Physiological and Transcriptomic Analyses Reveal the Effects of Elevated Root-Zone CO2 on the Metabolism of Sugars and Starch in the Roots of Oriental Melon Seedlings
Abstract
:1. Introduction
2. Results
2.1. Elevated Root-Zone CO2 Affects Root Growth and Vigor
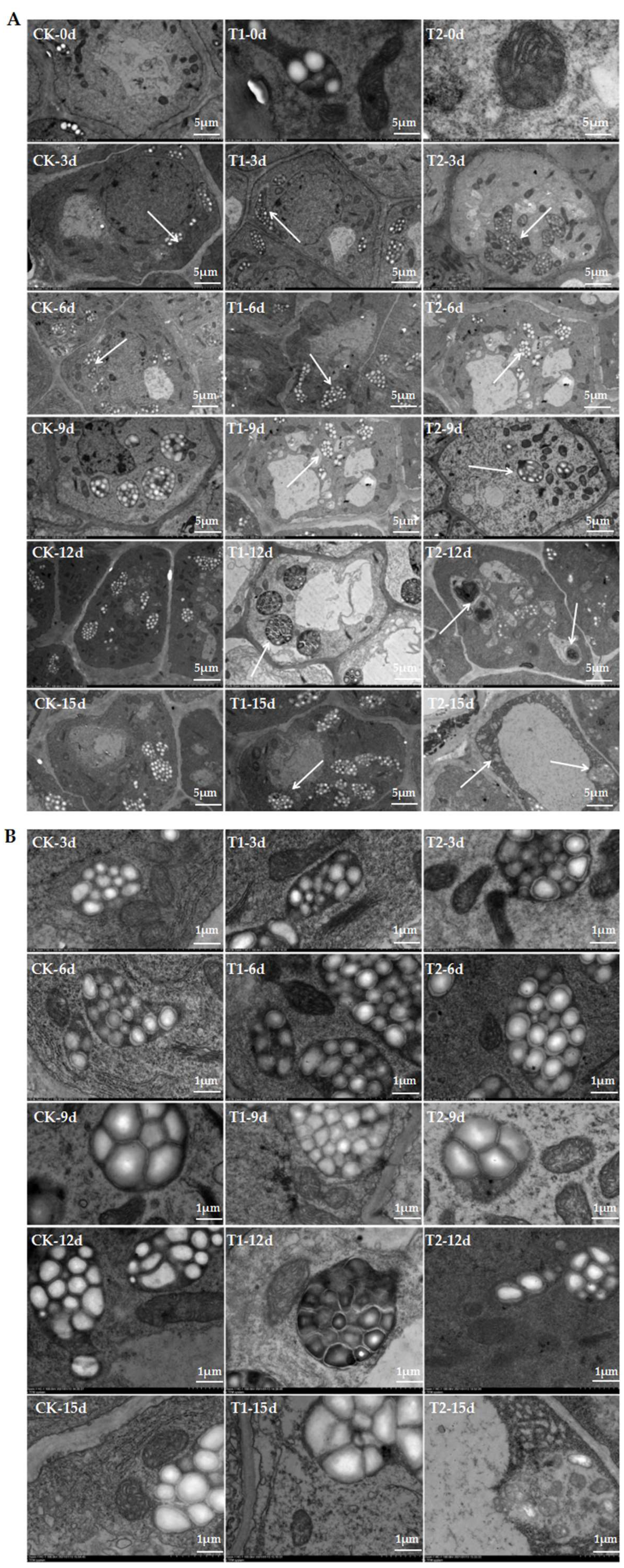
2.2. Elevated Root-Zone CO2 Affected Cellular Ultrastructure of Root Tips
2.3. Quality Analysis of Transcriptome Sequencing Data
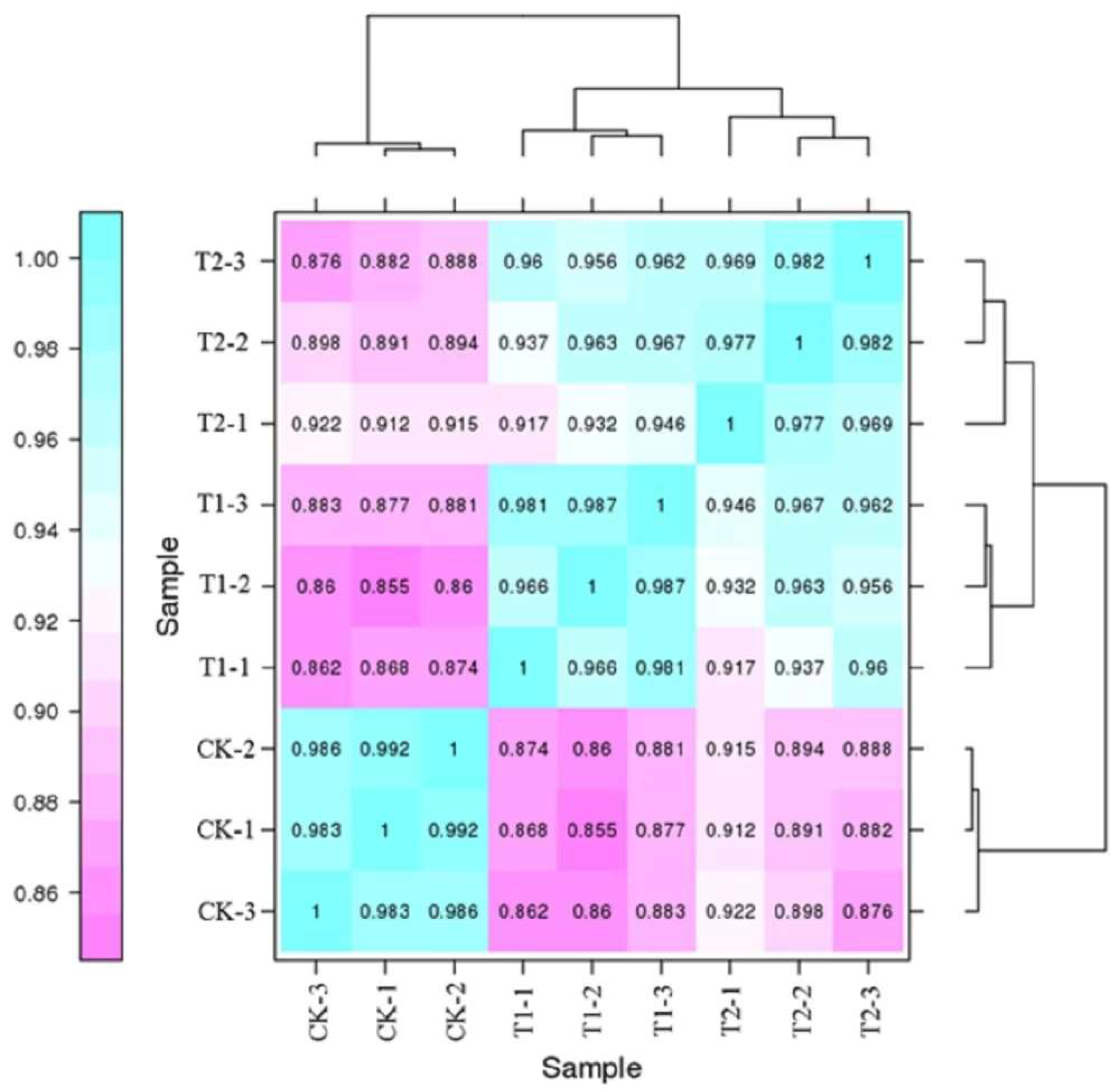
2.4. Sample Correlation Analysis
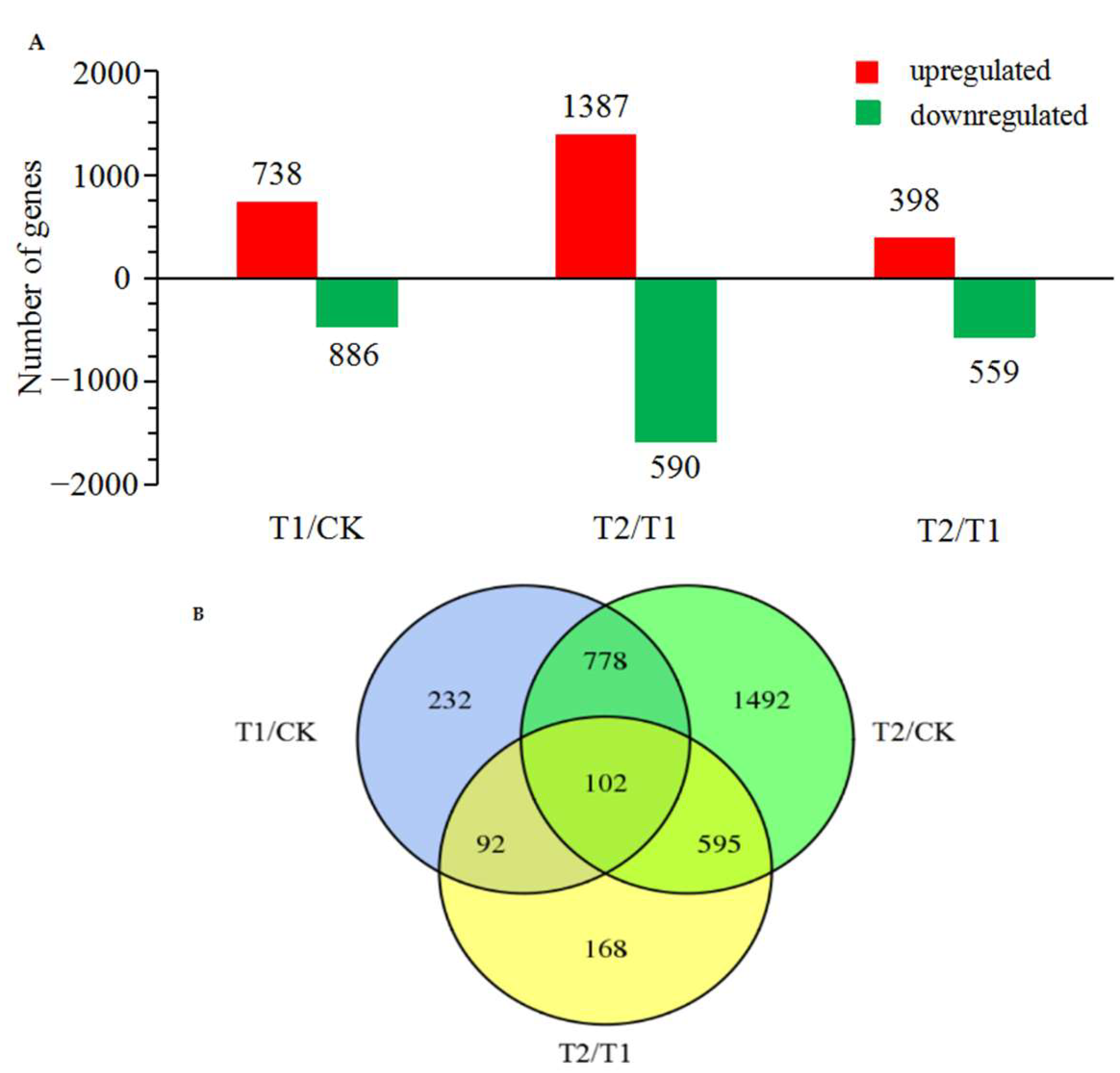
2.5. Screening of DEGs
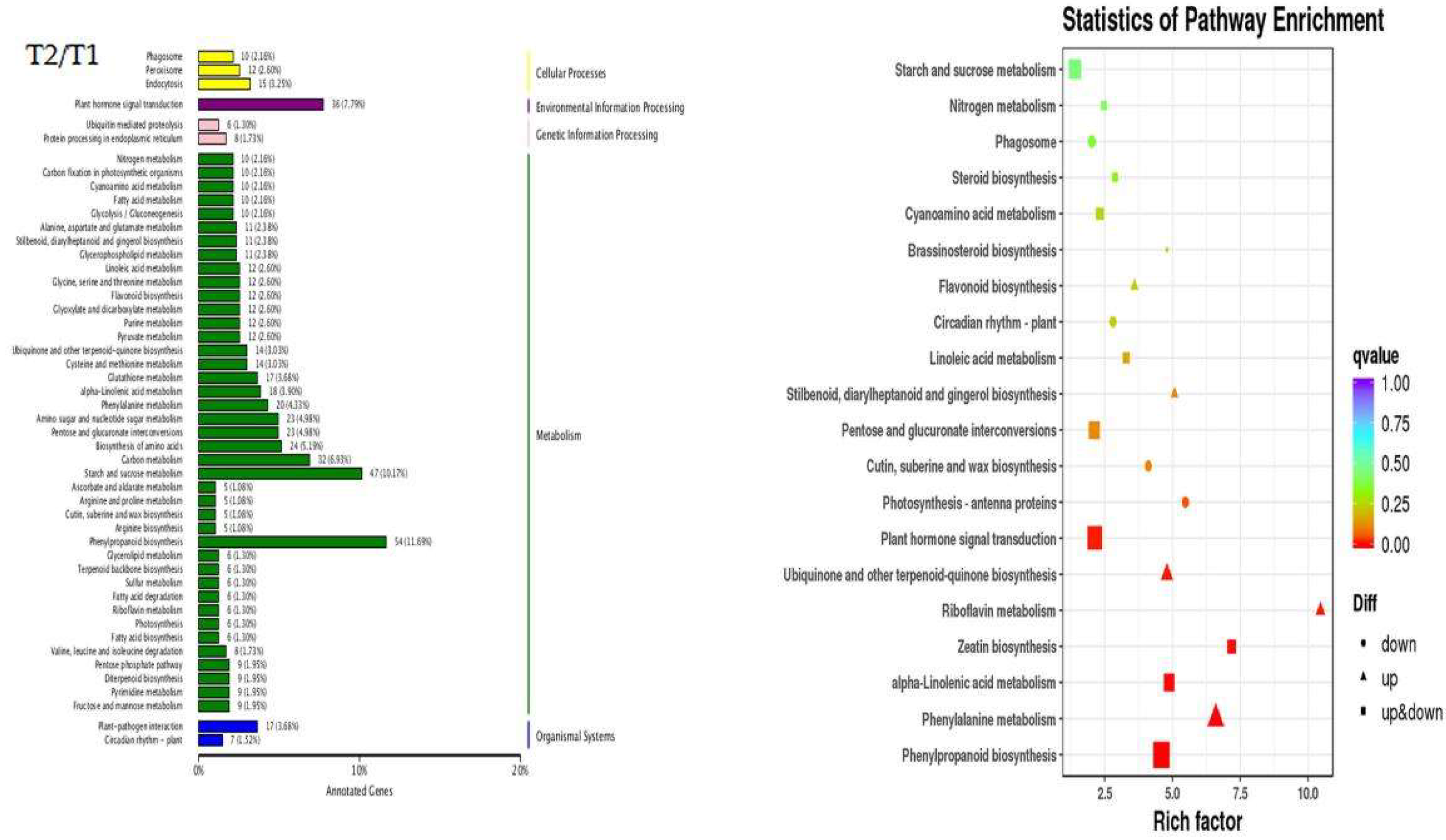
2.6. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Analysis of DEGs
2.7. DEGs Involved in Sugar and Starch Metabolism
2.8. Elevated Root-Zone CO2 Affected Contents of Soluble Sugars and Starch in Roots
2.9. Elevated Root-Zone CO2 Affected the Contents of Different Sugars in Roots
2.10. Elevated Root-Zone CO2 Affected Activities of Enzymes Related to Sugar and Starch Metabolism in Roots
2.11. qRT-PCR Analysis of Major Genes Related to Sugar and Starch Metabolism
3. Discussion
3.1. Elevated Root-Zone CO2 Affected Root Growth and Accumulation of Sugars and Starch
3.2. Elevated Root-Zone CO2 Affected the Metabolism of Sugar and Starch in the Roots
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Measurement of Plant Growth Indices
4.3. Analysis of Root Morphology
4.4. Observation of Ultrastructure of Root Tip Cells
4.5. Determination of Sugar and Starch Contents
4.6. Determination of Activities of Enzymes Related to Sugar and Starch Metabolism
4.7. Analysis of Transcriptome Sequencing Data
4.8. Analysis of Expression of Genes Related to Sugar and Starch Metabolism
4.9. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bouma, T.J.; Nielsen, K.L.; Eissenstat, D.M.; Lynch, J.P. Soil CO2 concentration does not affect growth or root respiration in bean or citrus. Plant Cell Environ. 2010, 12, 1495–1505. [Google Scholar] [CrossRef] [Green Version]
- Duenas, C.; Fernandez, M.C.; Cartetero, J.; Liger, E.; Perez, M. Emission of CO2 from some soils. Chemosphere 1995, 30, 1875–1889. [Google Scholar] [CrossRef]
- Johnson, D.; Geisinger, D.; Walker, R.; Newman, J.; Vose, J.; Elliot, K.; Ball, T. Soil pCO2, soil respiration, and root activity in CO2-futnigated and nitrogen-fertilized ponderosa pine. Plant Soil 1994, 165, 129–138. [Google Scholar] [CrossRef]
- Hamada, Y.; Tanaka, T. Dynamics of carbon dioxide in soil profiles based on long-term field observation. Hydrol. Process. 2001, 50, 1829–1845. [Google Scholar] [CrossRef]
- Boru, G.; Vantoai, T.; Alves, J. Responses of soybean to oxygen deficiency and elevated root-zone carbon dioxide concentration. Ann. Bot. 2003, 91, 447–453. [Google Scholar] [CrossRef]
- Cramer, M.D.; Shane, M.W.; Lambers, H. Physiological changes in white lupin associated with variation in root-zone CO2 concentration and cluster-root P mobilization. Plant Cell Environ. 2005, 28, 1203–1217. [Google Scholar] [CrossRef]
- Cramer, M.D.; Titus, C.H.A. Elevated root-zone dissolved inorganic carbon can ameliorate aluminium toxicity in tomato seedlings. New Phytol. 2001, 152, 29–39. [Google Scholar] [CrossRef]
- Leibar-Porcel, E.; McAinsh, M.R.; Dodd, I.C. Elevated root-zone dissolved inorganic carbon alters plant nutrition of lettuce and pepper grown hydroponically and aeroponically. Agronomy 2020, 10, 403. [Google Scholar] [CrossRef] [Green Version]
- Cramer, M.D.; Lips, S.H. Enriched rhizosphere CO2 concentrations can ameliorate the influence of salinity on hydroponically grown tomato plants. Physiol. Plant. 2010, 94, 425–432. [Google Scholar] [CrossRef]
- He, J.; Qin, L.; Lee, S.K. Root morphology, plant growth, nitrate accumulation and nitrogen metabolism of temperate lettuce grown in the tropics with elevated root-zone CO2 at different root-zone temperatures. Am. J. Plant Sci. 2016, 7, 1821–1833. [Google Scholar] [CrossRef]
- He, W.; Moonis, M.; Chung, H.; Yoo, G. Effects of high soil CO2 concentrations on seed germination and soil microbial activities. Int. J. Greenh. Gas Control 2016, 53, 117–126. [Google Scholar] [CrossRef]
- Grossiord, C.; Mareschal, L.; Epron, D. Transpiration alters the contribution of autotrophic and heterotrophic components of soil CO2 efflux. New Phytol. 2012, 194, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Robinson, D.; Hodge, A.; Fitter, A. Constraints on the Form and Function of Root Systems. In Root Ecology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–31. [Google Scholar]
- He, J.; Austin, P.T.; Nichols, M.A.; Lee, S.K. Effects of elevated root-zone CO2 and air temperature on photosynthetic gas exchange, nitrate uptake and total reduced nitrogen content in aeroponically grown lettuce plants. J. Exp. Bot. 2010, 61, 3959–3969. [Google Scholar] [CrossRef] [Green Version]
- Koch, K.E. Carbohydrate-modulated gene expression in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 45, 509–540. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.C.; Sheen, J. Sugar sensing in higher plants. Trends Plant Sci. 1997, 2, 208–214. [Google Scholar] [CrossRef] [Green Version]
- Sabine, K.; Edouard, P.; Kleczkowski, L.A. Functional dissection of sugar signals affecting gene expression in arabidopsis thaliana. PLoS ONE 2014, 9, e100312. [Google Scholar]
- Munir, R.; Konnerup, D.; Khan, H.A. Sensitivity of chickpea and faba bean to root-zone hypoxia, elevated ethylene, and carbon dioxide. Plant Cell Environ. 2019, 42, 85–97. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Yoo, G.; Moonis, M.; Kim, Y.; Chen, X. Impact assessment of high soil CO2 on plant growth and soil environment: A greenhouse study. Peer J. 2019, 7, e6311. [Google Scholar] [CrossRef] [Green Version]
- Kesh, H.; Kaushik, P. Advances in melon (Cucumis melo L.) breeding: An update. Sci. Hortic. 2021, 282, 110045. [Google Scholar] [CrossRef]
- Lake, J.A.; Steven, M.D.; Smith, K.L.; Lomax, B.H. Plant responses to elevated CO2 levels in soils: Distinct CO2 and O2-depletion effects. Int. J. Greenh. Gas Control 2017, 64, 333–339. [Google Scholar] [CrossRef]
- Kolb, R.M.; Dolder, H.; Cortelazzo, A.L. Effects of anoxia on root ultrastructure of four neotropical trees. Protoplasma 2004, 224, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yin, Z.; Yin, Y.; Xu, C.; Wang, W.; Liu, Y.; Li, T. Effects of elevated root-zone CO2 on root morphology and nitrogen metabolism revealed by physiological and transcriptome analysis in oriental melon seedling roots. Int. J. Mol. Sci. 2020, 21, 803. [Google Scholar] [CrossRef] [Green Version]
- Malamy, J. Intrinsic and environmental factors regulating root system growth. Plant Cell Env. 2005, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Zürcher, E.; Müller, B. Cytokinin synthesis, signaling, and function-advances and new insights. Int. Rev. Cell Mol. Biol. 2016, 324, 1–38. [Google Scholar] [PubMed]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2010, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Greenway, H.; Armstrong, W.; Colmer, T.D. Conditions leading to high CO2 (>5 kPa) in waterlogged–flooded soils and possible effects on root growth and metabolism. Ann. Bot. 2006, 98, 9–32. [Google Scholar] [CrossRef] [Green Version]
- Comparot-Moss, S.; Kotting, O.; Stettler, M. A Putative Phosphatase, LSF1, Is required for normal starch turnover in Arabidopsis leaves. Plant Physiol. 2010, 152, 685–697. [Google Scholar] [CrossRef] [Green Version]
- Geigenberger, P. Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 2003, 6, 247–256. [Google Scholar] [CrossRef]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Smith, S.M.; Smith, A.M. The diurnal metabolism of leaf starch. Biochem. J. 2007, 401, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Trethewey, R.N.; Smith, A.M. Starch Metabolism in Leaves. In Photosynthesis; Springer: Dordrecht, The Netherlands, 2000; pp. 205–231. [Google Scholar]
- Rosa, M.; Prado, C.; Podazza, G.; Interdonato, R.; González, J.A.; Hilal, M.; Prado, F.E. Soluble sugars metabolism, sensing and abiotic stress: A complex network in the life of plants. Plant Signal. Behav. 2009, 4, 388–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loreti, E.; de Bellis, L.; Alpi, A.; Perata, P. Why and how do plant cells sense sugars. Ann. Bot. 2001, 88, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.K.; Kaur, N. Sugar signalling and gene expression in relation to carbohydrate metabolism under abiotic stresses in plants. J. Biosci. 2005, 30, 761–776. [Google Scholar] [CrossRef]
- Wang, H.; Gong, M.; Xin, H.; Tang, L.; Dai, D.; Gao, Y.; Liu, C. Effects of chilling stress on the accumulation of soluble sugars and their key enzymes in Jatropha curcas seedlings. Physiol. Mol. Biol. Plants 2018, 24, 857–865. [Google Scholar]
- Wang, Y.; Feng, Y.; Yan, M.; Yu, J.; Zhou, X.; Bao, J.; Wu, C. Effect of saline–alkali stress on sugar metabolism of jujube fruit. Horticulturae 2022, 8, 474. [Google Scholar] [CrossRef]
- Yd, A.; Qiang, Z.A.; Lc, A.; Xy, A.; Wei, Z.B.; Bo, Z.C. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar]
- Albrecht, G.; Mustroph, A.; Fox, T.C. Sugar and fructan accumulation during metabolic adjustment between respiration and fermentation under low oxygen conditions in wheat roots. Physiol. Plant. 2010, 120, 93–105. [Google Scholar] [CrossRef]
- Mustroph, A.; Albrecht, G. Tolerance of crop plants to oxygen deficiency stress: Fermentative activity and photosynthetic capacity of entire seedlings under hypoxia and anoxia. Physiol. Plant. 2010, 117, 508. [Google Scholar] [CrossRef]
- Centeno, D.C.; Osorio, S.; Nunes-Nesi, A.; Bertolo, A.L.F.; Carneiro, R.T.; Araújo, W.L.; Steinhauser, M.C.; Michalska, J.; Rohrmann, J.; Geigenberger, P. Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell 2011, 23, 162–184. [Google Scholar] [CrossRef] [Green Version]
- Lake, J.; Walker, H.J.; Cameron, D.D.; Lomax, B.H. A novel root-to-shoot stomatal response to very high CO2 levels in the soil: Electrical, hydraulic and biochemical signalling. Physiol. Plant. 2016, 159, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkhani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Moore, B.D.; Sheen, J. Plant sugar sensing and signaling-a complex reality. Trends Plant Sci. 1999, 4, 250. [Google Scholar] [CrossRef]
- Baslam, M.; Mitsui, T.; Sueyoshi, K. Recent advances in carbon and nitrogen metabolism in C3 plants. Int. J. Mol. Sci. 2020, 22, 318. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Petreikov, M.; Zamski, E. Carbohydrate metabolism during early fruit development of sweet melon (Cucumis melo). Physiol. Plant. 1999, 106, 1–8. [Google Scholar]
- Lingle, S.E.; Dunlap, J.R. Sucrose metabolism in netted muskmelon fruit during development. Plant Phvsioh. 1987, 84, 386–389. [Google Scholar] [CrossRef] [Green Version]
- Ricard, B.; Rivoal, J.; Spiteri, A. Anaerobic stress induces the transcription and translation of sucrose synthasein rice. Plant Physiol. 1991, 95, 669–674. [Google Scholar] [CrossRef] [Green Version]
- Crespi, M.D.; Zabaleta, E.J.; Pontis, H.G. Sucrose synthase expression during cold acclimation in wheat. Plant Physiol. 1991, 96, 887–891. [Google Scholar] [CrossRef] [Green Version]
- Sringer, B.; Werr, W.; Stralinger, P. The shrunken gene on chromosome of Zea mays L. is expressed in various plant tissues and encodes an anaerobic protein. Mol. Gen. Genet. 1986, 205, 461–468. [Google Scholar] [CrossRef]
- Fernandez, O.; Vandesteene, L.; Feil, R.; Baillieul, F.; Lunn, J.E.; Clément, C. Trehalose metabolism is activated upon chilling in grapevine and might participate in Burkholderia phytofirmans induced chilling tolerance. Planta 2012, 236, 355–356. [Google Scholar] [CrossRef]
- Lester, G.E.; Arias, L.S.; Gomez-Lim, M. Muskmelon fruit soluble acid invertase and sucrose phosphate synthase activity and polypeptide profiles during growth and maturation. J. Am. Soc. Hortic. Sci. 2001, 126, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Schluepmann, H.; Van Dijken, A.; Aghdasi, M.; Wobbes, B.; Paul, M.; Smeekens, S. Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiol. 2004, 135, 879–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, L.D.; Gilday, A.; Feil, R.; Lunn, J.E.; Graham, I.A. AtTPS1-mediated trehalose 6-phosphate synthesis is essential for embryogenic and vegetative growth and responsiveness to ABA in germinating seeds and stomatal guard cells. Plant J. 2010, 64, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.W.; Nie, Y.X.; Wan, Y.Y.; Chen, S.Y.; Sun, Y.; Wang, X.J.; Bai, J.G. Exogenous glucose regulates activities of antioxidant enzyme, soluble acid invertase and neutral invertase and alleviates dehydration stress of cucumber seedlings. Sci. Hortic. 2013, 162, 20–30. [Google Scholar] [CrossRef]
- Dziedzoave, N.T.; Graffham, A.J.; Westby, A. Influence of variety and growth environment on β-amylase activity of flour from sweet potato (Ipomea batatas). Food Control 2010, 21, 162–165. [Google Scholar] [CrossRef]

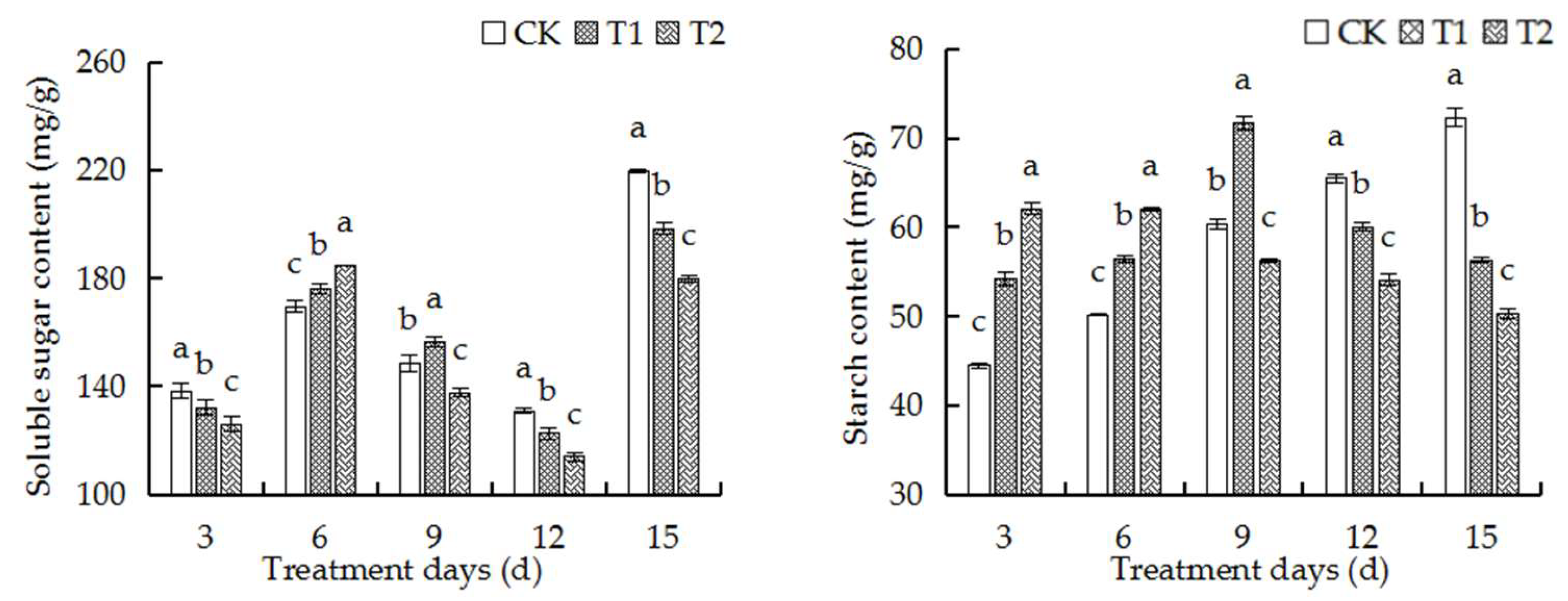
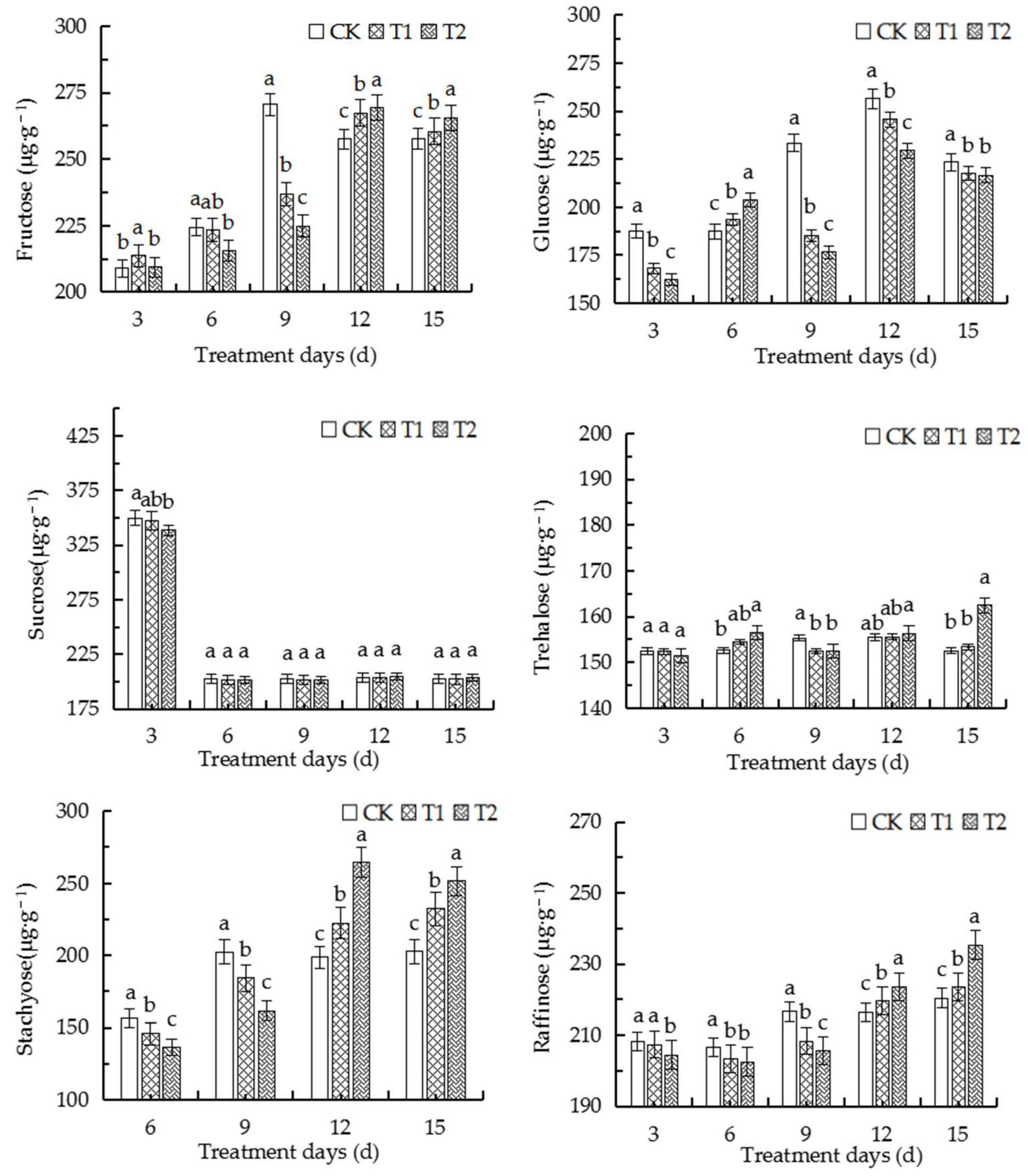

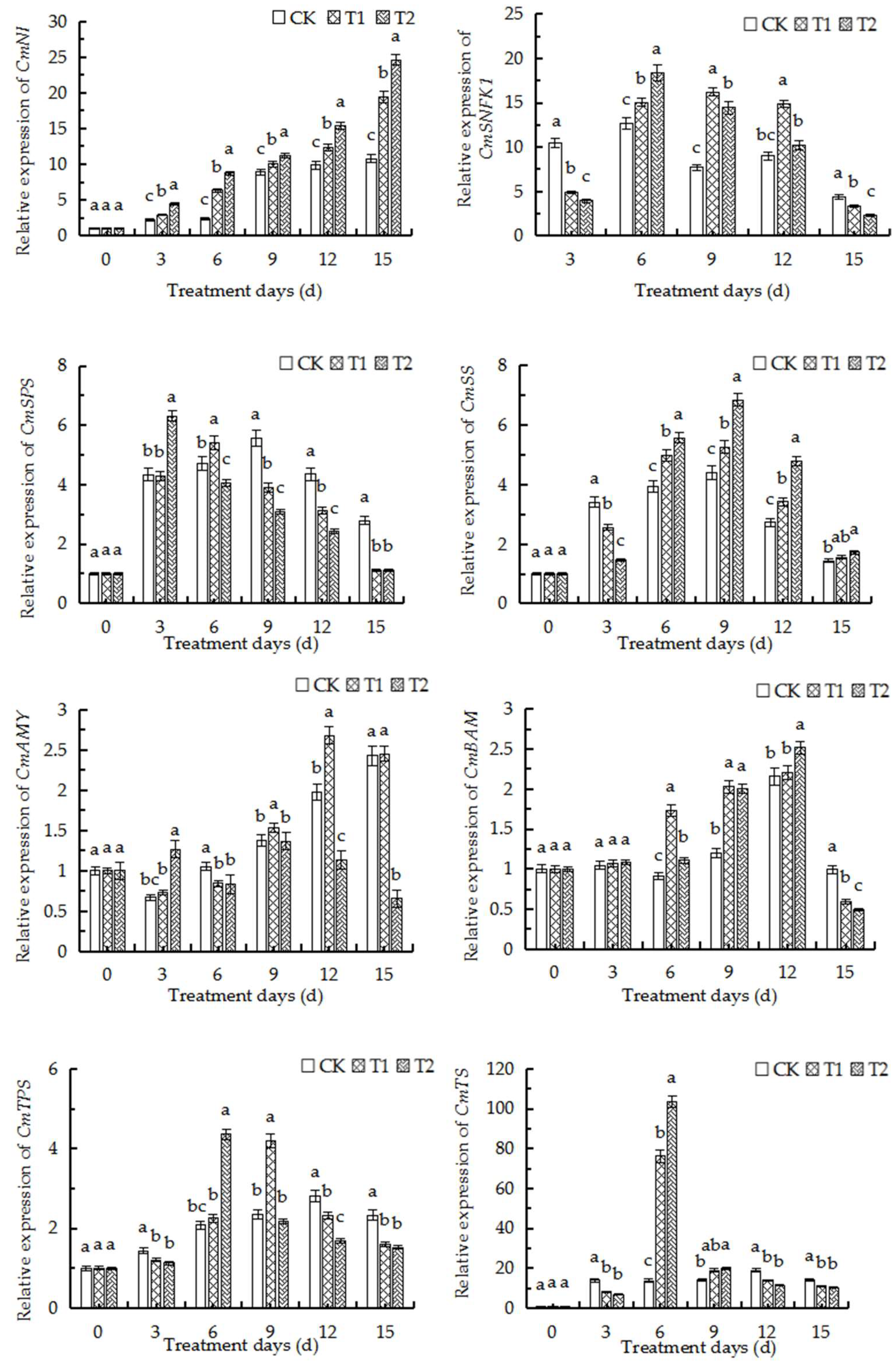

| Root Growth Indices | Treatment Groups | Days of Treatment (Day) | ||||
|---|---|---|---|---|---|---|
| 3 | 6 | 9 | 12 | 15 | ||
| Total root length (cm) | CK | 843.67 ± 1.53 c | 1107.33 ± 2.08 c | 1476.11 ± 2.21 a | 1597 ± 1.32 a | 1724.15 ± 2.65 a |
| T1 | 903.21 ± 1.24 b | 1175.55 ± 2.65 a | 1333.67 ± 3.06 b | 1396.67 ± 1.65 b | 1522.67 ± 3.06 b | |
| T2 | 1099.54 ± 1.21 a | 1127.33 ± 2.08 b | 1256.33 ± 2.52 c | 1322.24 ± 2.02 c | 1456.65 ± 2.03 c | |
| Total root surface area (cm2) | CK | 206.33 ± 2.52 c | 466.33 ± 1.53 a | 906 ± 1.23 a | 1467.67 ± 1.53 a | 1564.33 ± 1.35 a |
| T1 | 296.67 ± 3.21 b | 462.33 ± 2.58 b | 864 ± 3.61 b | 1434.33 ± 3.52 b | 1493.67 ± 4.04 b | |
| T2 | 436 ± 2.65 a | 455.67 ± 3.06 b | 714.67 ± 2.52 c | 1304.33 ± 0.58 c | 1363 ± 3.02 c | |
| Total root volume (cm3) | CK | 0.87 ± 0.03 c | 1.33 ± 0.04 b | 1.55 ± 0.02 b | 2.64 ± 0.02 a | 2.93 ± 0.02 a |
| T1 | 1.04 ± 0.01 b | 1.37 ± 0.01 b | 1.63 ± 0.01 a | 2.37 ± 0.02 b | 2.37 ± 0.02 b | |
| T2 | 1.26 ± 0.02 a | 1.42 ± 0.01 a | 1.37 ± 0.01 c | 1.73 ± 0.02 c | 2.05 ± 0.03 c | |
| Root vigor (μg·g−1·h−1) | CK | 12.66 ± 0.14 c | 13.67 ± 0.17 c | 15.08 ± 0.14 a | 5.98 ± 0.16 a | 16.45 ± 0.17 a |
| T1 | 13.46 ± 0.12 b | 14.86 ± 0.18 b | 14.56 ± 0.11 b | 15.26 ± 0.12 b | 15.76 ± 0.14 b | |
| T2 | 14.97 ± 0.12 a | 17.58 ± 0.11 a | 13.13 ± 0.13 c | 13.07 ± 0.12 c | 12.99 ± 0.12 c | |
| Total root tip number | CK | 867 ± 1.53 c | 1163 ± 2.08 c | 1446 ± 2.09 a | 1543 ± 1.53 a | 1685 ± 3.12 a |
| T1 | 1006 ± 1.28 b | 1277 ± 1.53 ab | 1403 ± 1.04 a | 1499 ± 1.35 b | 1543 ± 1.53 b | |
| T2 | 1222 ± 4.74 a | 1424 ± 3.21 a | 1295 ± 3.21 c | 1323 ± 3.21 c | 1312 ± 2.65 c | |
| 0–0.5 mm root tip number | CK | 255 ± 1.21 c | 276 ± 2.08 c | 246 ± 1.52 b | 265 ± 1.15 a | 244 ± 1.53 a |
| T1 | 337 ± 1.73 a | 354 ± 1.44 a | 274 ± 2.04 a | 235 ± 1.97 b | 221 ± 1.54 b | |
| T2 | 280 ± 1.09 b | 310 ± 1.14 b | 221 ± 1.11 c | 181 ± 0.54 c | 158 ± 1.73 c | |
| 0.5–2.0 mm root tip number | CK | 489 ± 1.21 c | 516 ± 2.28 c | 667 ± 1.44 a | 681 ± 1.71 a | 713 ± 1.62 a |
| T1 | 544 ± 2.05 b | 551 ± 1.15 b | 598 ± 1.61 b | 620 ± 1.34 b | 657 ± 2.04 b | |
| T2 | 590 ± 2.00 a | 655 ± 1.25 a | 567 ± 0.26 c | 580 ± 0.88 c | 602 ± 1.53 c | |
| Sample Name | Clean Reads | Clean Bases | % ≥Q30 | Mapped Reads | Unique Mapped Reads | GC Content |
|---|---|---|---|---|---|---|
| CK-1 | 20,972,383 | 6,281,865,348 | 92.95% | 39,476,959 (94.12%) | 38,780,024 (92.45%) | 43.84% |
| CK-2 | 21,989,492 | 6,587,534,178 | 92.62% | 41,278,506 (93.86%) | 40,506,664 (92.10%) | 44.19% |
| CK-3 | 19,324,296 | 5,782,784,588 | 92.88% | 36,309,424 (93.95%) | 35,665,517 (92.28%) | 43.79% |
| T1-1 | 21,680,097 | 6,481,877,992 | 92.94% | 40,640,140 (93.73%) | 39,880,455 (91.97%) | 44.48% |
| T1-2 | 22,140,865 | 6,624,929,878 | 92.68% | 41,229,200 (93.11%) | 40,478,885 (91.41%) | 44.45% |
| T1-3 | 21,089,895 | 6,312,995,220 | 92.76% | 39,347,813 (93.29%) | 38,609,369 (91.54%) | 44.37% |
| T2-1 | 21,309,551 | 6,376,761,952 | 93.05% | 39,664,868 (93.07%) | 38,978,316 (91.46%) | 44.52% |
| T2-2 | 21,762,284 | 6,517,388,230 | 92.82% | 40,803,032 (93.75%) | 40,125,589 (92.19%) | 43.96% |
| T2-3 | 22,392,078 | 6,709,294,358 | 92.91% | 42,016,764 (93.82%) | 45,076,178 (92.90%) | 44.39% |
| Comparison between Treatments | Gene ID | Gene Name | FDR | Log2Fold Change | Regulation |
|---|---|---|---|---|---|
| T1/CK | MELO3C006362.2 | BAM | 1.29 × 10−35 | 1.549877695 | up |
| MELO3C005751.2 | TS | 2.22 × 10−65 | 1.073184869 | up | |
| MELO3C026210.2 | SNFK1 | 1.76 × 10−45 | 1.019037441 | up | |
| MELO3C017002.2 | AMY | 2.21 × 10−7 | −1.237429382 | down | |
| T2/CK | MELO3C006362.2 | BAM | 1.39 × 10−17 | 1.210496866 | up |
| MELO3C005751.2 | TS | 0 | 3.029269697 | up | |
| MELO3C025673.2 | TPS | 5.77 × 10−185 | 1.669152326 | up | |
| MELO3C017942.2 | SS | 7.66 × 10−55 | 1.17349538 | up | |
| MELO3C026210.2 | SNFK1 | 3.87 × 10−94 | 1.44586252 | up | |
| MELO3C006727.2 | NI | 1.02 × 10−55 | 1.130833392 | up | |
| MELO3C017002.2 | AMY | 2.21 × 10−7 | −1.237429382 | down | |
| T2/T1 | MELO3C025673.2 | TPS | 5.77 × 10−185 | 1.669152326 | up |
| MELO3C005751.2 | TS | 0 | 3.029269697 | up | |
| MELO3C006727.2 | NI | 1.02 × 10−55 | 1.130833392 | up | |
| MELO3C020357.2 | SPS | 1.86 × 10−8 | −1.040248354 | down |
| Gene ID | Sequence (5′–3′) | |
|---|---|---|
| Actin | F | AAGGCAAACAGGGAGAAGATGA |
| R | AGCAAGGTCGAGACGTAGGATA | |
| MELO3C017002.2 | F | CCCAACCTTTTGTCCGTAGAG |
| R | CAATCCAATTTATTATCCGCTGT | |
| MELO3C013887.2 | F | TGATGATGCCGTTGGACAGT |
| R | CCGTGCTTTTTAGCCATTTCT | |
| MELO3C017942.2 | F | TTTACTCGTAGCCCCAGCGT |
| R | AATGGCGGCAGAACAATAGC | |
| MELO3C020357.2 | F | CGTGCTCGGCGTGGAGTA |
| R | GGAGAGGACCCATCGCTTGTA | |
| MELO3C005751.2 | F | AACAGAAGTCAGCCTCCCCTT |
| R | TGATGCGATTTCACGGTATAGAT | |
| MELO3C025673.2 | F | ATTGGCGTGATAAAGTTGTTTTG |
| R | ATATAGCGCACACAAGTGCATGA | |
| MELO3C026210.2 | F | GAAATGCGTCGAAGAAGGCT |
| R | TCGTGTTGTCCCAACGGTGT | |
| MELO3C006727.2 | F | CTTTATGTCTGTCGGAGGGTT |
| R | GCTTCACAATACGCTCTATGCACT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Wang, W.; Xu, C.; Han, X.; Li, Y.; Liu, Y.; Qi, H. Physiological and Transcriptomic Analyses Reveal the Effects of Elevated Root-Zone CO2 on the Metabolism of Sugars and Starch in the Roots of Oriental Melon Seedlings. Int. J. Mol. Sci. 2022, 23, 12537. https://doi.org/10.3390/ijms232012537
Gao L, Wang W, Xu C, Han X, Li Y, Liu Y, Qi H. Physiological and Transcriptomic Analyses Reveal the Effects of Elevated Root-Zone CO2 on the Metabolism of Sugars and Starch in the Roots of Oriental Melon Seedlings. International Journal of Molecular Sciences. 2022; 23(20):12537. https://doi.org/10.3390/ijms232012537
Chicago/Turabian StyleGao, Lijia, Wanxin Wang, Chuanqiang Xu, Xintong Han, Yanan Li, Yiling Liu, and Hongyan Qi. 2022. "Physiological and Transcriptomic Analyses Reveal the Effects of Elevated Root-Zone CO2 on the Metabolism of Sugars and Starch in the Roots of Oriental Melon Seedlings" International Journal of Molecular Sciences 23, no. 20: 12537. https://doi.org/10.3390/ijms232012537
APA StyleGao, L., Wang, W., Xu, C., Han, X., Li, Y., Liu, Y., & Qi, H. (2022). Physiological and Transcriptomic Analyses Reveal the Effects of Elevated Root-Zone CO2 on the Metabolism of Sugars and Starch in the Roots of Oriental Melon Seedlings. International Journal of Molecular Sciences, 23(20), 12537. https://doi.org/10.3390/ijms232012537





