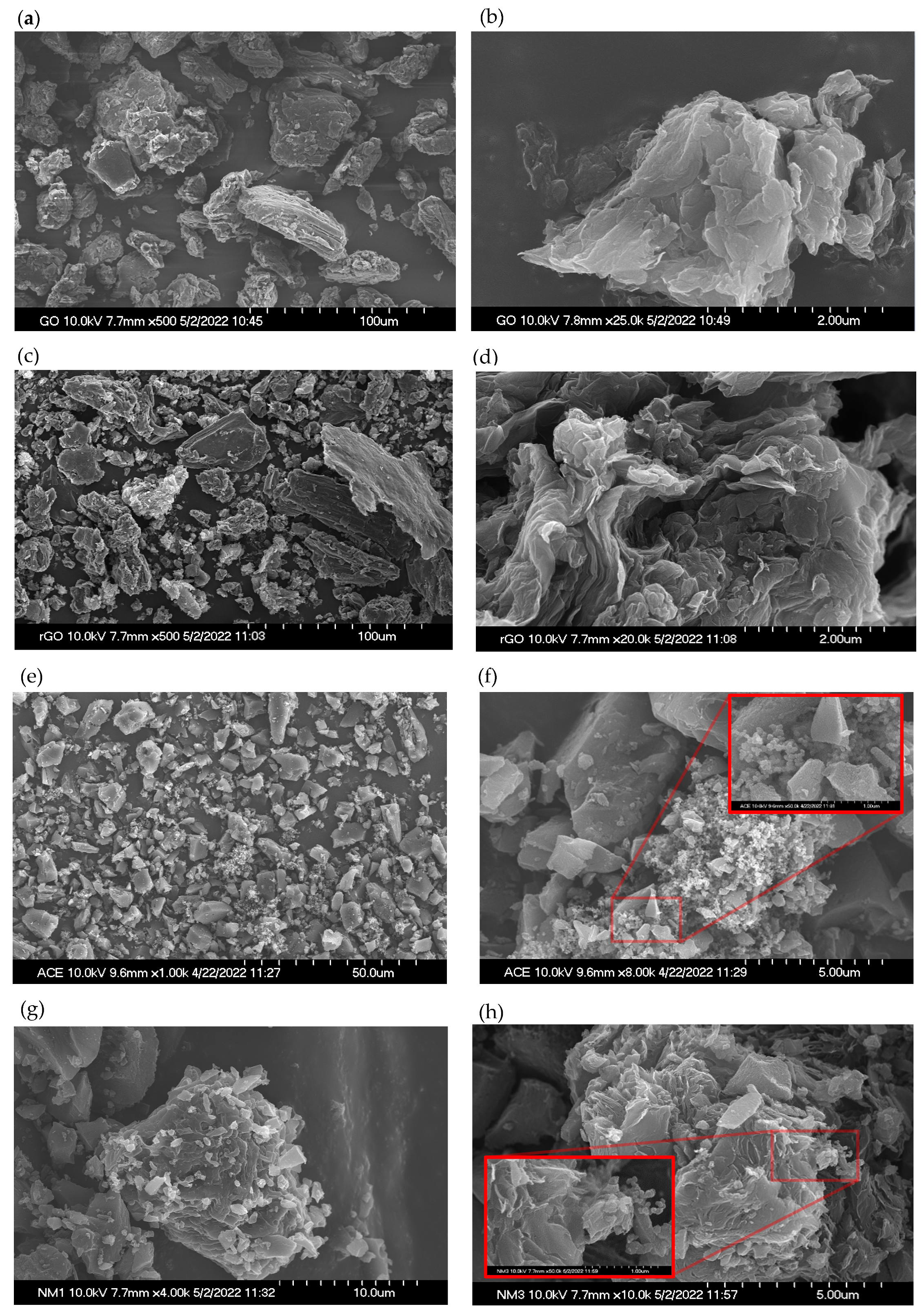
2.5. Material Characterization
2.6. Electrochemical Characterization
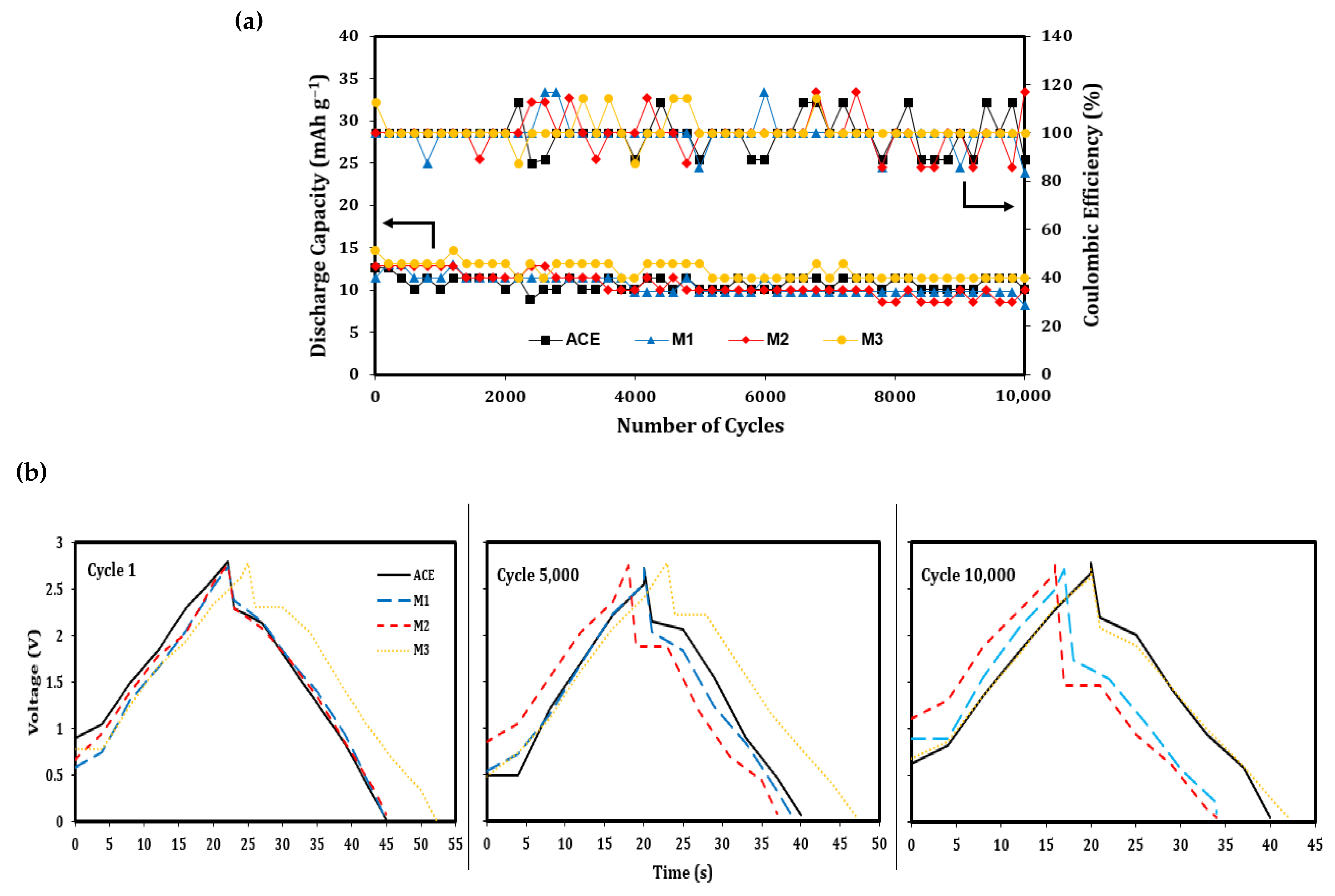
3. Results & Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dubey, R.; Guruviah, V. Review of carbon-based electrode materials for supercapacitor energy storage. Ionics 2019, 25, 1419–1445. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999. [Google Scholar]
- Borenstein, A.; Hanna, O.; Attias, R.; Luski, S.; Brousse, T.; Aurbach, D. Carbon-Based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Frackowiak, E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007, 9, 1774–1785. [Google Scholar] [CrossRef]
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Azaïs, P.; Duclaux, L.; Florian, P.; Massiot, D.; Lillo-Rodenas, M.-A.; Linares-Solano, A.; Peres, J.-P.; Jehoulet, C.; Béguin, F. Causes of supercapacitors ageing in organic electrolyte. J. Power Sources 2007, 171, 1046–1053. [Google Scholar] [CrossRef]
- Hahn, M.; Barbieri, O.; Gallay, R.; Kötz, R. A dilatometric study of the voltage limitation of carbonaceous electrodes in aprotic EDLC type electrolytes by charge-induced strain. Carbon 2006, 44, 2523–2533. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-Based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Gong, Q.; Weng, M.; Bai, J.; Liu, X.; Jiang, Y.; Wang, J.; Wang, D.; Shao, Y.; et al. Experimental and Correlative Analyses of the Ageing Mechanism of Activated Carbon Based Supercapacitor. Electrochim. Acta 2017, 228, 214–225. [Google Scholar] [CrossRef]
- Wang, Y.; Song, Y.; Xia, Y. Electrochemical capacitors: Mechanism, materials, systems, characterization and applications. Chem. Soc. Rev. 2016, 45, 5925–5950. [Google Scholar] [CrossRef]
- Lu, W.; Hartman, R.; Qu, L.; Dai, L. Nanocomposite Electrodes for High-Performance Supercapacitors. J. Phys. Chem. Lett. 2011, 2, 655–660. [Google Scholar] [CrossRef]
- Li, Q.; Liu, F.; Zhang, L.; Nelson, B.; Zhang, S.; Ma, C.; Tao, X.; Cheng, J.; Zhang, X. In situ construction of potato starch based carbon nanofiber/activated carbon hybrid structure for high-performance electrical double layer capacitor. J. Power Sources 2012, 207, 199–204. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Zhang, H.; Sun, X.; Zhang, D.; Ma, Y. High-Performance supercapacitors based on a graphene–activated carbon composite prepared by chemical activation. RSC Adv. 2012, 2, 7747–7753. [Google Scholar] [CrossRef]
- Zheng, C.; Zhou, X.; Cao, H.; Wang, G.; Liu, Z. Synthesis of porous graphene/activated carbon composite with high packing density and large specific surface area for supercapacitor electrode material. J. Power Sources 2014, 258, 290–296. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, D.; Yin, Z.; Yan, Q.; Zhang, H. Graphene and Graphene-Based Materials for Energy Storage Applications. Small 2014, 10, 3480–3498. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Shulga, Y.M.; Shulga, N.Y.; Parkhomenko, Y.N. Carbon nanostructures reduced from graphite oxide as electrode materials for supercapacitors. Mod. Electron. Mater. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, R.; Yadav, A.; Kumari, R. Recent advancement made in the field of reduced graphene oxide-based nanocomposites used in the energy storage devices: A review. J. Energy Storage 2020, 33, 102032. [Google Scholar] [CrossRef]
- Mokhtar, M.M.; Abo El Enein, S.A.; Hassaan, M.Y.; Morsy, M.S.; Khalil, M.H. Thermally Reduced Graphene Oxide: Synthesis, Structural and Electrical Properties. Int. J. Nanoparticles Nanotechnol. 2017, 3, 1–9. [Google Scholar]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Cheng, F.; Yang, X.; Zhang, S.; Lu, W. Boosting the supercapacitor performances of activated carbon with carbon nanomaterials. J. Power Sources 2020, 450, 227678. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Xiao, B.; Mishra, S.; Killam, A.; Pradhan, A.K. Resistive switching behavior of reduced graphene oxide memory cells for low power nonvolatile device application. Sci. Rep. 2016, 6, 26763. [Google Scholar] [CrossRef] [PubMed]
- Kaniyoor, A.; Baby, T.T.; Arockiadoss, T.; Rajalakshmi, N.; Ramaprabhu, S. Wrinkled Graphenes: A Study on the Effects of Synthesis Parameters on Exfoliation-Reduction of Graphite Oxide. J. Phys. Chem. C 2011, 115, 17660–17669. [Google Scholar] [CrossRef]
- Noori, A.; El-Kady, M.F.; Rahmanifar, M.S.; Kaner, R.B.; Mousavi, M.F. Towards establishing standard performance metrics for batteries, supercapacitors and beyond. Chem. Soc. Rev. 2019, 48, 1272–1341. [Google Scholar] [CrossRef] [PubMed]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Cao, N.; Zhang, Y. Study of Reduced Graphene Oxide Preparation by Hummers’ Method and Related Characterization. J. Nanomater. 2015, 2015, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Al-Gaashani, R.; Najjar, A.; Zakaria, Y.; Mansour, S.; Atieh, M.A. XPS and structural studies of high quality graphene oxide and reduced graphene oxide prepared by different chemical oxidation methods. Ceram. Int. 2019, 45, 14439–14448. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaria, R.; Granda, M.; Gutiérrez, M.D.; Rodriguez-Reinoso, F.; Menéndez, R. Critical temperatures in the synthesis of graphene-like materials by thermal exfoliation–reduction of graphite oxide. Carbon 2013, 52, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
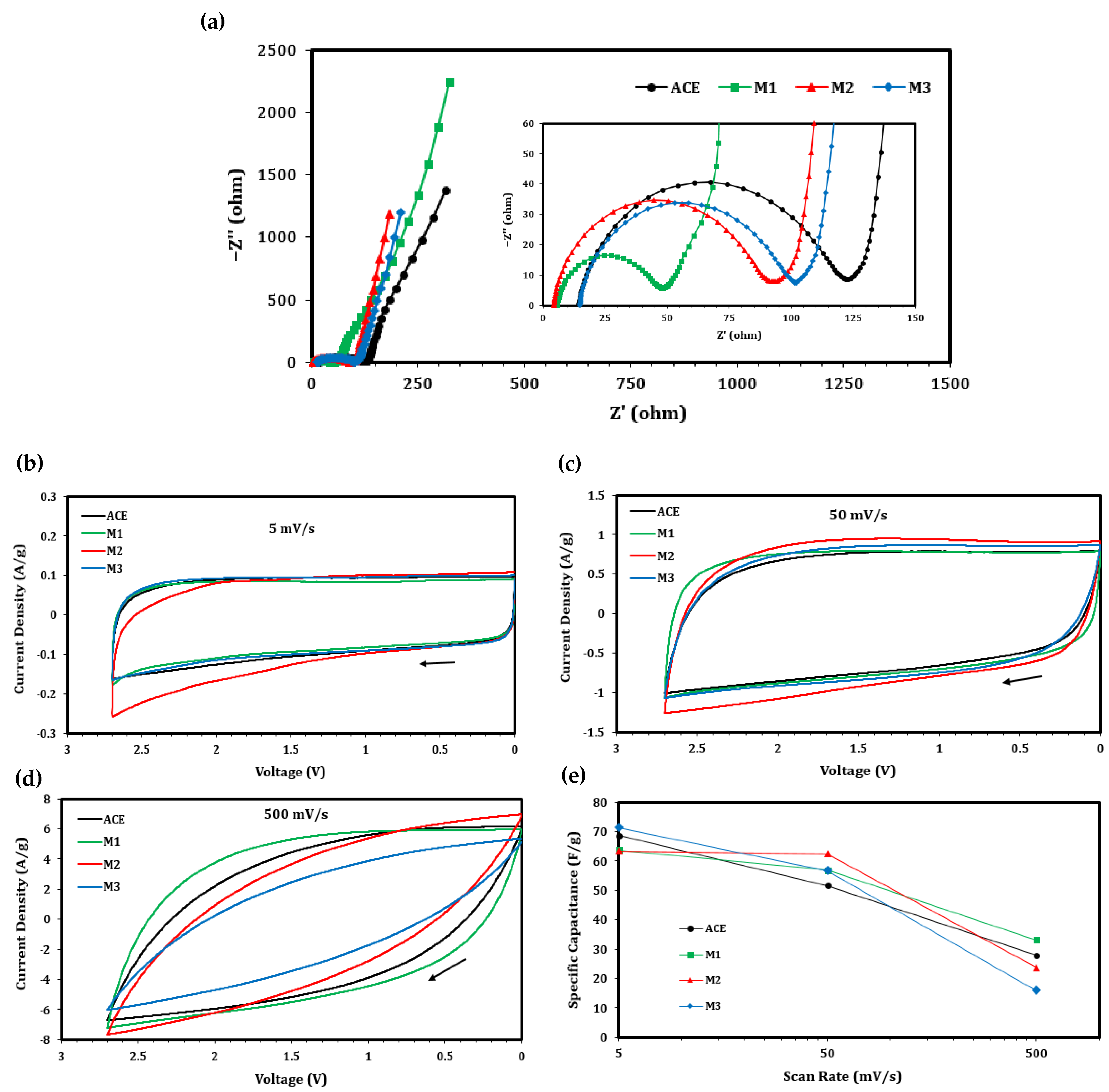
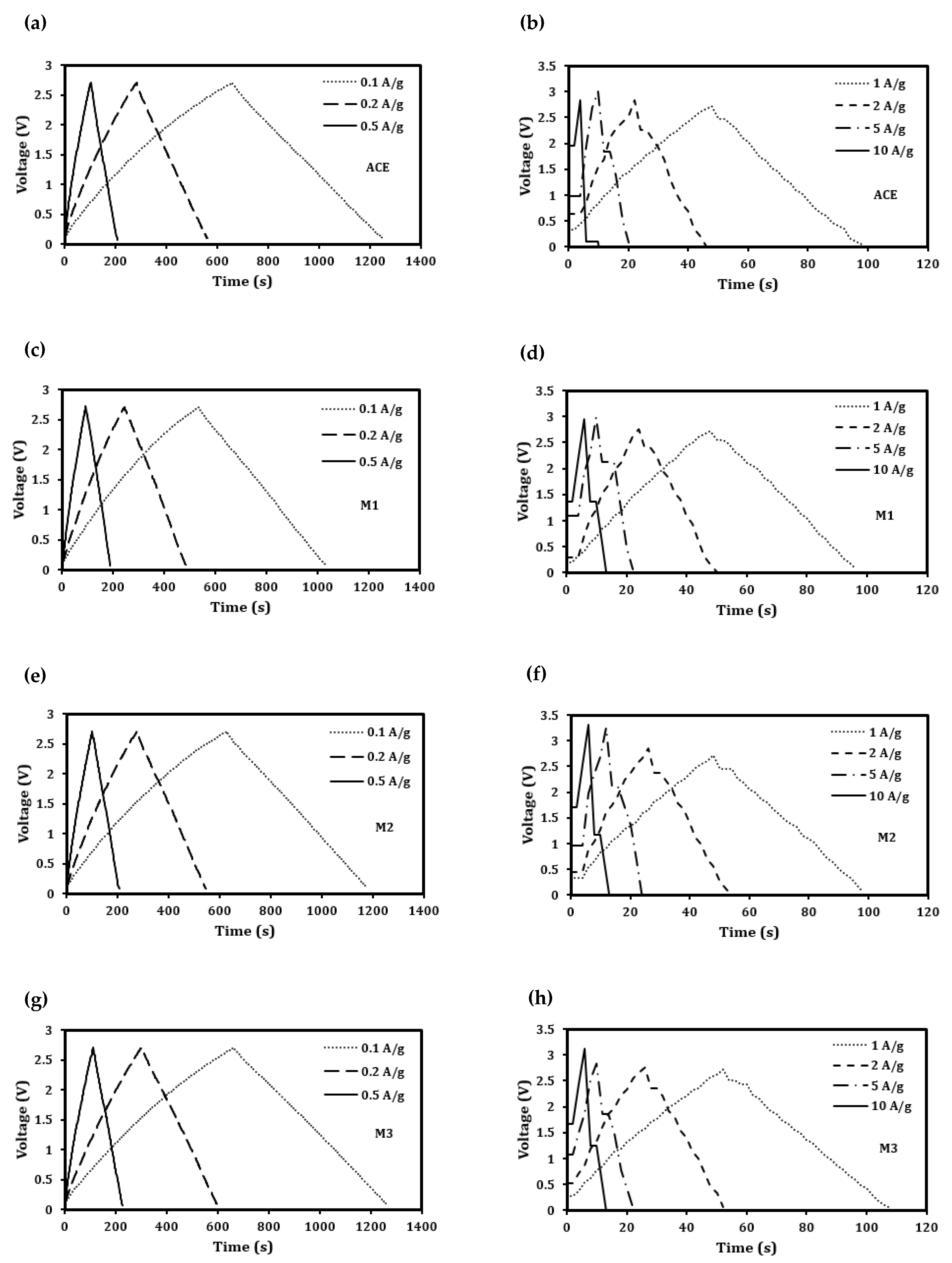
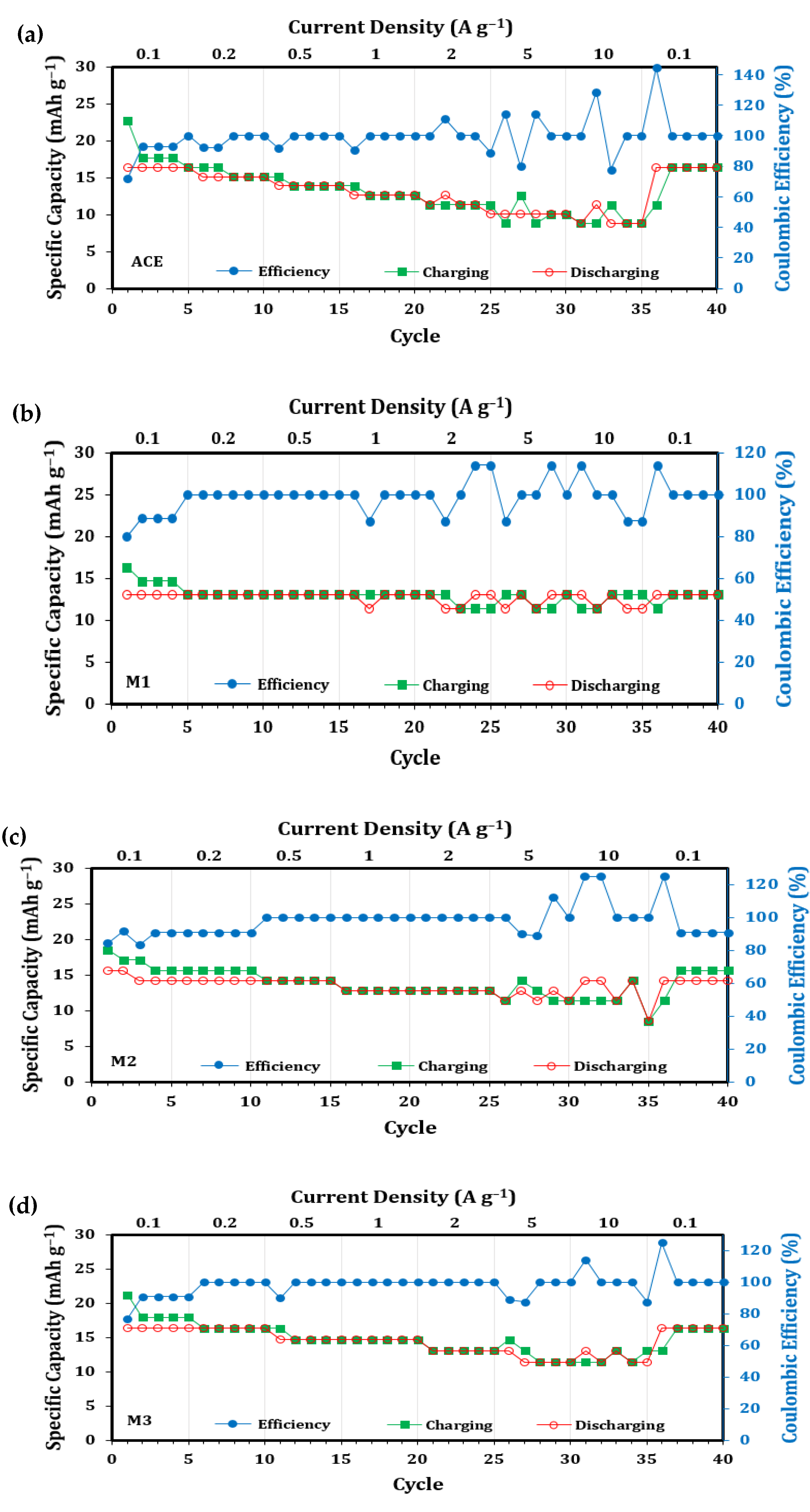
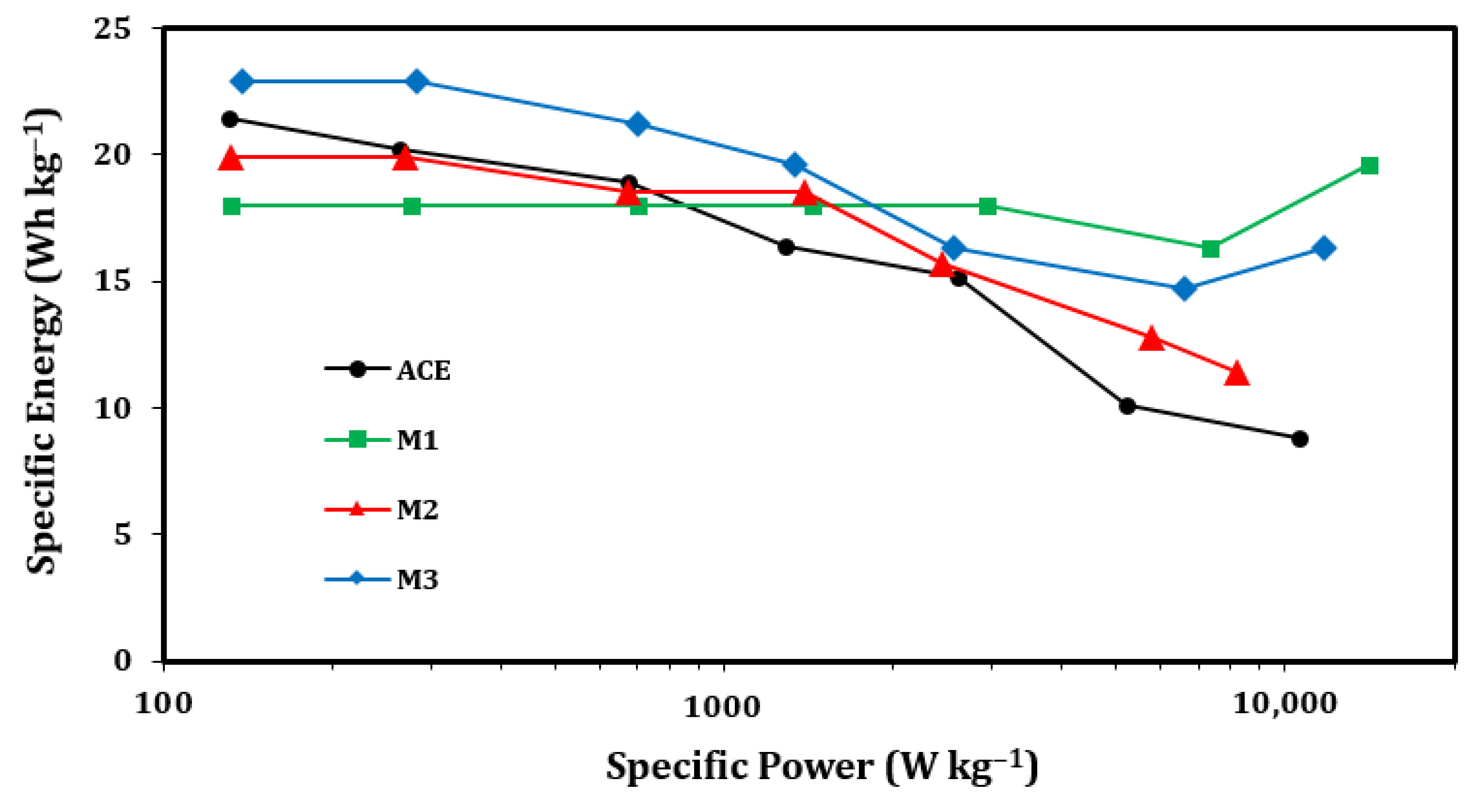
| Description | Label | Composition (wt.%) |
|---|---|---|
| Conventional AC electrode | ACE | AC/Super P (90/5) |
| GO added | M1 | AC/Super P/GO (90/2.5/2.5) |
| GO and rGO added (1:1) | M2 | AC/Super P/GO/rGO (90/2.5/1.25/1.25) |
| rGO added | M3 | AC/Super P/rGO (90/2.5/2.5) |
| Sample | Peak | Binding Energy (eV) | Atomic Conc. (%) | C/O Ratio |
|---|---|---|---|---|
| Pristine graphite (PG) | C 1s | 284.61 | 99.5 | 199 |
| O 1s | 533.51 | 0.5 | ||
| Graphene oxide (GO) | C 1s C-C/C=C | 284.59 | 27.5 | 2.19 |
| C 1s C-O | 286.61 | 35.1 | ||
| C 1s C=O | 288.37 | 5.4 | ||
| O 1s | 532.35 | 31.0 | ||
| Reduced graphene oxide (rGO) | C 1s C-C/C=C | 284.91 | 56.3 | 4.65 |
| C 1s C-O | 286.33 | 9.7 | ||
| C 1s C=O | 288.18 | 11.7 | ||
| C 1s O-C=O | 291.05 | 2.8 | ||
| O 1s | 531.59, 533 | 17.3 |
| Label | Slope (10−5) | Y-Intercept | R2 |
|---|---|---|---|
| ACE | −4.28 | 10.98 | 0.027 |
| M1 | −29.4 | 12.04 | 0.688 |
| M2 | −40.8 | 12.60 | 0.782 |
| M3 | −23.1 | 13.42 | 0.526 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strimaitis, J.; Danquah, S.A.; Denize, C.F.; Pradhan, S.K.; Bahoura, M. The Effects of Graphene Oxide and Reduced Graphene Oxide Conductive Additives on Activated Carbon Supercapacitors. Processes 2022, 10, 2190. https://doi.org/10.3390/pr10112190
Strimaitis J, Danquah SA, Denize CF, Pradhan SK, Bahoura M. The Effects of Graphene Oxide and Reduced Graphene Oxide Conductive Additives on Activated Carbon Supercapacitors. Processes. 2022; 10(11):2190. https://doi.org/10.3390/pr10112190
Chicago/Turabian StyleStrimaitis, Jacob, Samuel Adjepong Danquah, Clifford F. Denize, Sangram K. Pradhan, and Messaoud Bahoura. 2022. "The Effects of Graphene Oxide and Reduced Graphene Oxide Conductive Additives on Activated Carbon Supercapacitors" Processes 10, no. 11: 2190. https://doi.org/10.3390/pr10112190
APA StyleStrimaitis, J., Danquah, S. A., Denize, C. F., Pradhan, S. K., & Bahoura, M. (2022). The Effects of Graphene Oxide and Reduced Graphene Oxide Conductive Additives on Activated Carbon Supercapacitors. Processes, 10(11), 2190. https://doi.org/10.3390/pr10112190