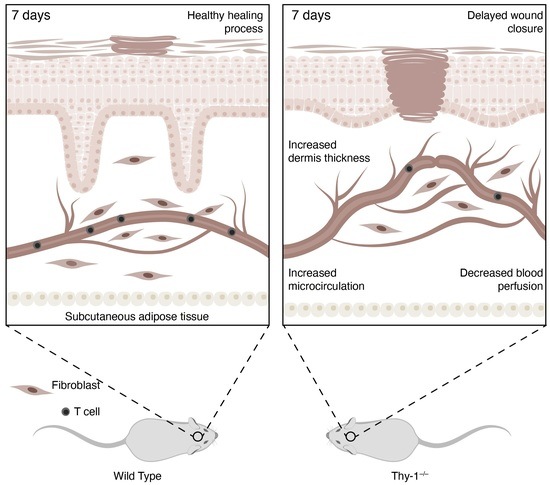
The GPI-Anchored Protein Thy-1/CD90 Promotes Wound Healing upon Injury to the Skin by Enhancing Skin Perfusion
Abstract
1. Introduction
2. Results
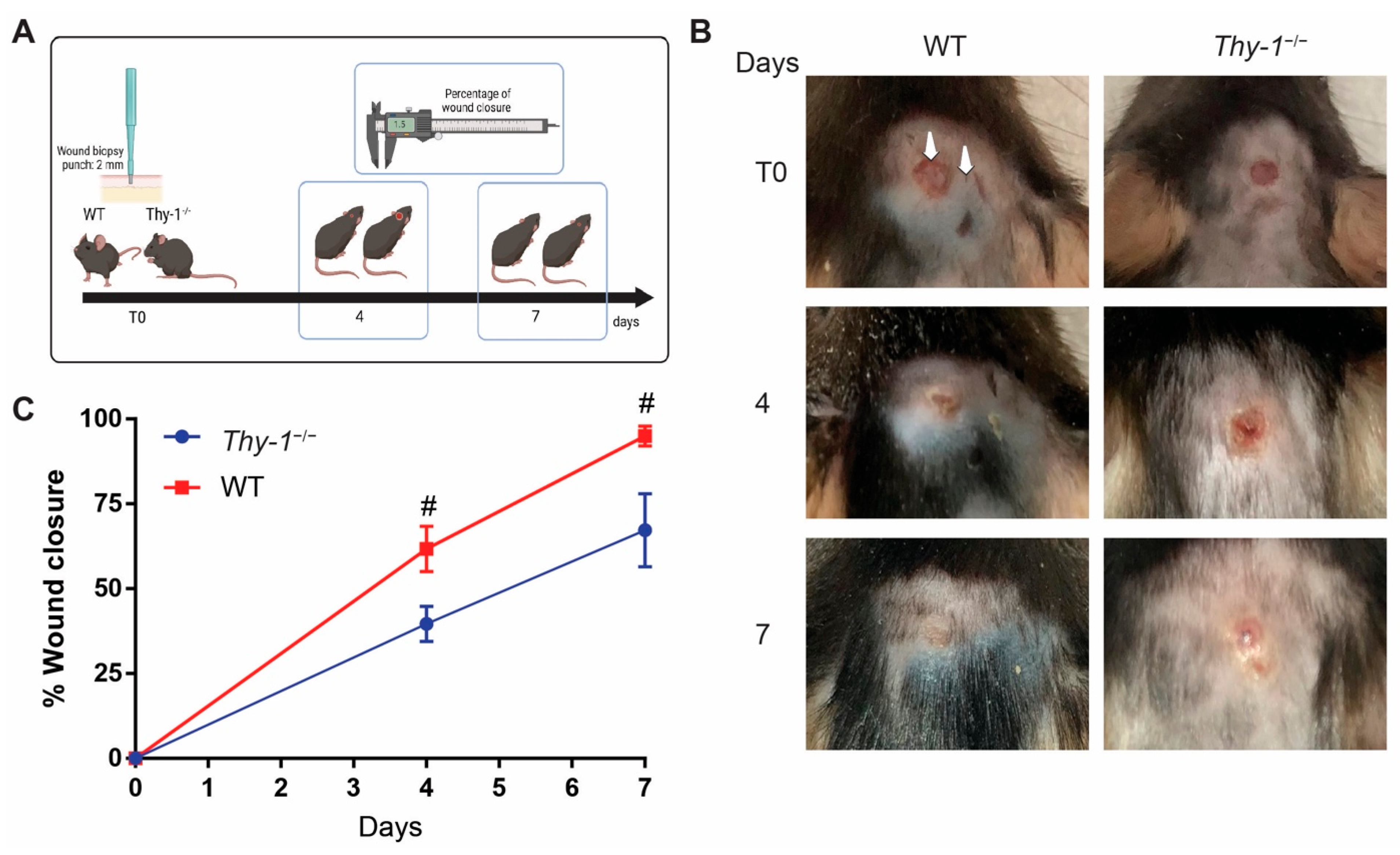
2.1. Lack of Thy-1 Expression Delays Wound Healing
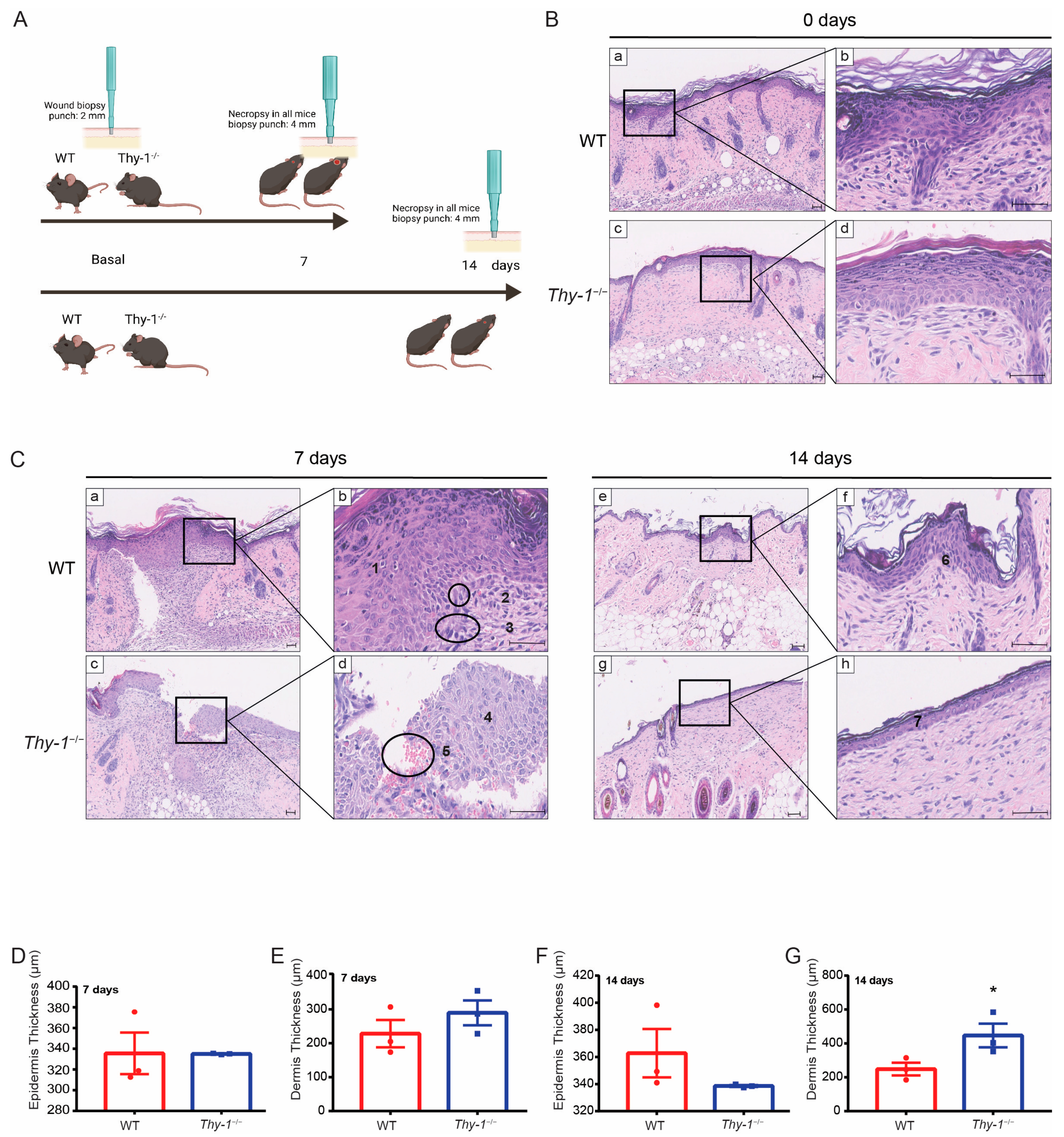
2.2. Skin Morphology during Wound Healing Is Altered in Mice Lacking Thy-1
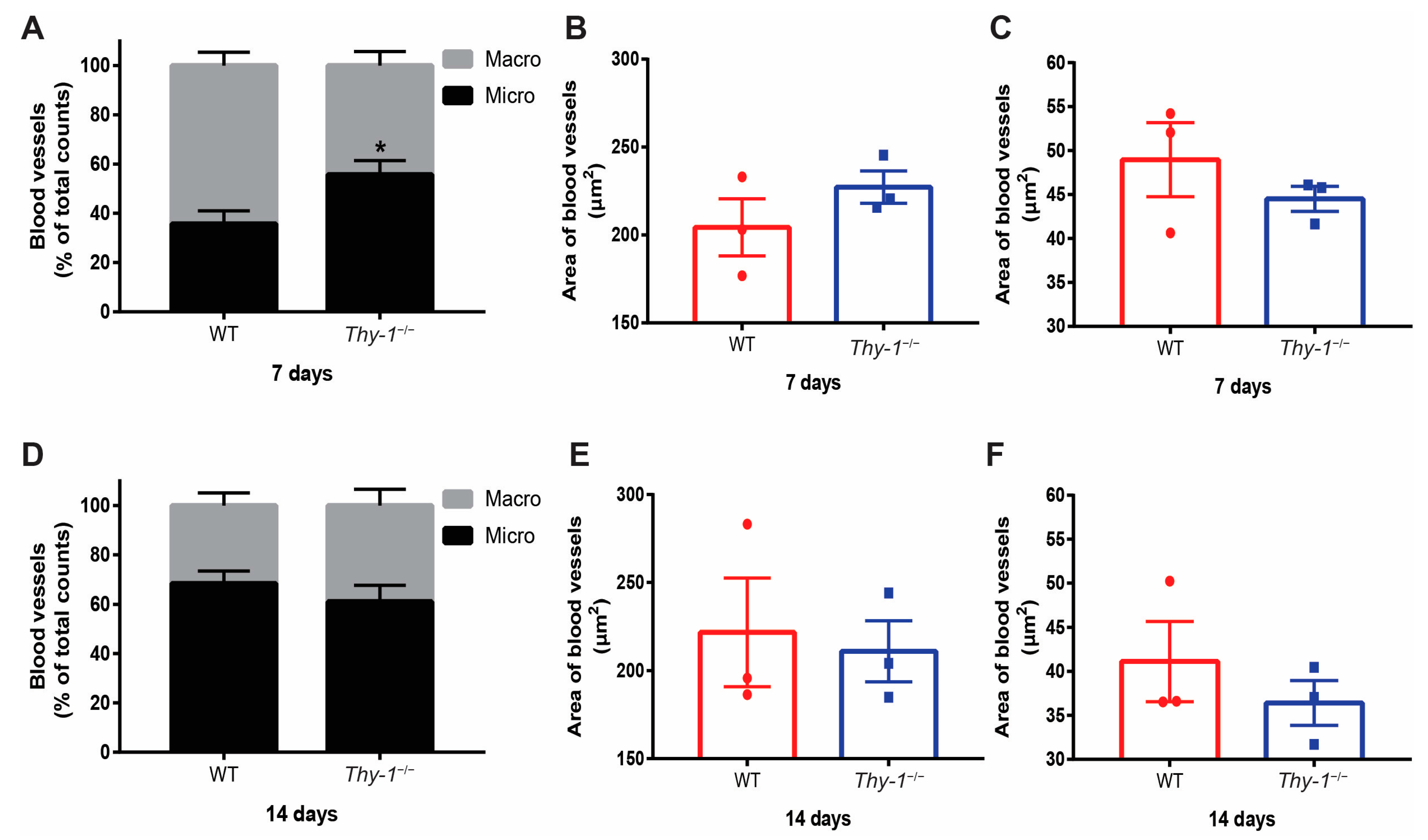
2.3. Micro and Macrovascular Vessels Are Regulated by Thy-1
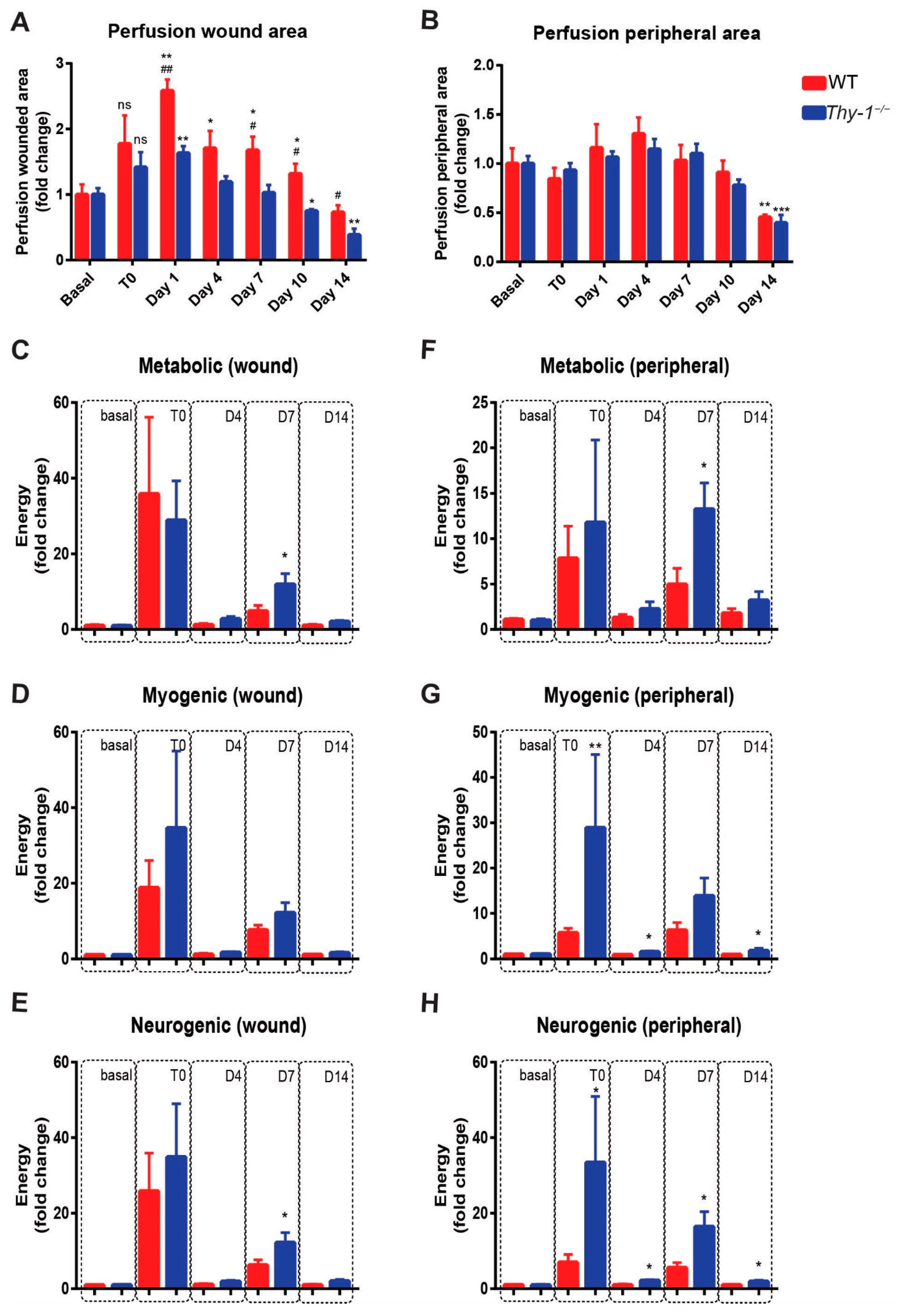
2.4. Absence of Thy-1 Decreases Blood Perfusion in Wound Healing
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. In Vivo Wound Healing Assay
4.3. Speckle Laser Perfusion Analysis
4.4. Wavelet Analysis
4.5. Histology
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schilrreff, P.; Alexiev, U. Chronic Inflammation in Non-Healing Skin Wounds and Promising Natural Bioactive Compounds Treatment. Int. J. Mol. Sci. 2022, 23, 4928. [Google Scholar] [CrossRef] [PubMed]
- Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Walmsley, G.G.; Marecic, O.; Hu, M.; Senarath-Yapa, K.; McArdle, A.; Tevlin, R.; et al. Wound healing: An update. Regen. Med. 2014, 9, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Mustoe, T.; Clark, R.A. Cutaneous wound healing in aging small mammals: A systematic review. Wound Repair Regen 2015, 23, 318–339. [Google Scholar] [CrossRef] [PubMed]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Tonnesen, M.G.; Feng, X.; Clark, R.A. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Chaseling, G.K.; Crandall, C.G.; Gagnon, D. Skin blood flow measurements during heat stress: Technical and analytical considerations. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R57–R69. [Google Scholar] [CrossRef]
- Mahe, G.; Rousseau, P.; Durand, S.; Bricq, S.; Leftheriotis, G.; Abraham, P. Laser speckle contrast imaging accurately measures blood flow over moving skin surfaces. Microvasc. Res. 2011, 81, 183–188. [Google Scholar] [CrossRef]
- Gnyawali, S.C.; Blum, K.; Pal, D.; Ghatak, S.; Khanna, S.; Roy, S.; Sen, C.K. Retooling Laser Speckle Contrast Analysis Algorithm to Enhance Non-Invasive High Resolution Laser Speckle Functional Imaging of Cutaneous Microcirculation. Sci. Rep. 2017, 7, 41048. [Google Scholar] [CrossRef]
- Lal, C.; Unni, S.N. Correlation analysis of laser Doppler flowmetry signals: A potential non-invasive tool to assess microcirculatory changes in diabetes mellitus. Med. Biol. Eng. Comput. 2015, 53, 557–566. [Google Scholar] [CrossRef]
- Kvernmo, H.; Stefanovska, A.; Bracic, M.; Kirkebøen, K.; Kvernebo, K. Spectral Analysis of the Laser Doppler Perfusion Signal in Human Skin before and after Exercise. Microvasc. Res. 1998, 56, 173–182. [Google Scholar] [CrossRef]
- Zhou, Y.; Hagood, J.S.; Lu, B.; Merryman, W.D.; Murphy-Ullrich, J.E. Thy-1-integrin alphav beta5 interactions inhibit lung fibroblast contraction-induced latent transforming growth factor-beta1 activation and myofibroblast differentiation. J. Biol. Chem. 2010, 285, 22382–22393. [Google Scholar] [CrossRef] [PubMed]
- Brenet, M.; Martinez, S.; Perez-Nunez, R.; Perez, L.A.; Contreras, P.; Diaz, J.; Avalos, A.M.; Schneider, P.; Quest, A.F.G.; Leyton, L. Thy-1 (CD90)-Induced Metastatic Cancer Cell Migration and Invasion Are beta3 Integrin-Dependent and Involve a Ca(2+)/P2X7 Receptor Signaling Axis. Front. Cell Dev. Biol. 2020, 8, 592442. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Gutknecht, D.; Koberle, M.; Anderegg, U.; Saalbach, A. Melanoma cells use Thy-1 (CD90) on endothelial cells for metastasis formation. Am. J. Pathol. 2013, 182, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Schubert, K.; Polte, T.; Bonisch, U.; Schader, S.; Holtappels, R.; Hildebrandt, G.; Lehmann, J.; Simon, J.C.; Anderegg, U.; Saalbach, A. Thy-1 (CD90) regulates the extravasation of leukocytes during inflammation. Eur. J. Immunol. 2011, 41, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Saalbach, A.; Haustein, U.F.; Anderegg, U. A ligand of human thy-1 is localized on polymorphonuclear leukocytes and monocytes and mediates the binding to activated thy-1-positive microvascular endothelial cells and fibroblasts. J. Investig. Dermatol. 2000, 115, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, A.; Chavakis, T.; Preissner, K.T.; Sticherling, M.; Haustein, U.F.; Anderegg, U.; Saalbach, A. Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J. Immunol. 2004, 172, 3850–3859. [Google Scholar] [CrossRef]
- Perez, L.A.; Leyton, L.; Valdivia, A. Thy-1 (CD90), Integrins and Syndecan 4 are Key Regulators of Skin Wound Healing. Front. Cell Dev. Biol. 2022, 10, 810474. [Google Scholar] [CrossRef]
- Lee, M.J.; Shin, J.O.; Jung, H.S. Thy-1 knockdown retards wound repair in mouse skin. J. Dermatol. Sci. 2013, 69, 95–104. [Google Scholar] [CrossRef]
- Lee, W.-S.; Jain, M.K.; Arkonac, B.M.; Zhang, D.; Shaw, S.-Y.; Kashiki, S.; Maemura, K.; Lee, S.-L.; Hollenberg, N.K.; Lee, M.-E.; et al. Thy-1, a Novel Marker for Angiogenesis Upregulated by Inflammatory Cytokines. Circ. Res. 1998, 82, 845–851. [Google Scholar] [CrossRef]
- Wong, R.; Geyer, S.; Weninger, W.; Guimberteau, J.C.; Wong, J.K. The dynamic anatomy and patterning of skin. Exp. Dermatol. 2016, 25, 92–98. [Google Scholar] [CrossRef]
- Roy, S.; Patel, D.; Khanna, S.; Gordillo, G.M.; Biswas, S.; Friedman, A.; Sen, C.K. Transcriptome-wide analysis of blood vessels laser captured from human skin and chronic wound-edge tissue. Proc. Natl. Acad. Sci. USA 2007, 104, 14472–14477. [Google Scholar] [CrossRef] [PubMed]
- Josvay, K.; Winter, Z.; Katona, R.L.; Pecze, L.; Marton, A.; Buhala, A.; Szakonyi, G.; Olah, Z.; Vizler, C. Besides neuro-imaging, the Thy1-YFP mouse could serve for visualizing experimental tumours, inflammation and wound-healing. Sci. Rep. 2014, 4, 6776. [Google Scholar] [CrossRef] [PubMed]
- Koumas, L.; Smith, T.J.; Feldon, S.; Blumberg, N.; Phipps, R.P. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. Am. J. Pathol. 2003, 163, 1291–1300. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Koren, E.; Feldman, A.; Yusupova, M.; Kadosh, A.; Sedov, E.; Ankawa, R.; Yosefzon, Y.; Nasser, W.; Gerstberger, S.; Kimel, L.B.; et al. Thy1 marks a distinct population of slow-cycling stem cells in the mouse epidermis. Nat. Commun. 2022, 13, 4628. [Google Scholar] [CrossRef]
- Kemp, S.S.; Aguera, K.N.; Cha, B.; Davis, G.E. Defining Endothelial Cell-Derived Factors That Promote Pericyte Recruitment and Capillary Network Assembly. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2632–2648. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Z.; Shou, K.; Jian, C.; Li, P.; Niu, Y.; Qi, B.; Yu, A. Negative pressure wound therapy: Regulating blood flow perfusion and microvessel maturation through microvascular pericytes. Int. J. Mol. Med. 2017, 40, 1415–1425. [Google Scholar] [CrossRef]
- Payne, L.B.; Zhao, H.; James, C.C.; Darden, J.; McGuire, D.; Taylor, S.; Smyth, J.W.; Chappell, J.C. The pericyte microenvironment during vascular development. Microcirculation 2019, 26, e12554. [Google Scholar] [CrossRef]
- Park, T.I.; Feisst, V.; Brooks, A.E.; Rustenhoven, J.; Monzo, H.J.; Feng, S.X.; Mee, E.W.; Bergin, P.S.; Oldfield, R.; Graham, E.S.; et al. Cultured pericytes from human brain show phenotypic and functional differences associated with differential CD90 expression. Sci. Rep. 2016, 6, 26587. [Google Scholar] [CrossRef]
- Wen, H.C.; Huo, Y.N.; Chou, C.M.; Lee, W.S. PMA inhibits endothelial cell migration through activating the PKC-delta/Syk/NF-kappaB-mediated up-regulation of Thy-1. Sci. Rep. 2018, 8, 16247. [Google Scholar] [CrossRef] [PubMed]
- Dinh, T.; Elder, S.; Veves, A. Delayed wound healing in diabetes: Considering future treatments. Diabetes Manag. 2011, 1, 509–519. [Google Scholar] [CrossRef]
- Pierpont, Y.N.; Dinh, T.P.; Salas, R.E.; Johnson, E.L.; Wright, T.G.; Robson, M.C.; Payne, W.G. Obesity and surgical wound healing: A current review. ISRN Obes. 2014, 2014, 638936. [Google Scholar] [CrossRef] [PubMed]
- Sedov, E.; Koren, E.; Chopra, S.; Ankawa, R.; Yosefzon, Y.; Yusupova, M.; Weiss, L.E.; Mahly, A.; Soffer, A.; Feldman, A.; et al. THY1-mediated mechanisms converge to drive YAP activation in skin homeostasis and repair. Nat. Cell Biol. 2022, 24, 1049–1063. [Google Scholar] [CrossRef]
- Wong, V.W.; Sorkin, M.; Glotzbach, J.P.; Longaker, M.T.; Gurtner, G.C. Surgical approaches to create murine models of human wound healing. J. Biomed. Biotechnol. 2011, 2011, 969618. [Google Scholar] [CrossRef]
- Aguilera, V.; Briceno, L.; Contreras, H.; Lamperti, L.; Sepulveda, E.; Diaz-Perez, F.; Leon, M.; Veas, C.; Maura, R.; Toledo, J.R.; et al. Endothelium trans differentiated from Wharton’s jelly mesenchymal cells promote tissue regeneration: Potential role of soluble pro-angiogenic factors. PLoS ONE 2014, 9, e111025. [Google Scholar] [CrossRef]
- Troncoso, F.; Herlitz, K.; Acurio, J.; Aguayo, C.; Guevara, K.; Castro, F.O.; Godoy, A.S.; San Martin, S.; Escudero, C. Advantages in Wound Healing Process in Female Mice Require Upregulation A2A-Mediated Angiogenesis under the Stimulation of 17beta-Estradiol. Int. J. Mol. Sci. 2020, 21, 7145. [Google Scholar] [CrossRef]
- Merino, M.; Martin, S.S.; Sandana, P.; Herlitz, K.; Aguayo, C.; Godoy, A.; Torres-Vergara, P.; Gonzalez, M.; Troncoso, F.; Acurio, J.; et al. Deletion of the adenosine A2A receptor increases the survival rate in a mice model of polymicrobial sepsis. Purinergic Signal. 2020, 16, 427–437. [Google Scholar] [CrossRef]
- Cumsille, P.; Lara, E.; Verdugo-Hernandez, P.; Acurio, J.; Escudero, C. A robust quantitative approach for laser speckle contrast imaging perfusion analysis revealed anomalies in the brain blood flow in offspring mice of preeclampsia. Microvasc. Res. 2022, 144, 104418. [Google Scholar] [CrossRef]
- Mena, M.; Lloveras, B.; Tous, S.; Bogers, J.; Maffini, F.; Gangane, N.; Kumar, R.V.; Somanathan, T.; Lucas, E.; Anantharaman, D.; et al. Development and validation of a protocol for optimizing the use of paraffin blocks in molecular epidemiological studies: The example from the HPV-AHEAD study. PLoS ONE 2017, 12, e0184520. [Google Scholar] [CrossRef]
- Masserdotti, C. Architectural patterns in cytology: Correlation with histology. Vet. Clin. Pathol. 2006, 35, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Popel, A.S.; Johnson, P.C. Microcirculation and Hemorheology. Annu. Rev. Fluid Mech. 2005, 37, 43–69. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez, L.A.; León, J.; López, J.; Rojas, D.; Reyes, M.; Contreras, P.; Quest, A.F.G.; Escudero, C.; Leyton, L. The GPI-Anchored Protein Thy-1/CD90 Promotes Wound Healing upon Injury to the Skin by Enhancing Skin Perfusion. Int. J. Mol. Sci. 2022, 23, 12539. https://doi.org/10.3390/ijms232012539
Pérez LA, León J, López J, Rojas D, Reyes M, Contreras P, Quest AFG, Escudero C, Leyton L. The GPI-Anchored Protein Thy-1/CD90 Promotes Wound Healing upon Injury to the Skin by Enhancing Skin Perfusion. International Journal of Molecular Sciences. 2022; 23(20):12539. https://doi.org/10.3390/ijms232012539
Chicago/Turabian StylePérez, Leonardo A., José León, Juan López, Daniela Rojas, Montserrat Reyes, Pamela Contreras, Andrew F. G. Quest, Carlos Escudero, and Lisette Leyton. 2022. "The GPI-Anchored Protein Thy-1/CD90 Promotes Wound Healing upon Injury to the Skin by Enhancing Skin Perfusion" International Journal of Molecular Sciences 23, no. 20: 12539. https://doi.org/10.3390/ijms232012539
APA StylePérez, L. A., León, J., López, J., Rojas, D., Reyes, M., Contreras, P., Quest, A. F. G., Escudero, C., & Leyton, L. (2022). The GPI-Anchored Protein Thy-1/CD90 Promotes Wound Healing upon Injury to the Skin by Enhancing Skin Perfusion. International Journal of Molecular Sciences, 23(20), 12539. https://doi.org/10.3390/ijms232012539