Abstract
Background: Omega-3 fatty acids are essential fatty acids that the body cannot create itself; thus, they must be obtained from functional foods. Therefore, the food processing industries are becoming more interested in the production of omega-3 enriched food products, as consumers and healthcare organizations are increasingly demanding functional foods with minimal fatty acid loss and higher oxidative stability. Moreover, the stability of long-chain, polyunsaturated fatty acids in functional foods is a major challenge for the food processing industries. Therefore, the spray drying method was used to prepare spray-dried microcapsules (SDMs) with the minimum loss of, and more stable, fatty acids. Methods: In this study, emulsion blends of chia seed oil (CSO, 50%) and fish oil (FO, 50%) were spray-dried using varied operating conditions, including the inlet air temperature (IAT) (125, 140, 155, 170, and 185 °C), wall material (WM) (5, 10, 15, 20, and 25%), pump speed (PS) (3, 4, 5, 6, and 7 mL/min), and needle speed (NS) (3, 5, 7, 9, and 11 S). Results: The maximum loss of ALA in the spray-dried microcapsules (SDMs) was observed (9.90 ± 0.40%) at 170 °C, and the minimum loss was 4.18 ± 0.20% in run order 9. A similar trend was observed in the maximum retention loss of EPA and DHA (9.71 ± 0.39% and 9.77 ± 0.39%) at a high temperature of 170 °C, while the minimum losses of EPA and DHA were observed in run order 9. Furthermore, the maximum peroxide value (PV) of the SDMs was observed at a lower temperature of 140 °C (1.45 ± 0.19 meq O2/kg), and the minimum PV was 1.33 ± 0.16 meq O2/kg. Conclusions: Overall, based on the results, we concluded that the oxidative stability of the SDMs was improved and that it can be used as a fortifying agent in the processing of many food products.
1. Introduction
Over the past decade, scientists have focused their research intentions on balancing the omega-3 (ω-3) and omega-6 (ω-6) fatty acids in the human diet. Healthcare organizations have also issued nutritional recommendations for balancing the essential fatty acids in foods processed at different industrial stages. Moreover, research scientists are also exploring new combinations of omega-3 fatty acid sources to optimize the intake of essential fatty acids through the diet on daily basis. For this purpose, the terrestrial edible seed oils and marine oils are considered excellent sources of omega-3 and omega-6 essential fatty acids [1,2]. Chia (Salvia hispanica L.) seeds belong to the mint family and contain 25% to 35% oil contents. Significantly, chia seed oil (CSO) contains alpha linolenic acid (ALA) and linoleic acid among its fatty acid composition. CSO also possesses natural antioxidants such as tocopherols, bioactive compounds, and a wide range of phytonutrients [3,4,5]. On the other hand, fish oil (FO) is naturally abundant in very-long-chain fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Previously, many research studies have reported that FO contains EPA in the range of 16–18% and up to 12% DHA, respectively, depending upon the fish species and growing conditions. From functional, nutraceutical, and health points of view, the ALA-, EPA-, and DHA-enriched foods can reduce the risk of inflammation, skin infections, diabetes, obesity, mental disorders, cardiovascular diseases, and certain types of cancer [6,7,8]. It is noteworthy that the blend of CSO and FO has been recognized as a rich source of essential fatty acids, mainly EPA, DHA, and ALA, among which the long-chain polyunsaturated fatty acids (LCPUFAs) are of great importance for the human body [9]. These oil compounds could potentially be used in various industries to develop value-added and nutrient-enriched products [10].
The oxidative quality, or stability, of chia, fish oil, and their blend is low due to the presence of high concentrations of LCPUFAs in chia and fish oil. LCPUFAs such as EPA and DHA are rapidly oxidized, closely followed by ALA, during thermal processing and storage [11,12]. Therefore, the limitation of chia and fish oil utilization in the food processing, pharmaceutical, and nutraceutical industries is related to the products’ low stability and strong fishy flavor. The oxidative stability of these oils and their blend can be improved under certain conditions by using the encapsulation method to conceal the off-flavor. In this method, solid particles are coated with various wall materials before being delivered into the environment. This method has the ability to form a barrier between the internal phase and its surroundings in order to prevent undesirable interactions with the food matrix. It is a useful method for improving the natural phenolic compounds and stability of the fatty acid profiles of food items. The process of encapsulation not only increases the bioavailability of oils and their blends but also improves their functionality in functional food products [13,14].
There are many methods of encapsulation available in the food processing industries, but spray drying is the method most commonly used to prepare spray-dried microcapsules (SDMs). This method is widely available, cheap, easy to operate, low in cost, and very fast in terms of its process [15]. For the production of SDMs, four different factors, namely the inlet air temperature (IAT), pump speed (PS), needle speed (NS), and wall material, affect the rate of production [16,17,18]. In this process, the emulsion is prepared for the microencapsulation of the oils and their blends, in which gum arabic (GA) and maltodextrin (MD) are used as wall materials. The stability of omega-3-rich oil in SDMs is closely related to the composition of the wall material [19,20,21]. Chia and fish oil blends contain a high concentration of LCPUFAs, which can constitute a problem, because unsaturated fatty acids are sensitive to oxidation. Therefore, the spray drying method was introduced to protect the raw oil from oxidative damage (lipid oxidation) and increase its oxidative stability with minimal loss in the fatty acid composition at high and low temperatures. The main objective of this study was to maintain the fatty acid profile of SDMs during the processing and to increase their oxidative quality.
2. Materials and Methods
2.1. Preparation of Raw Material
Chia seeds (Gazala’s Pantry, CS) and fish fillets (Labeo rohita) were procured from a registered super store in Faisalabad, Punjab, Pakistan. Organic and inorganic substances were removed from the surface of the high-quality raw materials. In this research, fish (Labeo rohita) of a length of 1 m and weight of about 2 kg were used to extract the oil.
2.2. Chia Seed Oil Extraction
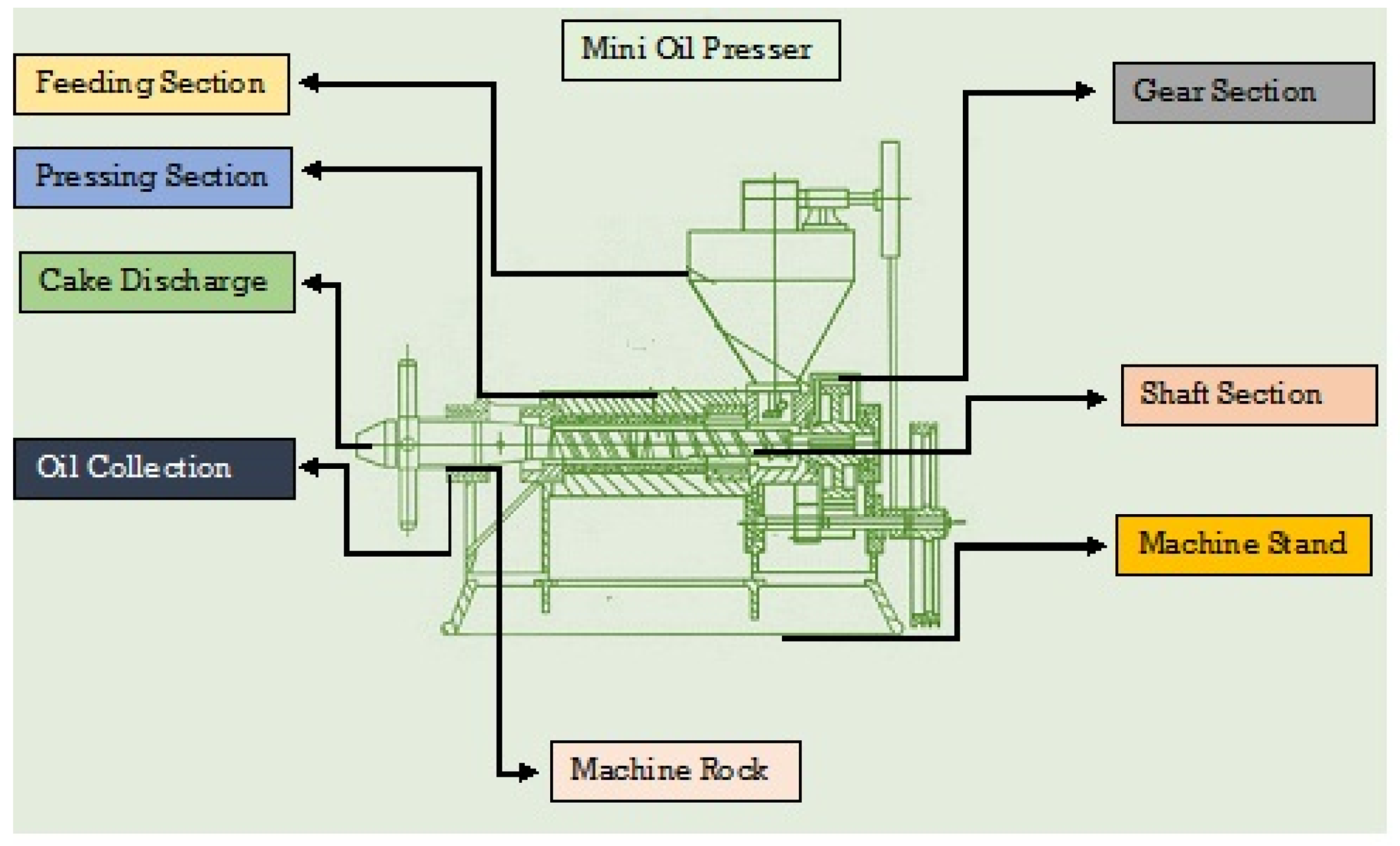
The mini oil presser model 6YL-550 (Wuhan Acme Agro-Tech Co., Ltd, Zhengzhou, China) was used to extract the oil from the chia seeds. The graphic diagram of the mini oil presser below (Figure 1) represents the oil extraction method. In this study, the oil was extracted from chia seeds following the procedure given in the research of Rahim et al. [22]. In this process, the chia seeds were poured into the squeeze chamber through a hopper. During this process, a great deal of friction was produced between the raw materials, aiding in thermal protein denaturation and improving the oil extraction rate. After extracting the chia seed oil (CSO), the sedimentation method was used to remove the suspended solids from the oil. The extracted CSO was purified using a series of processes involving various steps, such as degumming, neutralization, bleaching, and deodorization, in order to obtain a high-quality CSO [23]. These processes were also carried out in laboratory conditions. For the further processing, the CSO was sealed and labeled in screw-cap plastic bottles and kept at room temperature for 24 h.
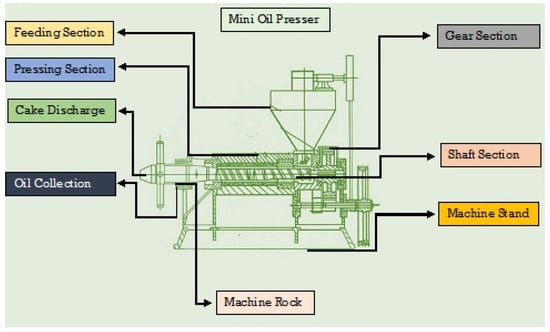
Figure 1.
Schematic diagram of the mini oil presser.
2.3. Fish Oil (FO) Extraction
Oil from ground fish by-product was obtained using the solvent extraction method according to the procedure given in the research of Quero-Jiménez et al. [24], with minor modifications. For the oil extraction, a mixture of solvent (100 mL of methanol) and chloroform (50 mL) was prepared under optimal conditions. A total of 50 g of ground fish sample was dipped in the prepared solution and left overnight (7 P.M.–11 A.M.). After that, the solvent was evaporated at 40 °C using a rotary evaporator. The remaining portion was the oil extracted from the ground fish samples. The extracted FO was purified using a series of processes involving various steps, including degumming, neutralization, bleaching, and deodorization, so as to obtain a high-quality FO. For the further processing, the FO was sealed in screw-cap plastic bottles and placed in a dark room at 25 °C for 24 h.
2.4. Characteristics of the Extracted Oil
2.4.1. Fatty Acid Composition
According to the method of Kostik et al. [25], the methyl esters of fatty acids were determined in the samples using a gas chromatography (GC) device. Furthermore, the ester of the oil was also analyzed by a gas chromatograph device according to the AOCS [26] method no. Ce 1f-96. In this method, the gas flow velocity and temperature were adjusted to 20 to 25 mL per min at 185 °C to determine the fatty acid composition of the oil. For the GC analysis, the sample was mixed thoroughly in n-hexane for 2 min at room temperature. After that, the hexane was separated and moved into the derivation tube. Then, the prepared solution was dried with nitrogen bursts. In screw-cape test tubes, 2 ml of solution was prepared by mixing sodium hydroxide (NaOH) in methanol, and the tubes were tightly sealed. After that, the solution was heated at 90 °C for 5 to 6 min and kept at room temperature until cooling. After cooling, a 2 ml solution of boron trifluoride (BF3) with methanol was mixed well into the heated solution, and it was again heated at 90 °C for half an hour. After heating, the solution was again kept at room temperature for cooling. After being left to cool, the surface of the solution was separated for the GC analysis. Then, 11.6 g/mL of standard methanol and ethanol were GC-injected as sample solvents, showing the broad peak shapes of long-chain fatty acids due to the polarity mismatch between the sample solvents and the stationary phase. The peak shapes of all the fatty acids under investigation were enhanced by using standard isopropanol as the sample solvent. This method was specifically applied to estimate the fatty acid profile of the oil. We calculated the peak value by estimating the concentration percentage.
2.4.2. Free Fatty Acids (FFAs)
The concentration of FFAs in the oils and their blends were evaluated using the Ca 5a–40 method, as explained in AOCS [27]. Typically, 1 g of oil sample was mixed into 25 mL of ethyl alcohol, followed by the dissolution of 50 μL of phenolphthalein indicator. After that, the prepared solution was titrated with 0.01 N of NaOH until a faint, permanent pink color appeared.
2.4.3. Peroxide Value (PV)
The PV was determined in the samples using the official method 965.33 [28]. A Spectrophotometric method was used to determine lipid peroxidation, as presented in the research of Low [29] and Scheme [30]. The conjugated double bonds were measured following the procedure described by Hamilton [31].
2.5. Preparation of Emulsion
For the spray drying process, the oil blend of fish (50%) and CSO (50%) oil was prepared under optimum processing conditions, according to the recent research of Rahim et al. [22]. A hydrated solution of emulsion continuous phase was prepared by dissolving gum arabic (GA) and maltodextrin (MD) in distilled water using a high-speed blender. A total of 500 mL of emulsion was prepared with 20% chia–fish oil blend. After that, soy lecithin was used as an emulsifier and added to the solution at the concentration of 1:1 as a mass ratio for all the chia–fish oil blend formulations, followed by stirring for 20 min. After mixing, the prepared solution was homogenized using a high-speed homogenizer (FSH-2A, Changzhou, China) at 10,000 rpm for 5 min.
2.6. Spray Drying of the Omega-3-Enriched Oil
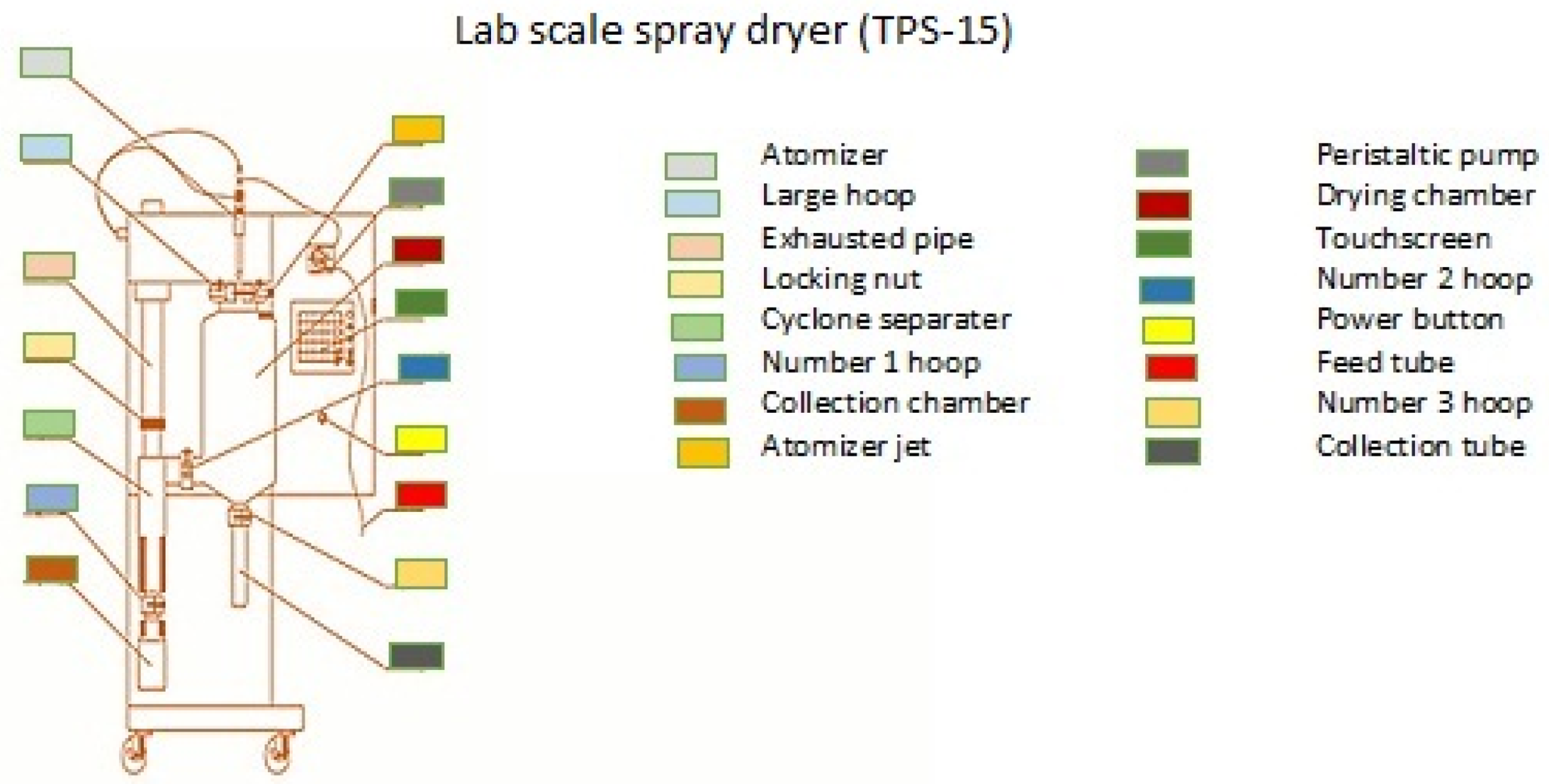
Spray-dried microcapsules (SDM) were obtained by spray drying the prepared liquid emulsion in a mini spray dryer (TS-15 Lab Spray Dryer, Shanghai, China). The schematic diagram of the spray dryer is depicted in Figure 2. The process of the microencapsulation of ALA, EPA, and DHA was selected as the diagnostic index using the method given in the research of the Lavanya et al. [32]. In this study, the spray drying operating factors, including the inlet air temperature (IAT) (125, 140, 155, 170, 185 °C), wall material (WM) (5, 10, 15, 20, 25%), pump speed (PS) (3, 4, 5, 6, 7 mL/min), and needle speed (NS) (3, 5, 7, 9, 11 S), were optimized as described in Table 1 [22].

Figure 2.
Schematic diagram of the spray dryer.

Table 1.
Coded and actual levels of independent variables for the optimization of the fatty acid composition determined using a central composite design (CCD).
2.7. Optimization of Spray-Dried Microcapsules (SDM)
The fatty acid composition, FFAs, and PV of the oil samples extracted from the SDM samples were observed according to the procedures described in the previous methodology description, Section 2.4, respectively.
2.8. Statistical Analysis
The results of this research were calculated using the central composite design (CCD) and response surface methodology, applying the optimized conditions of the spray dryer. The coded levels of the points of the factorial design were −2, −1, 0, +1, +2, and using the design of the experiment techniques, output variables (responses) were optimized with respect to the input variables (factors such as the IAT, WM, PS, and NS). Stat-Ease® and Matlab Software® were used to determine the level of significance and predict the R-squared value so as to determine the validity of the developed model’s calculation of the dependent and independent variables [33].
3. Results
3.1. Fatty Acid Composition of the Extracted Oil
The chia seed oil (CSO) was extracted using the mini oil presser model 6YL-550. The extraction of CSO by the cold-pressed method involves many unit operations, in which oil extraction is a very important step, as it measures the quality and quantity of the extracted oil [34]. In addition, numerous studies aiming to extract good-quality oil from ground fish with a maximum efficiency and yield have been conducted around the world, but the solvent extraction method has been found to be the most effective method. The solvent extraction method produces high-quality FO with a maximum yield or efficiency [29,35].
The fatty acid composition is an important attribute for measuring the quality of chia and fish oil. The results showed that the FO samples contained 3.40 ± 0.18% of EPA, 18.33 ± 0.34% of DHA, 7.1 ± 0.22% of FFAs, and 3.15 ± 0.29 meq O2/kg PV, as presented in Table 2. On the other hand, the CSO samples contained 62.22 ± 0.45% of α-Linolenic acid (ALA), 1.7 ± 0.12% of free fatty acids (FFAs), and 1.65 ± 0.10 meq O2/kg PV, while not containing EPA (eicosapentaenoic acid) or DHA (docosahexaenoic acid). Similarly, the chia and fish oil blend samples contained ALA (31.3 ± 0.64%), EPA (2.34 ± 0.23%), DHA (8.9 ± 0.38%), FFAs (2.1 ± 0.21%), and PV (2.06 ± 0.18 meq O2/kg), respectively, and were within the acceptable range for edible oil.

Table 2.
Fatty acid profile of fish oil and chia seed oil samples.
3.2. Optimization of Spray-Dried Microcapsules
3.2.1. Fatty Acid Composition
The spray-dried microcapsules (SDMs) were optimized using the central composite design (CCD), enabling a total of 30 experiments to be performed in order to obtain the factor effects on the ALA, EPA, and DHA (% of TFA) loss. All runs were randomized in order to avoid systematic errors. The mutual interaction effects between the spray dryer parameters related to the percent loss of ALA, EPA, and DHA for the SDMs were determined by rotating four independent factors, including the IAT (°C), WM (%), PS (mL/min), and NS (S), as described in Table 3, respectively.

Table 3.
Optimization of the spay drying operating conditions for the spray-dried microcapsules.
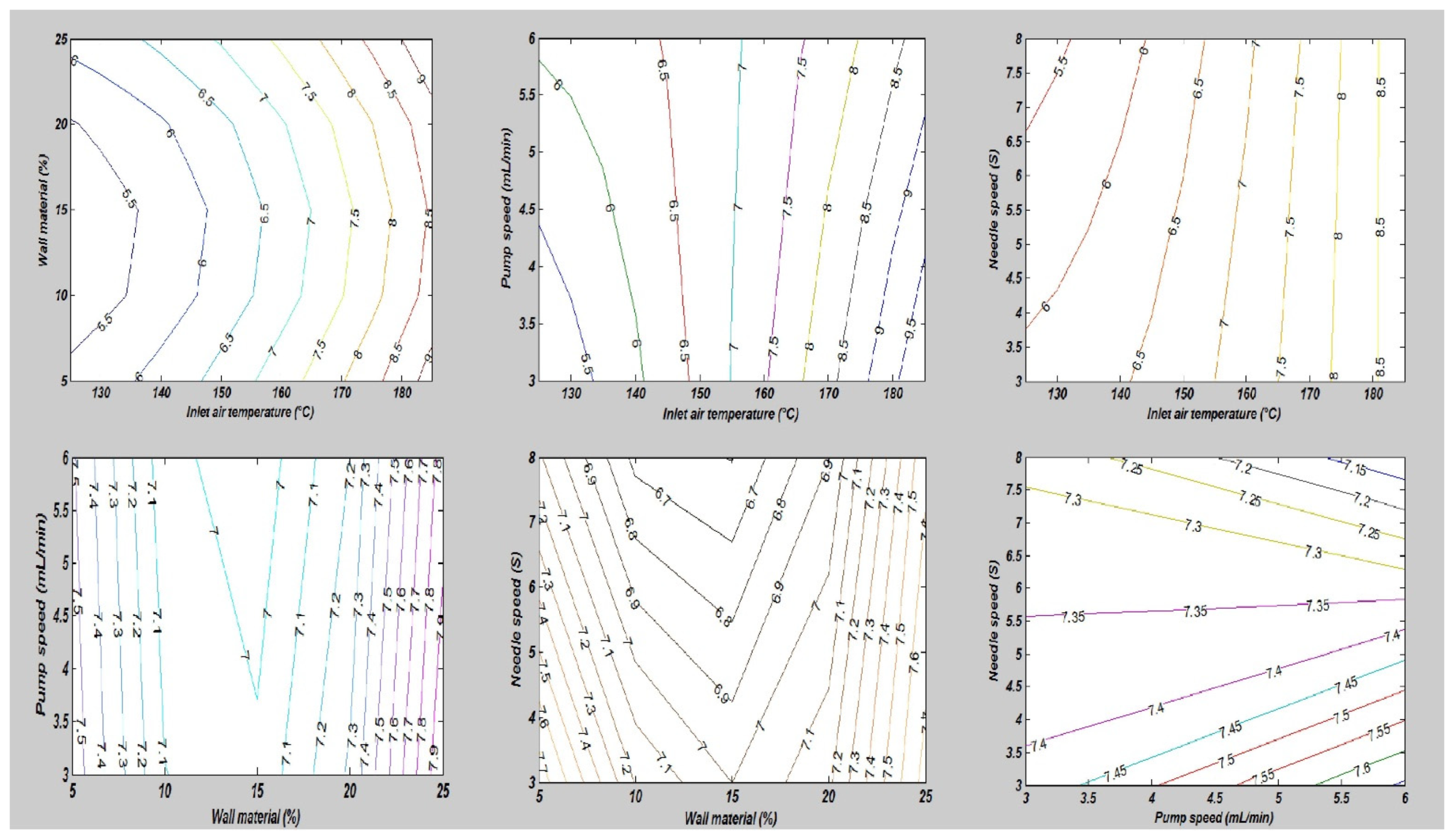
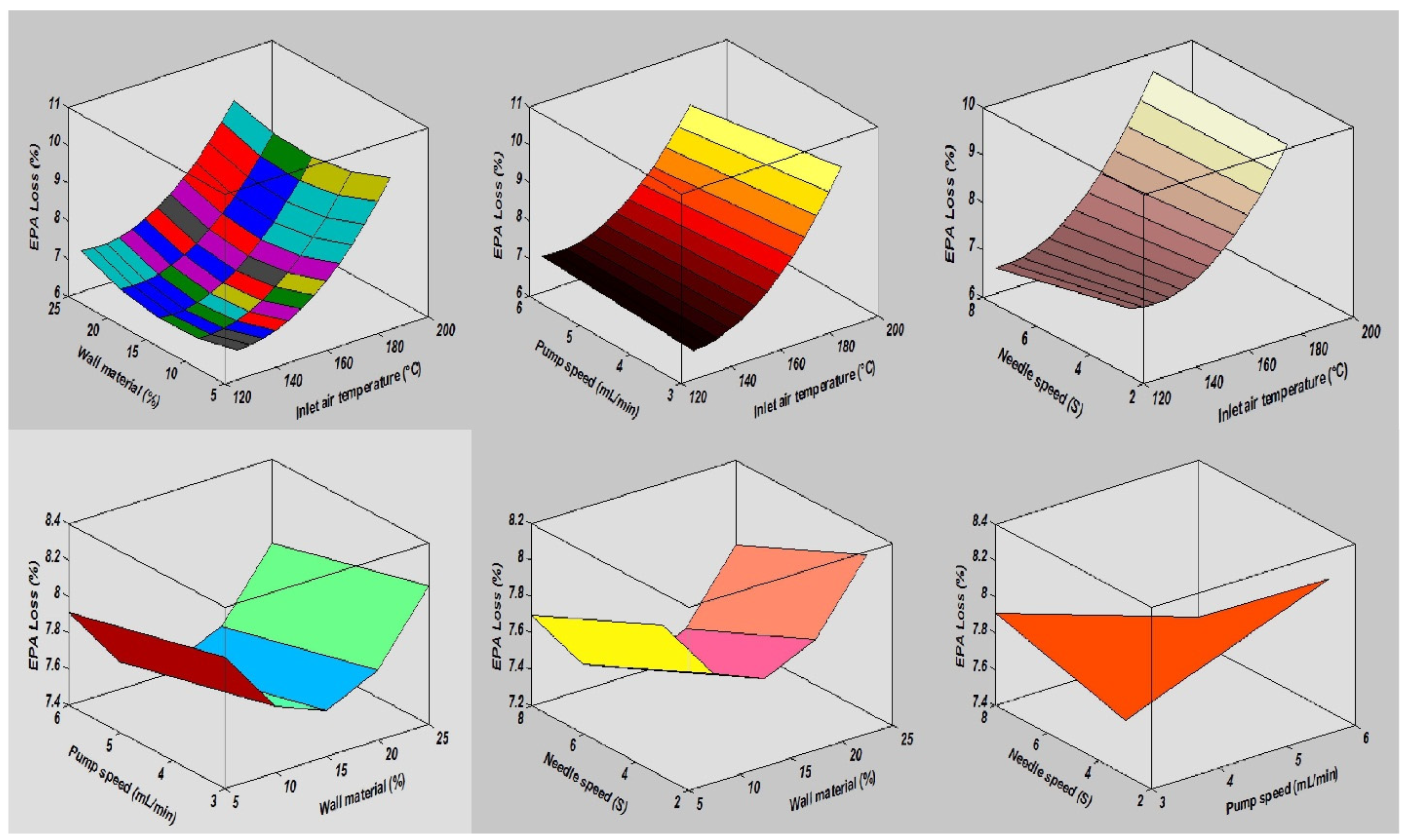
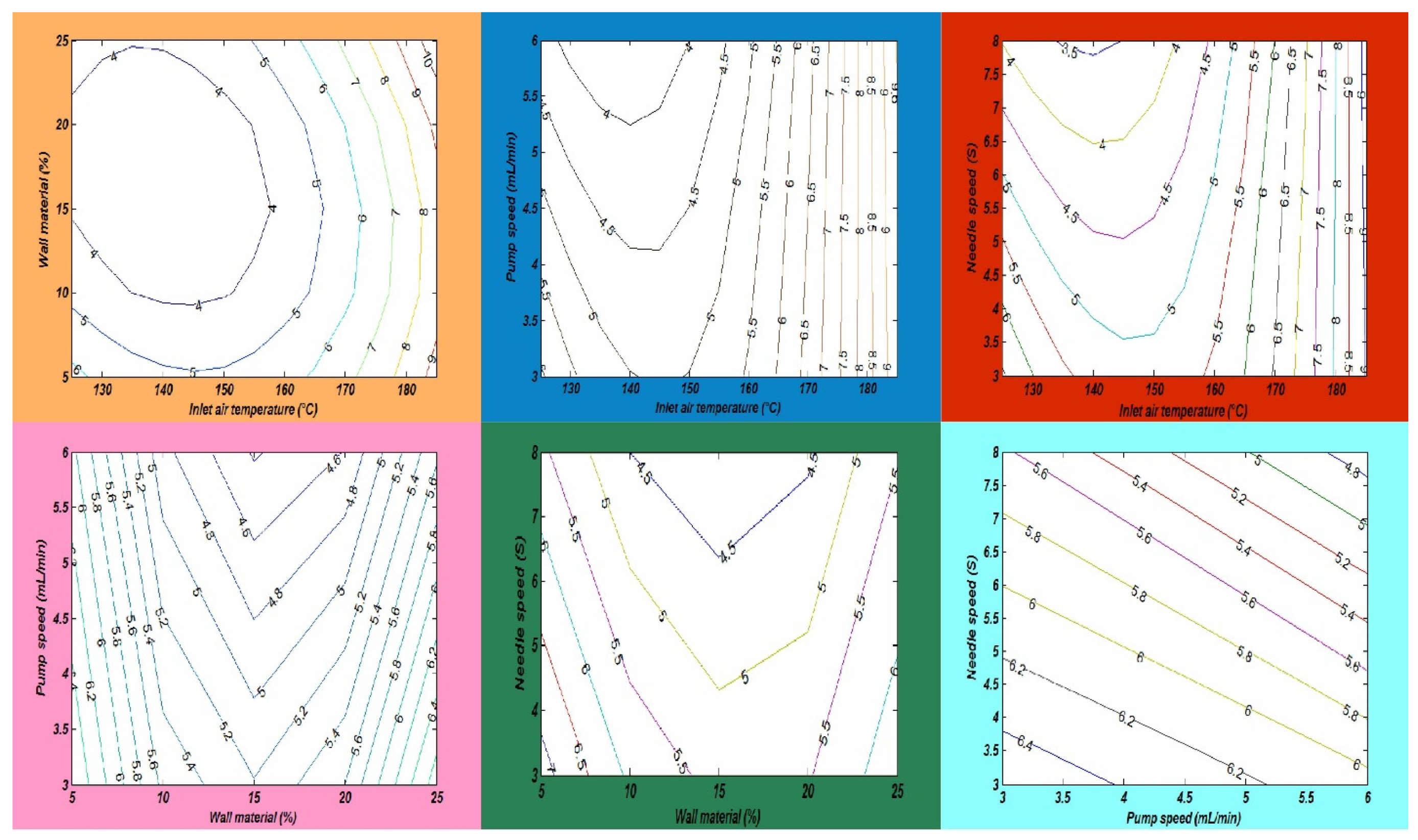
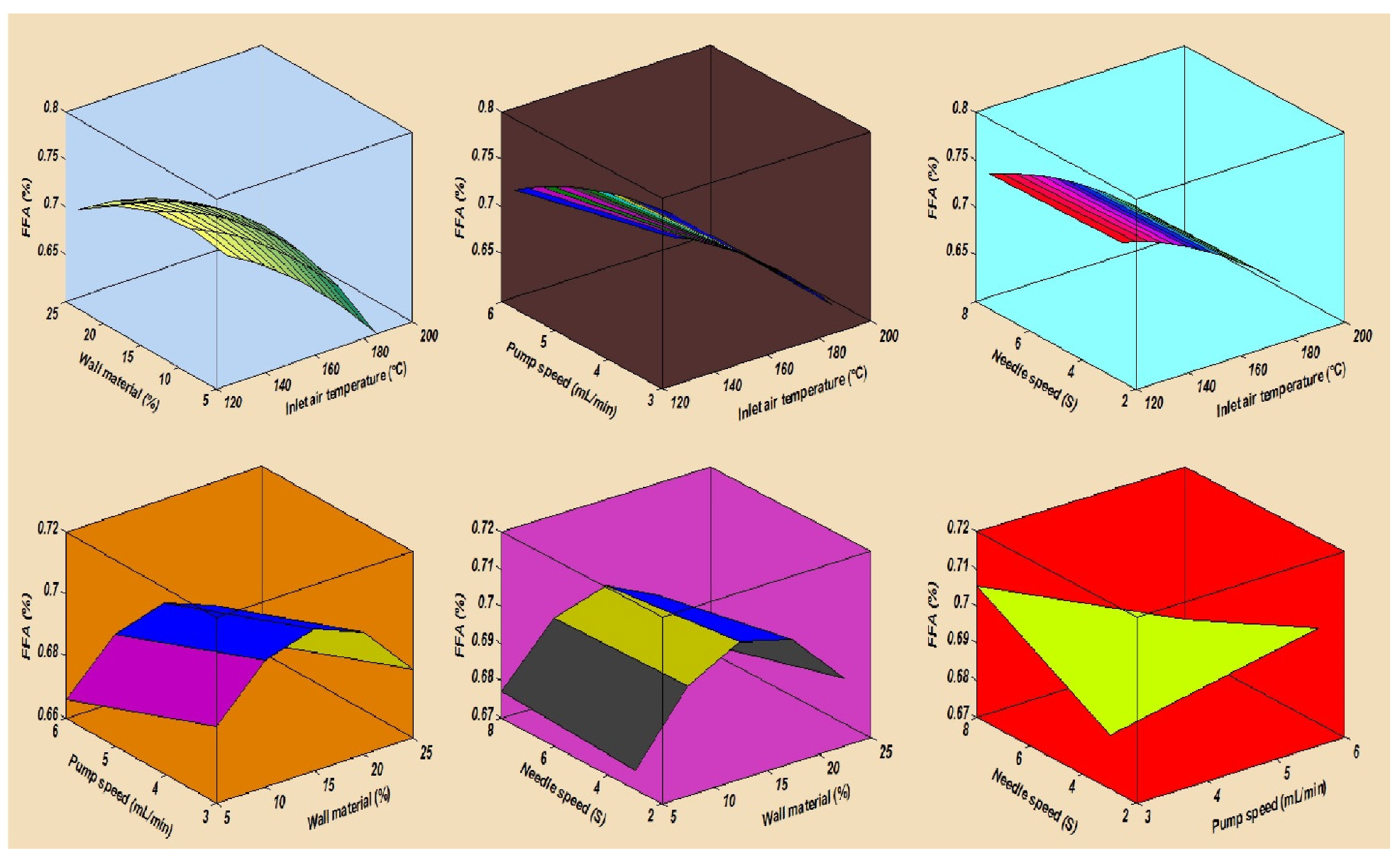
In this study, the combined effects of factors affecting the fatty acid composition of the SDMs showed that the minimum ALA retention loss was 4.18 ± 0.20% at 140 °C, 10 mL/min, 4%, and 5S. Furthermore, the greatest ALA content loss, obtained in run order 26, was 9.90 ± 0.40% at the high temperature of 170 °C. The contour plots of the ALA loss indicated the mutual interactions between operating factors, as presented in Figure 3. Furthermore, the lowest values of the EPA and DHA content loss in the microcapsules were calculated in run order 9 (5.71 ± 0.20% EPA and 0.44 ± 0.10% DHA), while the highest loss was measured in run order 26 (9.71 ± 0.39% EPA and 9.77 ± 0.39% DHA). The EPA and DHA retention losses were in the ranges from 5.71 to 9.71% and 0.44 to 9.77%, respectively. Figure 4 and Figure 5, showing the surface and contour plots, indicate the interaction effects of two independent variables on the EPA and DHA loss. Moreover, the maximum value of FFAs was 0.72 ± 0.15% in run order 9, and the minimum value of FFAs measured in run order 26 was 0.67 ± 0.11%. In addition, the surface plots of the FFAs indicated the mutual interactions between operating factors, as presented in Figure 6. At the end of the experiment, the results indicated that spray drying did not greatly affect the fatty acid composition of the SDMs. As shown in Table 3, the percentages of ALA, EPA, and DHA were similar to those of natural oil, showing only modest variation in the fatty acid composition in the SDMs. An ANOVA was conducted and revealed that the models were significant and that the lack of fit was not significant (Table 4). The significance and the magnitude of the estimated coefficients of each variable and all their possible linear interactions and quadratic effects of the response variables were estimated using the Design Expert Software.
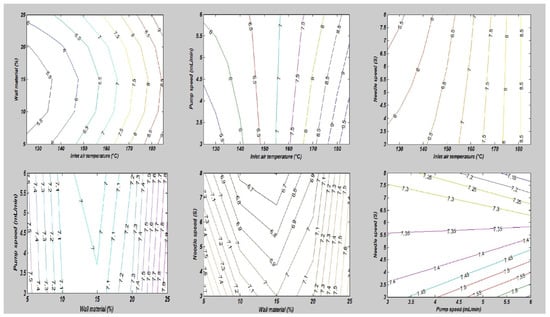
Figure 3.
The impact of the mutual interactions of the spray drying conditions on alpha-linolenic acid loss.
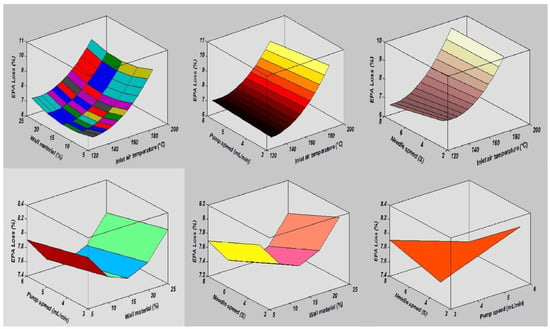
Figure 4.
The interaction effects of independent variables on eicosapentaenoic acid loss.
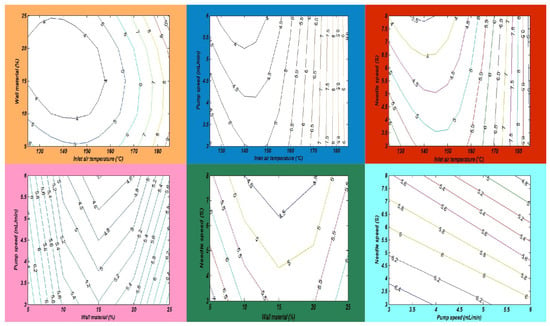
Figure 5.
The mutual interaction impacts of the spray drying parameters on docosahexaenoic acid loss.
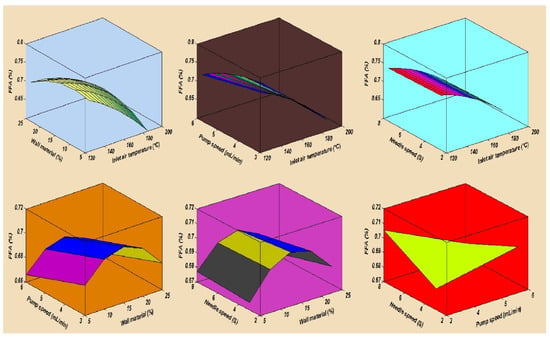
Figure 6.
The mutual interaction effects of spray drying conditions on the free fatty acids of spray-dried microcapsules.

Table 4.
Analysis of variance of the impacts of the spray drying conditions on the response parameters of the spray-dried microcapsules.
3.2.2. Oxidative Stability
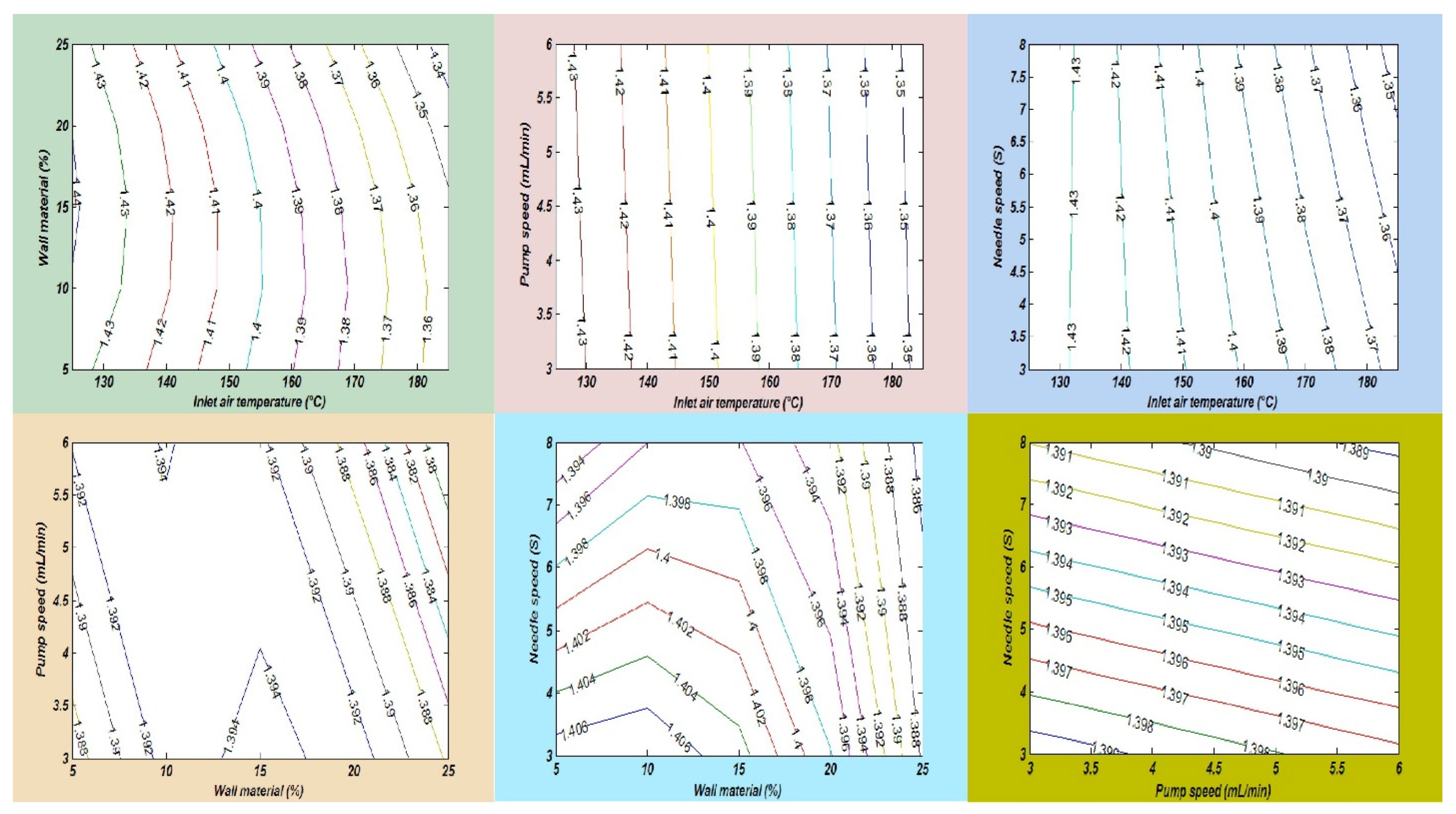
Oxidative stability is also known as the oil stability index, which measures the length of time before the oxidation process begins. Several factors are affected during oil processing and storage. An important factor is lipid oxidation, which has direct effects on the oil quality, flavor, odor, nutritional value, and loss of essential fatty acids and minimizes the shelf life during storage. Lipid oxidation is a process that is also called the rancidity of oil, in which oxygen produces free radicles in the cell membrane that destroy the cell membrane [36]. In this study, a fish and chia oil blend emulsion was prepared for subjection to a spray drying process in order to improve the oxidative stability of omega-3-enriched oil samples. Chia seed oil (CSO) has a lower oxidative stability than FO due to the differences in their fatty acid compositions, oil extraction methods, and the number of phytochemical compounds. In this research, the maximum peroxide value (PV) of the SDMs was found to be 1.45 ± 0.19 meq O2/kg in run order 9. Furthermore, the lowest PV value was measured in run order 26 (1.33 ± 0.16 meq O2/kg). The results showed that the oxidative stability of the SDMs was significantly improved, as compared with the raw oil blend sample. Finally, it was observed that an increase in the amount of wall material and the inlet temperature significantly increased the PV of the SDMs, as described in Table 3. The mutual interaction effects of the independent factors on the PV of the SDMs is also explained in Figure 7.
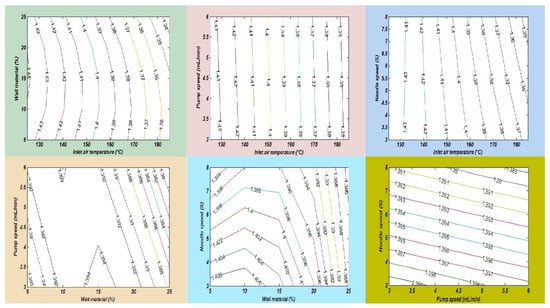
Figure 7.
The interaction impacts of independent variables on the peroxide value.
3.3. Model Fitting
Modified SDMs containing a chia and fish oil blend were produced by the spray drying method under optimal processing conditions. After conducting all the experiments, the best fitting model was determined via the regression equations described in Table 5. Regression equations for the specific levels of each factor can predict the response variable, in which the factors’ coded and actual levels are coded as −2 and +2, respectively. Furthermore, five coded levels of a CCD were used for the reactions between the independent variables and dependent variables. Our results were fitted to the quadratic polynomial model. This model shows the relationship between the dependent variables and the independent variables, with four independent factors. In this model, the predicted R2 value was estimated to be in the range from 0.1047 to 0.6609, with a small fraction of this response variable ranging from 0.6095 to 0.8694 with the adjusted R2, as can be expected in general. Moreover, the lack of fit F-value ranged from 0.92 to 10.16, results which were significant and non-significant relative to the pure error described in Table 4.

Table 5.
Coded and actual regression equations for the response factors according to the spray drying conditions.
4. Discussion
The present study verified that the fatty acid composition of fish and chia oil is influenced by the effects of factors such as the quality of the seed, weather conditions, storage temperature, and extraction system [37]. In a work by Bruno et al. [38], the results show that the head oil of labeo rohita contained 0.86% of EPA and 0.15% of DHA. Ghosh and Bhattacharyya [9] reported that the concentrations of FFAs and PV in FO increased significantly with increasing temperature. Moreover, in a similar study conducted by the Ixtaina et al. [39], screw-pressed CSO contained 64.5% ALA. In another similar work, the CSO contained 59.76% linolenic acid at 90 °C and 20.13% linoleic acid at 90 °C [40]. Another study found that the CSO was rich in ALA (61.1%) and linoleic acid (16.6%) [41]. According to the Ghena and MB [42], CSO contained 60.69% of ALA (unsaturated fatty acid). In another work, the fatty acid profile showed that the CSO contained 18.11 mg/g ALA [43]. In a similar study carried out by Carrillo et al. [44], the ALA in CSO was 54.08%. In addition, low PVs of 1.08–2.56 meq O2/kg have been noted in CSO samples [45,46]. In a similar work by Ghosh et al. [47], the highest PV was noted in samples of the blended oil containing FO and CSO in the ratio of 2:1, which was within the acceptable range for edible oil.
Most importantly, omega-3 and omega-6 have different benefits for human health. These LCPUFAs are essential for animal and human physiology due to their structural and regulatory functions. These valuable essential fatty acid components play vital roles in preventing or inhibiting health risks such as heart disease, chronic cancer, inflammation, and dementia [48,49]. Moreover, these essential omega-3 fatty acids are very important for cell repair and development, the body composition, and the function of the human body. These fatty acids play a very important role in the chemical reactions of the metabolism process. However, the intake of the wrong balance of essential fatty acids causes several of diseases in human body [2]. According to health services, a daily intake of EPA and DHA of 150 to 500 mg/day can reduce the triglyceride levels and the risk of blood clot formation [50]. Long-chain PUFAs are essential for visual and neurological development in the human body [1]. The ω-3 and ω-6 found in FO play important roles in the development, maintenance, and function of the brain, eyes, and cardiovascular and nervous systems [51]. Gamma-linolenic acid, ω-3, and ω-6 supplementations are very effective against skin diseases such as acne, itchy and scaly patches, rashes, swollen skin, and skin irritation [52].
The fatty acid profile of SDMs is influenced by the presence of non-triglyceride compounds, temperature (during scanning), and fatty acid double-bond chains [53]. Furthermore, in the research of Karthik and Anandharamakrishnan [54], it was concluded that during the preparation of emulsion for microencapsulation, temperature and physical stresses affect the fatty acid profiles of oil samples. The fatty acid profile of chia oil and seed was maintained under high-temperature spray drying operating conditions [55]. The results were in strong agreement with the research of Lavanya et al. [32] and Ali et al. [56], who also concluded that there was no significant change in the fatty acid profile of a chia and fish oil blend after increasing and decreasing the temperature in the spray drying process. In another study, the fatty acid profiles of chia seed oil and eggs were significantly decreased by increasing the temperature during spray drying, and high temperature conditions in the spray dryer caused higher losses of PUFAs [57]. Similarly, the fatty acid profile of fish oil was observed after the spray drying process at a high temperature, with no significant losses when compared to the bulk oil [58]. In addition, several recent studies have shown that the losses of LCPUFAs were increased by increasing the temperature in the spray drying processing [59,60].
Humans cannot synthesize these omega-3 fatty acids, and they must be obtained from food sources. Seed and marine oils are good natural sources of these omega-3 fatty acids. These essential fatty acids are found in large quantities in, for example, flax, rapeseed, chia, perella, walnuts, and fish meal. When the human body consumes PUFAs, these essential fatty acids are converted into oleic acid, linoleic acid, ALA, EPA, DHA, but this is a slow process. Therefore, a diet high in ω-3 and ω-6 fatty acids can be very effective against many diseases, such as hyperlipoproteinemia, atherosclerotic disease, obesity, hypertriglyceridemia, and platelet pro-aggregatory and prothrombotic diseases [61]. Therefore, many food industries have shown an increased interest in the production of PUFA-enriched functional food products [62]. Chia and fish oil by-products are an excellent natural sources of LCPUFAs, especially ALA, EPA, and DHA. Due to the presence of LCPUFAs, the stability of these oils is very low in terms of the products created in the food processing industry. The solution to this issue is the microencapsulation of oil using the various methods to increase the stability of fatty acids. However, food industries prefer to use encapsulated oils in fortified food products due to their longer shelf life, as compared to the raw oils [14,63,64]. In addition, the use of functional foods to ensure a heathy lifestyle has been increasing over the past few years [65]. Health services or organizations have also recommended that consumers consume more functional foods in which omega-3 fatty acids are balanced and less foods with unbalanced fatty acid profiles to ensure a good health and lifestyle [66].
These results are in agreement with the study of Shi et al. [67], in which the oxidative stability of spray-dried FO was improved at a low inlet temperature (140 °C) using various types of core materials (mixture of matcha and maltodextrin). Moreover, a work by Takashige et al. [68] reported that the stability of FO was increased after the spray drying of samples with maltodextrin. In another study, the oxidative stability of spray-dried fish oil microcapsules was improved during storage compared to the crude FO [69]. Furthermore, in the research of Hogan et al. [70] and Encina et al. [71], it was concluded that the PV of FO after spray drying was increased, as compared with raw oil samples. Moreover, in another work, microcapsules of CSO were prepared using a small-scale spray dryer, and the PV of the functional oil was 42.5 meq/kg and the PV of the microencapsulated oil was 10.3 meq/kg under optimal conditions. Hence, the oxidative stability of CSO was improved after spray drying [72]. A research work of Timilsena et al. [60] concluded that the PV of CSO microcapsules was improved during thermal processing and storage.
All over the world, various encapsulation processes are used to increase the stability of omega 3 fatty acids in oils. Most commonly, the spray drying process is used in the food processing industry to produce fortified products that are high in omega-3 fatty acids. This process is low in cost and easy to perform, requires less energy consumption, and produces good-quality microcapsules. Omega-3 fatty acids are essential for human health; thus consumers eat nutritious foods to keep the body healthy [73]. The fortification of food with PUFAs is considered as a good a way to increase one’s dietary of these products intake and reduce the risk of many chronic diseases. Therefore, consumers consume functional foods which contain PUFAs because these fatty acids play a very important role in the development of brain functions, reduce the risk of cardiovascular diseases, and boost the immunity of the body [8]. In another study, FO was deemed to be the main source of EPA and DHA, which have positive impacts in controlling the cholesterol levels and lowering the blood pressure. Several studies have claimed that FO reduces the risk of many types of cancer. Therefore, functional food products are developed as microcapsules of EPA and DHA for those who do not eat fish because they cannot purchase fish. These omega-3-enriched products influence several risk factors, such as bad cholesterol levels, and they can also control the blood pressure, platelet aggregation, and triglyceride levels [74].
5. Conclusions
Chia and fish oil are high in ALA, EPA, and DHA, which are essential for the human body and protect it against many diseases. In this study, GA and MD were used as wall materials in the preparation of emulsion for spray drying. According to the results, there was no significant loss in the fatty acid composition of the SDMs, with a retention of 94 ± 5% after spray drying. Furthermore, the concentration of FFAs in the SDMs was lower compared to the blend of chia and fish oil. Finally, the PV of the SDMs indicated that the oxidative stability was higher than that of the chia and fish oil blend. Further studies should focus on the development of innovative substances and techniques to improve the process of industrial-scale encapsulation, to produce fortified products encapsulating essential oils in the future, and to provide non-lethal therapeutic agents for the treatment of numerous diseases.
Author Contributions
Conceptualization, M.I. and M.A.R.; methodology, M.A.R., M.I. and W.K.; software, M.I. and W.K.; validation, W.K. and M.H.A.; formal analysis, M.A.R., M.K.K., J.M.L. and M.N.; investigation, W.K., J.M.L. and M.N.; resources, M.A.R., W.K., M.O.A. and M.K.K.; data curation, J.M.L. and M.H.A.; writing—original draft preparation, M.A.R.; writing—review and editing, W.K., M.M.A., A.A.-F. and M.N.; visualization, M.I. and M.A.R.; supervision, M.I. and M.K.K., and project administration, W.K.; funding acquisition, M.O.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project Number PNURSP2022R251, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend thanks to the Princess Nourah bint Abdulrahman University Researchers Supporting Project Number PNURSP2022R251, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nagy, K.; Tiuca, I.D. Importance of fatty acids in physiopathology of human body. In Fatty Acids; IntechOpen: London, UK, 2017. [Google Scholar]
- Kaur, N.; Chugh, V.; Gupta, A.K. Essential fatty acids as functional components of foods—A review. J. Food Sci. Technol. 2014, 51, 2289–2303. [Google Scholar] [CrossRef] [PubMed]
- Valdivia-López, M.Á.; Tecante, A. Chia (Salvia hispanica): A review of native Mexican seed and its nutritional and functional properties. Adv. Food Nutr. Res. 2015, 75, 53–75. [Google Scholar] [PubMed]
- Parker, J.; Schellenberger, A.N.; Roe, A.L.; Oketch-Rabah, H.; Calderón, A.I. Therapeutic perspectives on chia seed and its oil: A review. Planta Med. 2018, 84, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M. Chia (Salvia hispanica) Oil. In Fruit Oils: Chemistry and Functionality; Springer: Cham, Switzerland, 2019; pp. 303–316. [Google Scholar]
- Zeb, J.; Tufail, S.; Saboohi, N.; Samuel, Z.; Azeem, A.; Amir, Y.; Akram, S. Effect of Varying Levels of Lipids and Proteins on the Growth Indices and Fatty Acid Profile of Labeo rohita (Rohu). Pak. J. Zool. 2021, 54, 615–623. [Google Scholar] [CrossRef]
- Chughtai, M.I.; Maqbool, U.; Ahmed, R. Compositional properties of three freshwater carp species grown in brackish water. J. Microbiol. Biotechnol. Food Sci. 2021, 2021, 532–535. [Google Scholar]
- Manuelli, M.; Della, G.L.; Cena, H. Enriching diet with n-3 PUFAs to help prevent cardiovascular diseases in healthy adults: Results from clinical trials. Int. J. Mol. Sci. 2017, 18, 1552. [Google Scholar] [CrossRef]
- Ghosh, N.; Bhattacharyya, D.K. Effect of heating time and heat on the physicochemical and antioxidative property of fish (Labeo rohita) skin oil. Int. J. Pharm. Pharmac. Sci. 2019, 11, 87–89. [Google Scholar] [CrossRef][Green Version]
- Ghosh, N.; Roy, M.; Bhattacharyya, D.K. Formulation, production, and characterization of nutritionally enriched spread product with blends of fish skin (Labeo rohita) oil and chia seed (Salvia hispanica) oil. In Advances in Bioprocess Engineering and Technology; Springer: Singapore, 2021; pp. 209–217. [Google Scholar]
- Ismail, A.; Bannenberg, G.; Rice, H.B.; Schutt, E.; MacKay, D. Oxidation in EPA-and DHA-rich oils: An overview. Lipid Technol. 2016, 28, 55–59. [Google Scholar] [CrossRef]
- Catala, A. Fatty Acids; BoD–Books on Demand: Rejika, Crotia, 2017. [Google Scholar]
- Mittal, V. Encapsulation Nanotechnologies; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Moghadam, F.V.; Pourahmad, R.; Mortazavi, A.; Davoodi, D.; Azizinezhad, R. Use of fish oil nanoencapsulated with gum arabic carrier in low fat probiotic fermented milk. Food Sci. Anim. Resour. 2019, 39, 309. [Google Scholar] [CrossRef]
- Kwak, H.S. Nano-and Microencapsulation for Foods; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Anandharamakrishnan, C. Spray Drying Techniques for Food Ingredient Encapsulation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Santos, D.; Maurício, A.C.; Sencadas, V.; Santos, J.D.; Fernandes, M.H.; Gomes, P.S. Spray Drying: An Overview; Pignatello, R., Ed.; Biomaterials-Physics and Chemistry-New Edition; InTech: London, UK, 2018; pp. 9–35. [Google Scholar]
- Salaün, F. Microencapsulation: Processes, Technologies and Industrial Applications; BoD–Books on Demand: London, UK, 2019. [Google Scholar]
- Mohammed, N.K.; Tan, C.P.; Manap, Y.A.; Muhialdin, B.J.; Hussin, A.S.M. Spray drying for the encapsulation of oils—A review. Molecules 2020, 25, 3873. [Google Scholar] [CrossRef]
- Pourashouri, P.; Shabanpour, B.; Razavi, S.H.; Jafari, S.M.; Shabani, A.; Aubourg, S.P. Impact of wall materials on physicochemical properties of microencapsulated fish oil by spray drying. Food Bioprocess Technol. 2014, 7, 2354–2365. [Google Scholar] [CrossRef]
- Bordón, M.G.; Alasino, N.P.; Martínez, V.; Peter, R.G.; Iturralde, R.; Ribotta, P.D.; Martínez, M.L. Influence of the spray drying operating conditions on the estimated drying kinetics of emulsion single droplets and the properties of microencapsulated chia oil. Powder Technol. 2021, 383, 302–317. [Google Scholar] [CrossRef]
- Rahim, M.A.; Imran, M.; Khan, M.K.; Ahmad, M.H.; Ahmad, R.S. Impact of spray drying operating conditions on encapsulation efficiency, oxidative quality, and sensorial evaluation of chia and fish oil blends. J. Food Process. Preserv. 2021, 16, 16248. [Google Scholar] [CrossRef]
- Dijkstra, A.J. The purification of edible oils and fats. Lipid Technol. 2013, 25, 271–273. [Google Scholar] [CrossRef]
- Quero-Jiménez, P.C.; Felipe, L.A.A.; López, L.R. Oil extraction and derivatization method: A review. Open Access J. Sci. 2020, 4, 110–120. [Google Scholar] [CrossRef]
- Kostik, V.; Memeti, S.; Bauer, B. Fatty acid composition of edible oils and fats. J. Hyg. Eng. Des. 2013, 4, 112–116. [Google Scholar]
- American Oil Chemists’ Society. Official Methods and Recommended Practices of the AOCS; American Oil Chemists’ Society: Urbana, IL, USA, 1997. [Google Scholar]
- AOCS. Determination of Oil Content in Oilseeds; American Oil Chemists’ Society: Urbana, IL, USA, 2000. [Google Scholar]
- AOAC (Association of Analytical Chemists). Official Method 965.33 Peroxide Value in Oils and Fats/Pearson’s Composition and Analysis of Foods, 9th ed.; Association of Analytical Chemists: Rockville, MD, USA, 2000; p. 64. [Google Scholar]
- Low, L.K. Analysis of oils: Determination of peroxide value. In Laboratory Manual on Analytical Methods and Procedures for Fish and Fish Products; Marine Fisheries Research Department, Southeast Asian Fisheries Development Center: Samut Prakan, Thailand, 1992. [Google Scholar]
- Scheme, R. IFRA analytical method: Determination of the peroxide value. Int. Fragr. Assoc. Rep. 2011, 17, 1–5. [Google Scholar]
- Hamilton, R.J. Lipid Analysis in Oils and Fats; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Lavanya, M.N.; Kathiravan, T.; Moses, J.A.; Anandharamakrishnan, C. Influence of spray-drying conditions on microencapsulation of fish oil and chia oil. Dry Technol. 2019, 38, 279–292. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Muhammad, I.; Muhammad, N.; Manzoor, M.F.; Amna, J.; Zafar, A.; Akhtar, M.N.; Yasir, H. Fatty acids characterization, oxidative perspectives and consumer acceptability of oil extracted from pre-treated chia (Salvia hispanica L.) seeds. Lipids Health Dis. 2016, 15, 162. [Google Scholar]
- Ivanovs, K.; Blumberga, D. Extraction of fish oil using green extraction methods: A short review. Energy Procedia 2017, 128, 477–483. [Google Scholar] [CrossRef]
- Hu, M.; Jacobsen, C. Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Tocher, D.R.; Glencross, B.D. Lipids and fatty acids. In Dietary Nutrients, Additives, and Fish Health; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 47–94. [Google Scholar]
- Bruno, S.F.; Kudre, T.G.; Bhaskar, N. Impact of pretreatment-assisted enzymatic extraction on recovery, physicochemical and rheological properties of oil from Labeo rohita head. J. Food Process Eng. 2019, 42, 12990. [Google Scholar] [CrossRef]
- Ixtaina, V.Y.; Martínez, M.L.; Spotorno, V.; Mateo, C.M.; Maestri, D.M.; Diehl, B.W.; Tomás, M.C. Characterization of chia seed oils obtained by pressing and solvent extraction. J. Food Comp. Anal. 2011, 24, 166–174. [Google Scholar] [CrossRef]
- Ghafoor, K.; Ahmed, I.A.M.; Özcan, M.M.; Al-Juhaimi, F.Y.; Babiker, E.E.; Azmi, I.U. An evaluation of bioactive compounds, fatty acid composition and oil quality of chia (Salvia hispanica L.) seed roasted at different temperatures. Food Chem. 2020, 333, 127531. [Google Scholar] [CrossRef] [PubMed]
- Abad, A.; Shahidi, F. Compositional characteristics and oxidative stability of chia seed oil (Salvia hispanica L.). Food Prod. Process. Nutr. 2020, 2, 9. [Google Scholar] [CrossRef]
- Ghena, M.; Amany, M.B. Chia (Salvia hispanica L.) Seed oil a new source of omega-3. Plant Arch. 2020, 20, 2678–2683. [Google Scholar]
- Souza, M.F.; Francisco, C.R.L.; Sanchez, J.L.; Guimarães-Inácio, A.; Valderrama, P.; Bona, E.; Gonçalves, O.H. Fatty acids profile of chia oil-loaded lipid microparticles. Braz. J. Chem. Eng. 2017, 34, 659–669. [Google Scholar] [CrossRef]
- Carrillo, W.; Cardenas, M.; Carpio, C.; Morales, D.; Alvarez, M.; Silva, M. Content of nutrients component and fatty acids in chia seeds (Salvia hispanica L.) cultivated in Ecuador. Asian J. Pharm. Clin. Res. 2018, 11, 1–4. [Google Scholar]
- Da Silva Marineli, R.; Moraes, É.A.; Lenquiste, S.A.; Godoy, A.T.; Eberlin, M.N.; Maróstica, M.R., Jr. Chemical characterization and antioxidant potential of Chilean chia seeds and oil (Salvia hispanica L.). LWT-Food Sci. Technol. 2014, 59, 1304–1310. [Google Scholar] [CrossRef]
- Basuny, A.M.; Arafat, S.M.; Hikal, D.M. Chia (Salvia hispanica L.) seed oil rich in omega-3 fatty acid: A healthy alternative for milk fat in ice milk. Food Nutr. Sci. 2021, 12, 479–493. [Google Scholar] [CrossRef]
- Ghosh, N.; Roy, M.; Ghosh, M.; Bhattacharyya, D.K. Study on physicochemical and antioxidant properties of blend of fish skin (Labeo rohita) oil and chia seed (Salvia hispanica) oil. In Biotechnology and Biological Sciences; CRC Press: London, UK, 2019; pp. 121–126. [Google Scholar]
- Raatz, S.; Bibus, D. Fish and Fish Oil in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Varnham, A. Seed Oil: Biological Properties, Health Benefits and Commercial Applications; Nova Science Publishers: New York, NY, USA, 2014. [Google Scholar]
- Skulas-Ray, A.C.; Kris-Etherton, P.M.; Harris, W.S.; Vanden, H.J.P.; Wagner, P.R.; West, S.G. Dose-response effects of omega-3 fatty acids on triglycerides, inflammation, and endothelial function in healthy persons with moderate hypertriglyceridemia. Am. J. Clin. Nutr. 2011, 93, 243–252. [Google Scholar] [CrossRef]
- Bentsen, H. Dietary polyunsaturated fatty acids, brain function and mental health. Microb. Ecol. Health Dis. 2017, 28, 1281916. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić, M.Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Azizian, H.; Kramer, J.K.; Winsborough, S. Factors influencing the fatty acid determination in fats and oils using Fourier transform near-infrared spectroscopy. Eur. J. Lipid Sci. Technol. 2007, 109, 960–968. [Google Scholar] [CrossRef]
- Karthik, P.; Anandharamakrishnan, C. Enhancing omega-3 fatty acids nanoemulsion stability and in-vitro digestibility through emulsifiers. J. Food Eng. 2016, 187, 92–105. [Google Scholar] [CrossRef]
- Fernandes, S.S.; Greque, L.; Santos, M.D.F.C.; de Novais, L.M.; D’Oca, C.D.; Prentice, C.; Salas-Mellado, M.D.L.M. Effect of the spray drying conditions on the physicochemical and structural characteristics and the stability of chia oil microparticles. J. Appl. Polym. Sci. 2021, 138, 51015. [Google Scholar] [CrossRef]
- Ali, M.; Imran, M.; Khan, M.K.; Ahmad, M.H.; Muhammad, N. Functional and Oxidative Quality Characterization of Spray-Dried Omega-3-Enriched Milk Powder. J. Food Qual. 2021, 2021, 6693960. [Google Scholar] [CrossRef]
- Javed, A.; Imran, M.; Ahmad, N.; Hussain, A.I. Fatty acids characterization and oxidative stability of spray dried designer egg powder. Lipids Health Dis. 2018, 17, 282. [Google Scholar] [CrossRef]
- Botrel, D.A.; Borges, S.V.; Yoshida, M.I.; Feitosa, J.P.D.A.; Fernandes, R.V.D.B.; de Souza, H.J.B.; de Paula, R.C.M. Properties of spray-dried fish oil with different carbohydrates as carriers. J. Food Sci. Technol. 2017, 54, 4181–4188. [Google Scholar] [CrossRef]
- Babushkina, E.A.; Belokopytova, L.V.; Grachev, A.M.; Meko, D.M.; Vaganov, E.A. Variation of the hydrological regime of Bele-Shira closed basin in Southern Siberia and its reflection in the radial growth of Larix sibirica. Reg. Environ. Change 2017, 17, 1725–1737. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Barrow, C.J.; Adhikari, B. Microencapsulation of chia seed oil using chia seed protein isolate chia seed gum complex coacervates. Int. J. Biol. Macromol. 2016, 91, 347–357. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The omega-6/omega-3 fatty acid ratio: Health implications. Oléagineux Corps Gras Lipides 2010, 17, 267–275. [Google Scholar] [CrossRef]
- Hernandez, E.; Hosokawa, M. Omega-3 Oils: Applications in Functional Foods; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Barrow, C.J.; Nolan, C.; Holub, B.J. Bioequivalence of encapsulated and microencapsulated fish-oil supplementation. J. Funct. Foods 2009, 1, 38–43. [Google Scholar] [CrossRef]
- Tanwar, B.; Goyal, A. Oilseeds: Health Attributes and Food Applications; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef]
- Calder, P.C.; Yaqoob, P. Marine omega-3 fatty acids and coronary heart disease. Curr. Opin. Cardiol. 2012, 27, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Ying, D.; Hlaing, M.M.; Ye, J.; Sanguansri, L.; Augustin, M.A. Oxidative stability of spray dried matcha-tuna oil powders. Food Res. Int. 2020, 132, 109050. [Google Scholar] [CrossRef] [PubMed]
- Takashige, S.; Iwamoto, S.; Shiga, H.; Kakizaki, Y.; Yamaya, Y.; Ushirosako, A.; Yoshii, H. Stability of Fish Oil Encapsulated in Spray-dried Powders Coated with Starch Particles. Food Sci. Technol. Res. 2019, 25, 363–371. [Google Scholar] [CrossRef]
- Kolanowski, W.; Ziolkowski, M.; Weißbrodt, J.; Kunz, B.; Laufenberg, G. Microencapsulation of fish oil by spray drying—Impact on oxidative stability Part 1. Eur. Food Res. Technol. 2006, 222, 336–342. [Google Scholar] [CrossRef]
- Hogan, S.A.; O’riordan, E.D.; O’sullivan, M. Microencapsulation and oxidative stability of spray-dried fish oil emulsions. J. Microencapsul. 2003, 20, 675–688. [Google Scholar] [CrossRef]
- Encina, C.; Vergara, C.; Márquez-Ruiz, G.; Holgado, F.; Robert, P.; Giménez, B. Influence of solvent and lecithin in microencapsulation of fish oil by spray-drying. RSC Adv. 2018, 8, 4172–4181. [Google Scholar] [CrossRef]
- Yue, H.; Qiu, B.; Jia, M.; Liu, J.; Wang, J.; Huang, F.; Xu, T. Development and optimization of spray-dried functional oil microcapsules: Oxidation stability and release kinetics. Food Sci. Nutr. 2020, 8, 4730–4738. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M. Handbook on Spray Drying Applications for Food Industries; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Lakkis, J.M. Encapsulation and controlled release applications in confectionery and oral care products. Encapsulation Control. Release Technol. Food Syst. 2016, 2, 236. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).