Ultrasound-Assisted Preparation of Maillard Reaction Products Derived from Hydrolyzed Soybean Meal with Meaty Flavor in an Oil-In-Water System
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fatty Acids Composition Analysis
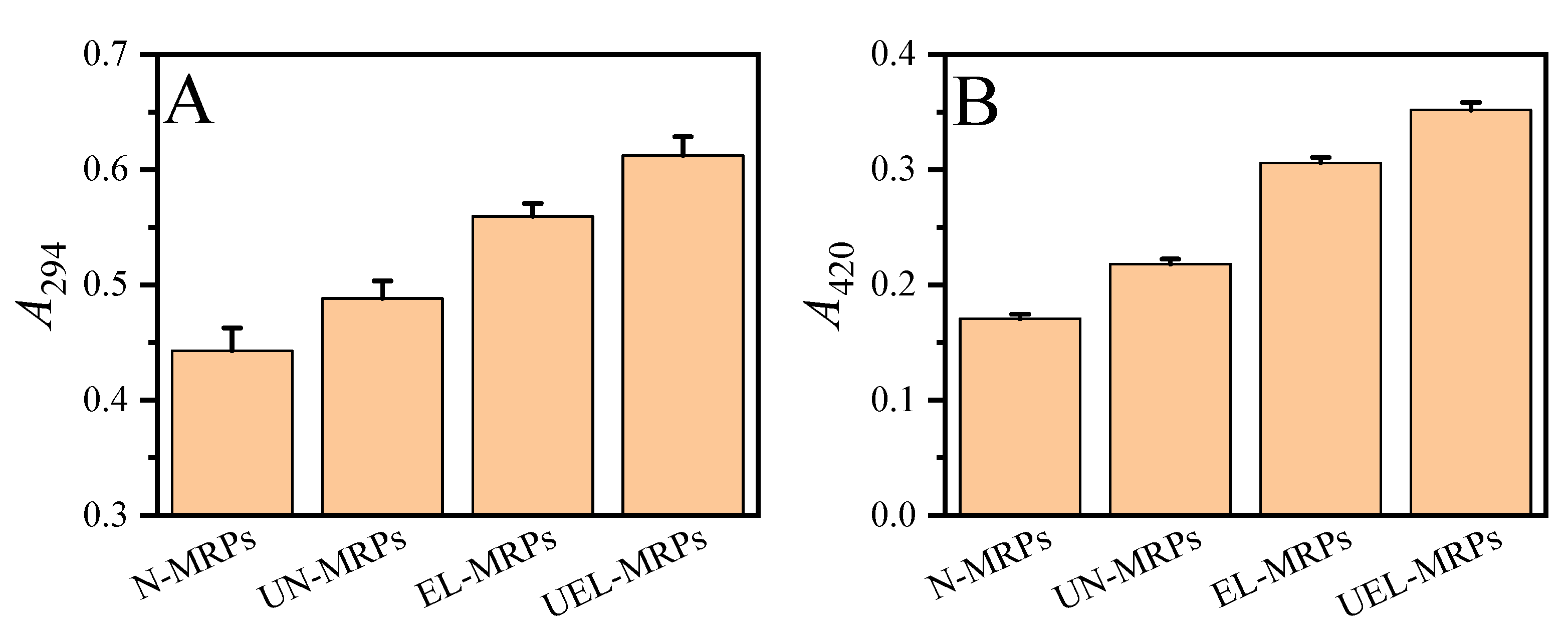
2.2. Browning Intensity of the MRPs
2.3. Changes in Color
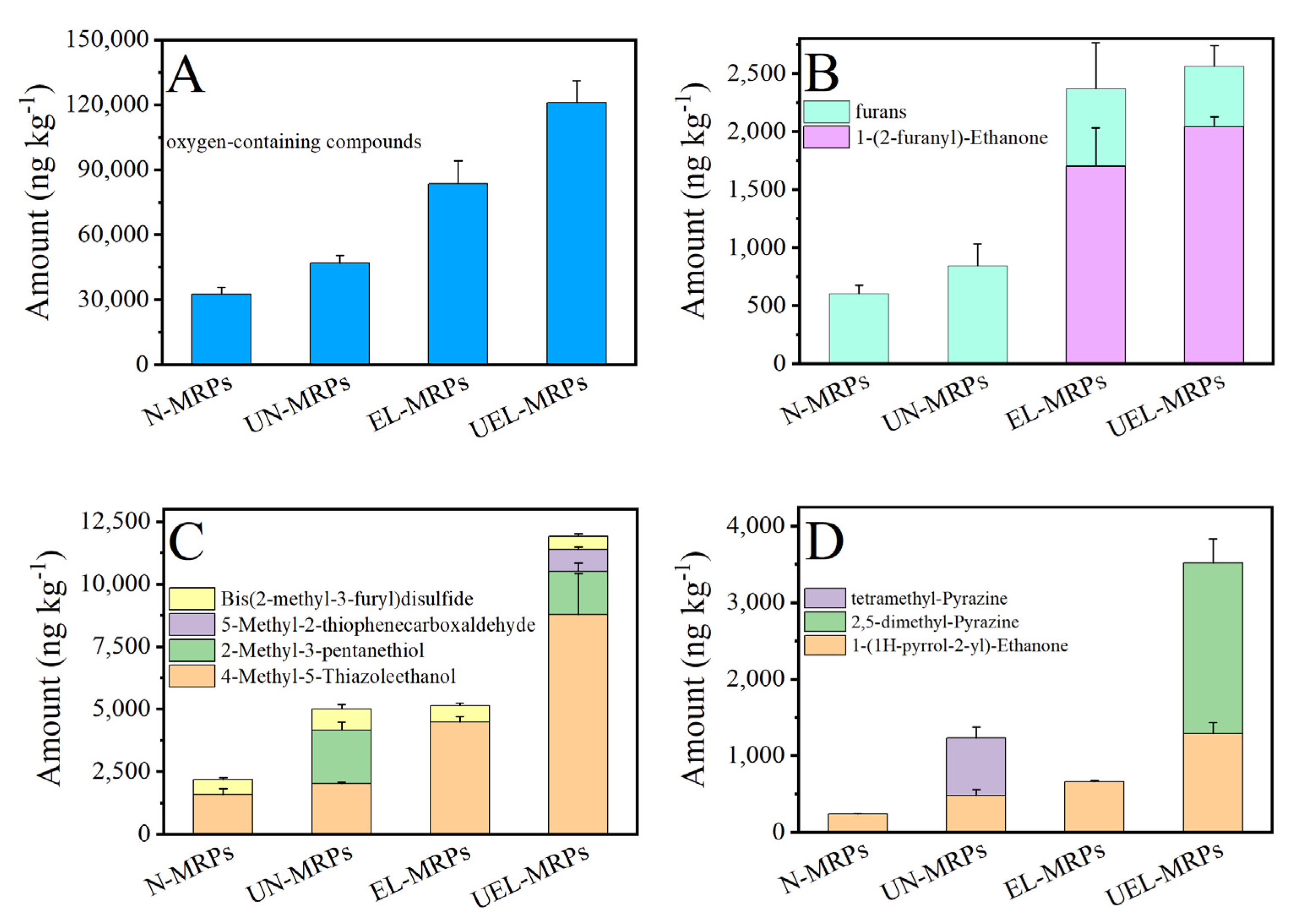
2.4. GC–MS/SPME Analysis of the Volatile Components in the MRPs
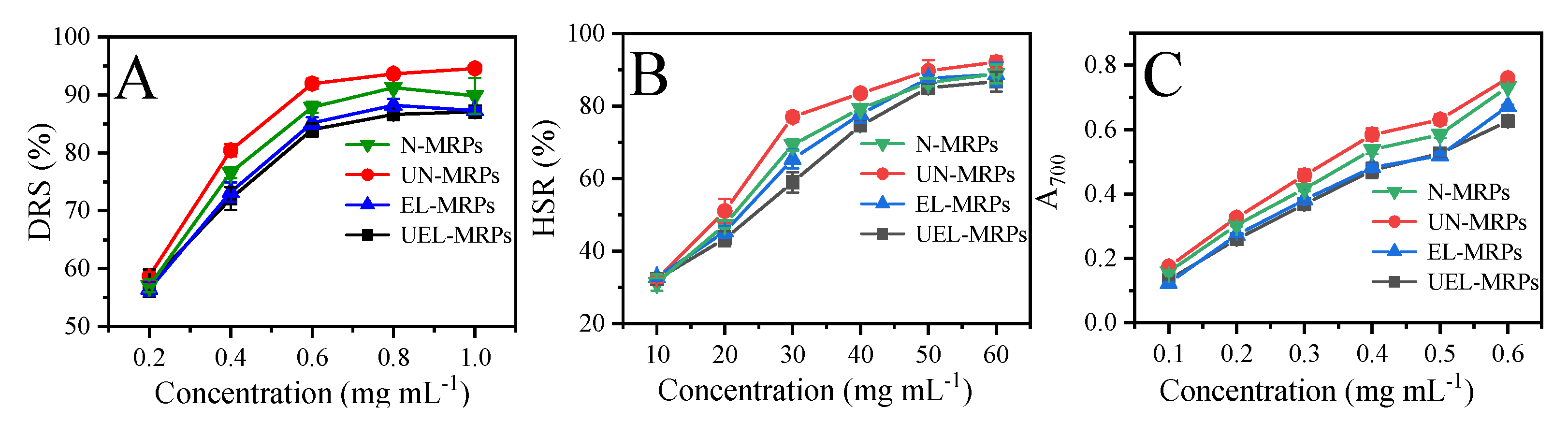
2.5. Sensory Evaluation
2.6. Sensory Evaluation
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Sample Preparation
3.2.1. Preparation of Enzymatic Hydrolyzed Lard
3.2.2. Preparation of Soybean Meal Hydrolysates
3.2.3. Preparation of Maillard Reaction Products
3.3. Analysis Methods
3.3.1. Analysis of the Fatty Acid Composition in Various Lard
3.3.2. Determination of Browning Intensity and Color of MRPs
3.3.3. Determination of DPPH Radical-Scavenging Activity
3.3.4. Determination of Hydroxyl Radical Scavenging Ability
3.3.5. Determination of Ferric Ion Reducing Ability
3.3.6. Analysis of Volatile Compounds by GC–MS/SPME
3.3.7. Descriptive Sensory Analysis of the MRPs
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Siewe, F.B.; Kudre, T.G.; Bettadaiah, B.K.; Narayan, B. Effects of Ultrasound-Assisted Heating on Aroma Profile, Peptide Structure, Peptide Molecular Weight, Antioxidant Activities and Sensory Characteristics of Natural Fish Flavouring. Ultrason. Sonochem. 2020, 65, 105055. [Google Scholar] [CrossRef] [PubMed]
- Habinshuti, I.; Chen, X.; Yu, J.; Mukeshimana, O.; Duhoranimana, E.; Karangwa, E.; Muhoza, B.; Zhang, M.; Xia, S.; Zhang, X. Antimicrobial, Antioxidant and Sensory Properties of Maillard Reaction Products (MRPs) Derived from Sunflower, Soybean and Corn Meal Hydrolysates. LWT 2019, 101, 694–702. [Google Scholar] [CrossRef]
- Xia, B.; Ni, Z.J.; Hu, L.T.; Elam, E.; Thakur, K.; Zhang, J.G.; Wei, Z.J. Development of Meat Flavors in Peony Seed-Derived Maillard Reaction Products with the Addition of Chicken Fat Prepared under Different Conditions. Food Chem. 2021, 363, 130276. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Ye, S.; Wanyan, Z.; Ping, H.; Xu, Z.; He, S.; Cao, X.; Chen, X.; Hu, W.; Wei, Z. Producing Beef Flavors in Hydrolyzed Soybean Meal-Based Maillard Reaction Products Participated with Beef Tallow Hydrolysates. Food Chem. 2022, 378, 132119. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; He, S.; Tang, M.; Zhang, Z.; Zhu, Y.; Sun, H. Antioxidant Activity and Sensory Characteristics of Maillard Reaction Products Derived from Different Peptide Fractions of Soybean Meal Hydrolysate. Food Chem. 2018, 243, 249–257. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, D.; Xu, P.; Geng, Z.; Xiong, G.; Zou, Y. Structural and Antimicrobial Properties of Maillard Reaction Products in Chicken Liver Protein Hydrolysate after Sonication. Food Chem. 2021, 343, 128417. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Y.; Ahmed, Z.; Geng, W.; Tang, W.; Liu, Y.; Jin, H.; Jiang, F.; Wang, J.; Wang, Y. Purification and Identification of Kokumi-Enhancing Peptides from Chicken Protein Hydrolysate. Int. J. Food Sci. Technol. 2019, 54, 2151–2158. [Google Scholar] [CrossRef]
- Wei, C.K.; Ni, Z.J.; Thakur, K.; Liao, A.M.; Huang, J.H.; Wei, Z.J. Aromatic Effects of Immobilized Enzymatic Oxidation of Chicken Fat on Flaxseed (Linum Usitatissimum L.) Derived Maillard Reaction Products. Food Chem. 2020, 306, 125560. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, Q.; Lei, S.; Wu, P.; Fan, G.; Xu, X.; Pan, S. Effects of Lard on the Formation of Volatiles from the Maillard Reaction of Cysteine with Xylose. J. Sci. Food Agric. 2011, 91, 2241–2246. [Google Scholar] [CrossRef]
- Ma, X.; Zhan, P.; Tian, H.; Wei, Z.; Wang, P. Effects of Different Enzymatic Hydrolyses of Mutton Tallow on the Aroma Characteristics of the Maillard Reaction of Xylose–Cysteine Based on GC-MS, E-Nose, and Statistical Analysis. Eur. J. Lipid Sci. Technol. 2020, 122, 1900212. [Google Scholar] [CrossRef]
- Song, S.; Tang, Q.; Hayat, K.; Karangwa, E.; Zhang, X.; Xiao, Z. Effect of Enzymatic Hydrolysis with Subsequent Mild Thermal Oxidation of Tallow on Precursor Formation and Sensory Profiles of Beef Flavours Assessed by Partial Least Squares Regression. Meat Sci. 2014, 96, 1191–1200. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Tang, Q.; Fan, L.; Xu, X.; Song, Z.; Hayat, K.; Feng, T.; Wang, Y. Identification of Pork Flavour Precursors from Enzyme-Treated Lard Using Maillard Model System Assessed by GC–MS and Partial Least Squares Regression. Meat Sci. 2017, 124, 15–24. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, J.; Zhang, L.; Du, R.; Cao, C.; Wang, M.; Acree, T.; Sun, B. Aromatic Effect of Fat and Oxidized Fat on a Meat-like Model Reaction System of Cysteine and Glucose. Flavour Fragr. J. 2015, 30, 320–329. [Google Scholar] [CrossRef]
- Yu, H.; Zhong, Q.; Liu, Y.; Guo, Y.; Xie, Y.; Zhou, W.; Yao, W. Recent Advances of Ultrasound-Assisted Maillard Reaction. Ultrason. Sonochem. 2020, 64, 104844. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.Y.; Liu, W.; Zhou, Y.J.; Ren, H.; Li, M.Y.; Liu, Y. Effects of Ultrasonic Treatment on Maillard Reaction and Product Characteristics of Enzymatic Hydrolysate Derived from Mussel Meat. J. Food Process Eng. 2019, 42, 1–11. [Google Scholar] [CrossRef]
- Yu, H.; Seow, Y.-X.; Ong, P.K.C.; Zhou, W. Effects of High-Intensity Ultrasound and Oil Type on the Maillard Reaction of d-Glucose and Glycine in Oil-in-Water Systems. npj Sci. Food 2018, 2, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanic-Vucinic, D.; Prodic, I.; Apostolovic, D.; Nikolic, M.; Cirkovic Velickovic, T. Structure and Antioxidant Activity of β-Lactoglobulin-Glycoconjugates Obtained by High-Intensity-Ultrasound-Induced Maillard Reaction in Aqueous Model Systems under Neutral Conditions. Food Chem. 2013, 138, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Trushenski, J.T.; Rombenso, A.N.; Page, M.; Jirsa, D.; Drawbridge, M. Traditional and Fermented Soybean Meals as Ingredients in Feeds for White Seabass and Yellowtail Jack. N. Am. J. Aquac. 2014, 76, 312–322. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Li, J.; Xia, C.; Li, J. Evaluation Performance of Soybean Meal and Peanut Meal Blends-Based Wood Adhesive. Polym. Test. 2022, 109, 107543. [Google Scholar] [CrossRef]
- Huang, A.; Sun, L.; Lin, F.; Guo, J.; Jiang, J.; Shen, B.; Chen, J. Medical Image Recognition Technology in the Effect of Substituting Soybean Meal for Fish Meal on the Diversity of Intestinal Microflora in Channa Argus. J. Healthc. Eng. 2021, 2021, 5269169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Elfalleh, W.; He, S.; Tang, M.; Zhao, J.; Wu, Z.; Wang, J.; Sun, H. Heating and Cysteine Effect on Physicochemical and Flavor Properties of Soybean Peptide Maillard Reaction Products. Int. J. Biol. Macromol. 2018, 120, 2137–2146. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Seow, Y.X.; Ong, P.K.C.; Zhou, W. Effects of Ultrasonic Processing and Oil Type on Maillard Reaction of D-Glucose and L-Alanine in Oil-in-Water Systems. Food Bioprocess Technol. 2019, 12, 325–337. [Google Scholar] [CrossRef]
- Chen, K.; Yang, Q.; Hong, H.; Feng, L.; Liu, J.; Luo, Y. Physicochemical and Functional Properties of Maillard Reaction Products Derived from Cod (Gadus Morhua L.) Skin Collagen Peptides and Xylose. Food Chem. 2020, 333, 127489. [Google Scholar] [CrossRef] [PubMed]
- Perusko, M.; Al-Hanish, A.; Velickovic, T.C.; Stanic-Vucinic, D. Macromolecular Crowding Conditions Enhance Glycation and Oxidation of Whey Proteins in Ultrasound-Induced Maillard Reaction. Food Chem. 2015, 177, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Abdelhedi, O.; Mora, L.; Jemil, I.; Jridi, M.; Toldrá, F.; Nasri, M.; Nasri, R. Effect of Ultrasound Pretreatment and Maillard Reaction on Structure and Antioxidant Properties of Ultrafiltrated Smooth-Hound Viscera Proteins-Sucrose Conjugates. Food Chem. 2017, 230, 507–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karangwa, E.; Zhang, X.; Murekatete, N.; Masamba, K.; Raymond, L.V.; Shabbar, A.; Zhang, Y.; Duhoranimana, E.; Muhoza, B.; Song, S. Effect of Substrate Type on Sensory Characteristics and Antioxidant Capacity of Sunflower Maillard Reaction Products. Eur. Food Res. Technol. 2015, 240, 939–960. [Google Scholar] [CrossRef]
- Karangwa, E.; Raymond, L.V.; Huang, M.; Cheserek, M.J.; Hayat, K.; Savio, N.D.; Amédée, M.; Zhang, X. Sensory Attributes and Antioxidant Capacity of Maillard Reaction Products Derived from Xylose, Cysteine and Sunflower Protein Hydrolysate Model System. Food Res. Int. 2013, 54, 1437–1447. [Google Scholar] [CrossRef]
- Zhang, W.; Leong, S.M.; Zhao, F.; Zhao, F.; Yang, T.; Liu, S. Viscozyme L Pretreatment on Palm Kernels Improved the Aroma of Palm Kernel Oil after Kernel Roasting. Food Res. Int. 2018, 107, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Hu, L.; Xia, B.; Ni, Z.; Elam, E.; Thakur, K.; Zhang, J.; Wei, Z. Effects of Different Sulfur-Containing Substances on the Structural and Flavor Properties of Defatted Sesame Seed Meal Derived Maillard Reaction Products. Food Chem. 2021, 365, 130463. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, T.; Xie, J.; Xiao, Q.; Cheng, J.; Chen, F.; Wang, S. Formation Mechanism of Aroma Compounds in a Glutathione-Glucose Reaction with Fat or Oxidized Fat. Food Chem. 2019, 270, 436–444. [Google Scholar] [CrossRef]
- Lee, S.E.; Chung, H.; Kim, Y.S. Effects of Enzymatic Modification of Wheat Protein on the Formation of Pyrazines and Other Volatile Components in the Maillard Reaction. Food Chem. 2012, 131, 1248–1254. [Google Scholar] [CrossRef]
- Yu, H.; Seow, Y.X.; Ong, P.K.C.; Zhou, W. Effects of High-Intensity Ultrasound on Maillard Reaction in a Model System of d-Xylose and l-Lysine. Ultrason. Sonochem. 2017, 34, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Xu, Y.; Ren, F.; Zhang, H. Characteristics and Antioxidant Activity of Maillard Reaction Products from α-Lactalbumin and 2′-Fucosyllactose. Food Chem. 2020, 316, 126341. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Li, F.; Yu, Q.; Li, D. In Vitro Antioxidant and Cytoprotective Properties of Maillard Reaction Products from Phloridzin-Amino Acid Model Systems. J. Sci. Food Agric. 2018, 98, 590–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jiang, Y.; Guan, H.; Li, F.; Sun-Waterhouse, D.; Chen, Y.; Li, D. Enhancing the Antioxidative Effects of Foods Containing Rutin and α-Amino Acids via the Maillard Reaction: A Model Study Focusing on Rutin-Lysine System. J. Food Biochem. 2020, 44, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cui, Y.; Su, D.; Bin, T.; Yuan, Y.; He, S. Process Optimization and Anti-Oxidative Activity of Peanut Meal Maillard Reaction Products. LWT 2018, 97, 573–580. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Ren, Z.; Wang, R.; Zhang, Y.; Li, J.; Ma, F.; Liu, X. Physicochemical Properties and Antioxidant Activity of Maillard Reaction Products Derived from Dioscorea Opposita Polysaccharides. LWT 2021, 149, 111833. [Google Scholar] [CrossRef]

| Fatty Acids | Percentage (%) | ||
|---|---|---|---|
| FL | EL | UEL | |
| Saturated fatty acid | |||
| C10:0 decanoic acid | 0.020 ± 0.001 a | 0.021 ± 0.000 a | 0.017 ± 0.000 b |
| C12:0 lauric acid | 0.076 ± 0.000 a | 0.077 ± 0.003 a | 0.075 ± 0.000 a |
| C14:0 myristic acid | 0.898 ± 0.005 a | 0.906 ± 0.005 a | 0.904 ± 0.008 a |
| C15:0 pentadecanoic acid | 0.041 ± 0.005 a | 0.040 ± 0.002 a | 0.041 ± 0.003 a |
| C16:0 palmitic acid | 36.271 ± 0.090 b | 36.861 ± 0.16 a | 36.958 ± 0.142 a |
| C17:0 margaric acid | 0.129 ± 0.012 a | 0.130 ± 0.003 a | 0.136 ± 0.006 a |
| C18:0 stearic acid | 8.150 ± 0.020 c | 8.345 ± 0.026 b | 8.402 ± 0.005 a |
| C21:0 n-heneicosanoic acid | 0.293 ± 0.007 ab | 0.318 ± 0.026 a | 0.279 ± 0.003 b |
| C22:0 behenic acid | 0.137 ± 0.002 a | 0.118 ± 0.000 b | 0.116 ± 0.001 b |
| Total | 46.016 ± 0.123 b | 46.814 ± 0.014 a | 46.928 ± 0.136 a |
| Unsaturated fatty acid | |||
| C14:1 myristoleic acid | 0.017 ± 0.001 a | 0.017 ± 0.000 a | 0.018 ± 0.005 a |
| C16:1 palmitoleic acid | 1.066 ± 0.026 a | 1.036 ± 0.001 a | 1.046 ± 0.018 a |
| C17:1 heptadecenoic acid | 0.060 ± 0.003 a | 0.056 ± 0.005 a | 0.058 ± 0.003 a |
| C18:1 oleic acid | 37.328 ± 0.103 a | 36.754 ± 0.018 b | 36.722 ± 0.135 b |
| C18:2 linoleic acid | 14.663 ± 0.027 a | 14.441 ± 0.004 b | 14.413 ± 0.009 b |
| C20:1 eicosenoic acid | 0.185 ± 0.005 b | 0.211 ± 0.003 a | 0.164 ± 0.016 c |
| C18:3 α-linolenic acid | 0.633 ± 0.003 a | 0.638 ± 0.015 a | 0.626 ± 0.001 a |
| C20:3 carbonium | 0.033 ± 0.003 a | 0.033 ± 0.000 a | 0.025 ± 0.001 b |
| Total | 53.984 ± 0.123 a | 53.186 ± 0.014 b | 53.072 ± 0.136 b |
| Sample | ΔL* | Δa* | Δb* | ΔE* |
|---|---|---|---|---|
| N-MRPs | −2.61 ± 0.06 a | −0.06 ± 0.02 a | −2.07 ± 0.06 c | 3.33 ± 0.02 c |
| UN-MRPs | −2.68 ± 0.03 a | −0.14 ± 0.02 c | −2.21 ± 0.01 d | 3.47 ± 0.02 b |
| EL-MRPs | −3.42 ± 0.08 b | −0.11 ± 0.02 b | −1.60 ± 0.03 b | 3.78 ± 0.09 a |
| UEL-MRPs | −3.53 ± 0.02 c | −0.08 ± 0.01 ab | −1.53 ± 0.02 a | 3.85 ± 0.02 a |
| No. | Volatile Compounds | 1 KIs | 2 Odors | Relative Concentration [ng kg−1] (Mean ± SD) | |||
|---|---|---|---|---|---|---|---|
| N-MRPs | UN-MRPs | EL-MRPs | UEL-MRPs | ||||
| Aldehydes (7) | 16,325.90 ± 2633.65 b | 23,312.20 ± 2256.92 b | 42,201.86 ± 6487.71 a | 51,094.08 ± 6451.28 a | |||
| 1 | 2-undecenal | 1311 | waxy | ---- | ---- | ---- | 1526.60 ± 439.78 |
| 2 | nonanal | 1104 | fatty, citrus | 2544.39 ± 789.48 c | 3150.21 ± 1627.73 c | 13,575.11 ± 744.44 a | 8164.12 ± 1311.46 b |
| 3 | octanal | 92 | fatty, citrus, honey | ---- | 628.01 ± 58.54 c | 5827.26 ± 424.38 a | 2740.49 ± 624.90 b |
| 4 | benzaldehyde | 982 | almond | 13,383.74 ± 2478.88 b | 18,797.82 ± 385.02 b | 21,529.94 ± 5270.65 a | 38,662.87 ± 8787.69 a |
| 5 | 4-methoxy-benzaldehyde | 1171 | hawthorn | ---- | 214.49 ± 59.06 | ---- | ---- |
| 6 | decanal | 1204 | fatty, sweet orange | 397.76 ± 65.39 b | 521.67 ± 154.22 b | 868.86 ± 65.75 a | ---- |
| 7 | (E)-2-octenal | 1013 | ---- | ---- | ---- | 400.68 ± 178.09 | ---- |
| Ketones (4) | 500.64 ± 33.39 b | 592.36 ± 40.27 b | 635.48 ± 117.50 b | 4302.12 ± 1105.77 a | |||
| 8 | 2H-pyran-2,6(3H)-dione | 1098 | ---- | 91.11 ± 20.19 b | 135.62 ± 13.81 b | 140.31 ± 40.39 b | 386.13 ± 76.23 a |
| 9 | acetoin | 717 | buttery | 112.13 ± 23.42 c | 106.88 ± 20.41 c | 495.16 ± 77.50 b | 756.99 ± 160.41 a |
| 10 | 1-hydroxy-2-propanone | 698 | ---- | 297.40 ± 9.22 | ---- | ---- | 3159.00 ± 875.38 |
| 11 | 6-methyl-5-hepten-2-one | 938 | fatty, green, citrus-like | ---- | 349.86 ± 48.08 | ---- | ---- |
| Alcohols (11) | 4696.10 ± 431.42 d | 10,404.52 ± 1020.19 c | 13,917.84 ± 1352.76 b | 37,637.68 ± 2448.57 a | |||
| 12 | 2-furanmethanol | 885 | burnt, caramel | 951.36 ± 27.19 b | 1242.79 ± 330.92 b | 2623.38 ± 348.82 a | 1000.58 ± 152.83 b |
| 13 | 1-pentanol | 761 | ---- | ---- | 621.58 ± 175.56 c | 4269.34 ± 411.52 b | 9280.11 ± 316.91 a |
| 14 | 1-octen-3-ol | 969 | mushroom | ---- | ---- | ---- | 7635.86 ± 1535.68 |
| 15 | 2-methyl-3-pentanethiol | 793 | ---- | ---- | 2140.45 ± 320.99 | ---- | 1730.77 ± 342.90 |
| 16 | 1-hexanol | 860 | green, fruity | 1106.16 ± 39.61 c | 2882.99 ± 293.59 b | 1959.74 ± 285.49 bc | 6247.56 ± 933.66 a |
| 17 | 1,4-butanediol | 904 | ---- | 112.70 ± 27.90 | ---- | ---- | 424.19 ± 15.77 |
| 18 | benzyl alcohol | 1036 | fruity | ---- | ---- | 1134.57 ± 175.23 | ---- |
| 19 | phenylethyl alcohol | 1136 | roses | 529.12 ± 17.16 | 649.71 ± 92.40 | ---- | ---- |
| 20 | 1-heptanol | 960 | weak alcoholic | ---- | ---- | ---- | 4115.44 ± 414.03 |
| 21 | maltol | 1063 | caramel | 1559.63 ± 345.82 c | 2331.50 ± 259.26 c | 3930.81 ± 601.33 b | 7203.18 ± 538.75 a |
| 22 | 1-dodecanol | 1457 | fatty | 437.12 ± 145.13 | 535.49 ± 89.77 | ---- | ---- |
| Esters (3) | 1413.30 ± 226.14 c | 1755.50 ± 99.63 bc | 2245.81 ± 515.19 mb | 5204.68 ± 231.95 a | |||
| 23 | butyrolactone | 825 | ---- | 1003.15 ± 223.50 c | 1192.48 ± 212.87 c | 2245.81 ± 630.98 b | 3749.90 ± 193.20 a |
| 24 | 5-ethyldihydro-2(3H)-furanone | 986 | caramel | ---- | ---- | ---- | 573.71 ± 44.52 |
| 25 | hexadecanoic acid, methyl ester | 1878 | ---- | 410.15 ± 12.07 b | 563.03 ± 154.90 b | ---- | 881.07 ± 9.14 a |
| Acids (9) | 8519.72 ± 535.84 b | 10,217.13 ± 741.44 b | 15,040.33 ± 997.20 a | 14,338.67 ± 1294.21 a | |||
| 26 | isovaleric acid | 811 | rancid | 5806.91 ± 786.41 b | 5495.16 ± 592.05 b | 8090.93 ± 853.77 a | 7441.90 ± 54.91 a |
| 27 | n-decanoic acid | 1372 | fatty, rancid | ---- | 378.11 ± 26.06 | 721.44 ± 142.74 | ---- |
| 28 | hexanoic acid | 974 | fatty, waxy, | 1480.04 ± 399.09 c | 1781.70 ± 230.72 bc | 2802.08 ± 545.34 a | 2394.52 ± 288.82 ab |
| 29 | octanoic acid | 1173 | waxy, fatty | 476.11 ± 97.97 c | 1156.04 ± 155.08 b | 2114.29 ± 498.28 a | 2560.38 ± 451.35 a |
| 30 | nonanoic acid | 1272 | ---- | 264.56 ± 8.58 c | 378.17 ± 62.92 b | 461.28 ± 40.42 a | 485.55 ± 17.18 a |
| 31 | heptanoic acid | 1073 | waxy, fruity, fatty | 226.64 ± 24.61 c | 421.16 ± 68.00 b | 538.76 ± 96.81 b | 673.93 ± 65.34 a |
| 32 | pentanoic acid | 875 | ---- | 139.37 ± 23.12 | 606.79 ± 71.25 | ---- | ---- |
| 33 | butanoic acid | 811 | rancid | ---- | ---- | 311.54 ± 20.75 | ---- |
| 34 | pentadecanoic acid | 1869 | waxy | 126.09 ± 34.85 | ---- | ---- | ---- |
| Pyrazines (2) | ---- | 756.89 ± 144.74 | ---- | 2235.33 ± 311.44 | |||
| 35 | 2,5-dimethyl-pyrazine | 894 | Cocoa, roasted, nutty | ---- | ---- | ---- | 2235.33 ± 311.44 |
| 36 | tetramethyl-pyrazine | nutty, chocolate, coffee | ---- | 756.89 ± 144.74 | ---- | ---- | |
| Furans (2) | 600.96 ± 73.22 b | 842.49 ± 191.62 c | 2369.50 ± 398.75 a | 2565.21 ± 176.92 a | |||
| 37 | bis(2-methyl-3-furyl) disulfide | 1745 | roasty, meat, sulfur | 600.96 ± 73.22 b | 842.49 ± 191.62 a | 666.67 ± 91.68 ab | 524.40 ± 94.07 b |
| 38 | 1-(2-furanyl)-Ethanone | 878 | almond, nut, roasted | ---- | ---- | 1702.83 ± 329.22 | 2040.81 ± 89.87 |
| Hydrocarbons (3) | ---- | 678.87 ± 208.19 ab | 1047.89 ± 327.45 a | 314.59 ± 50.54 b | |||
| 39 | n-hexane | 618 | gasoline | ---- | ---- | ---- | 314.59 ± 50.54 |
| 40 | nonadecane | 1910 | ---- | ---- | 678.87 ± 208.19 | ---- | ---- |
| 41 | pentadecane | 1512 | ---- | ---- | ---- | 1047.89 ± 327.45 | ---- |
| Phenols (4) | 1094.01 ± 59.50 d | 2181.28 ± 357.30 c | 6203.81 ± 445.34 b | 7909.26 ± 221.45 a | |||
| 42 | butylated hydroxytoluene | 1668 | ---- | ---- | ---- | 3796.72 ± 348.50 | 3963.23 ± 502.73 |
| 43 | phenol | 901 | ---- | 395.39 ± 30.41 c | 378.72 ± 92.58 c | 636.09 ± 131.79 b | 1090.62 ± 146.61 a |
| 44 | p-cresol | 1014 | ---- | ---- | 526.95 ± 189.71 | ---- | 695.62 ± 166.81 |
| 45 | 2-methoxy-phenol | 1090 | ---- | 698.62 ± 65.46 d | 1275.61 ± 247.70 c | 1770.99 ± 41.55 b | 2159.78 ± 92.60 a |
| Thiazoles (1) | |||||||
| 46 | 4-methyl-5-thiazoleethanol | 1264 | meaty, roasted | 1582.85 ± 236.87 c | 2018.86 ± 74.48 c | 4492.16 ± 215.17 b | 8787.13 ± 1646.45 a |
| Ethers (1) | |||||||
| 47 | 3-tert-butyl-4-hydroxyanisole | 1417 | ---- | ---- | ---- | 752.29 ± 76.05 | 742.80 ± 18.10 |
| Pyrroles (1) | |||||||
| 48 | 1-(1H-pyrrol-2-yl)-ethanone | 1035 | walnuts, toast | 237.32 ± 5.26 d | 477.30 ± 83.31 c | 665.75 ± 10.75 b | 1288.37 ± 146.92 a |
| Thiophenes (1) | |||||||
| 49 | 5-methyl-2-thiophenecarboxaldehyde | 1072 | almond, fruity, nutty | ---- | ---- | ---- | 873.56 ± 95.92 |
| Sensory Indicators | Judging Controls | Scoring Criteria/Point |
|---|---|---|
| Off-flavor | The unaccepted flavor of rotten eggs, prepared by putting broken eggs (100 g) at 50 °C for 7 days, was used as odor intensity evaluation. | Strong odor: 0–2 |
| Medium odor: 2–5 | ||
| Lighter odor: 5–7 | ||
| Odorless: 7–10 | ||
| Meaty | Take certain pork lean meat, cut into 2.5 cm cubes, cook in water for 2 h, and then use as a meat flavor evaluation control. | Strong odor: 7–10 |
| Umami | The umami used sodium glutamate solution (1%, w/v) as the umami note. | Medium odor: 5–7 |
| Salty | Salty taste is the taste of 0.5% (w/v) sodium chloride solution. | Lighter odor: 2–5 |
| Total acceptance | Evaluation based on meaty, umami, salty, and off-flavor. | Odorless: 0–2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Y.; Dai, S.; Zhang, H.; He, S.; Hu, W.; Cao, X.; Wei, Z. Ultrasound-Assisted Preparation of Maillard Reaction Products Derived from Hydrolyzed Soybean Meal with Meaty Flavor in an Oil-In-Water System. Molecules 2022, 27, 7236. https://doi.org/10.3390/molecules27217236
Ye Y, Dai S, Zhang H, He S, Hu W, Cao X, Wei Z. Ultrasound-Assisted Preparation of Maillard Reaction Products Derived from Hydrolyzed Soybean Meal with Meaty Flavor in an Oil-In-Water System. Molecules. 2022; 27(21):7236. https://doi.org/10.3390/molecules27217236
Chicago/Turabian StyleYe, Yongkang, Shengquan Dai, Hongyan Zhang, Shudong He, Wanwan Hu, Xiaodong Cao, and Zhaojun Wei. 2022. "Ultrasound-Assisted Preparation of Maillard Reaction Products Derived from Hydrolyzed Soybean Meal with Meaty Flavor in an Oil-In-Water System" Molecules 27, no. 21: 7236. https://doi.org/10.3390/molecules27217236
APA StyleYe, Y., Dai, S., Zhang, H., He, S., Hu, W., Cao, X., & Wei, Z. (2022). Ultrasound-Assisted Preparation of Maillard Reaction Products Derived from Hydrolyzed Soybean Meal with Meaty Flavor in an Oil-In-Water System. Molecules, 27(21), 7236. https://doi.org/10.3390/molecules27217236








