Effect of Heat Starvation Stress on Physiological Immunity and Metabolism of Mizuhopecten yessoensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Measurement, and Collection
2.2. Analysis of Biochemical Indicators
2.3. RNA Extraction, Library Construction and RNA-Seq
2.4. Data Preprocessing
2.5. Differentially Expressed Genes
2.6. Function Enrichment Analysis
3. Results
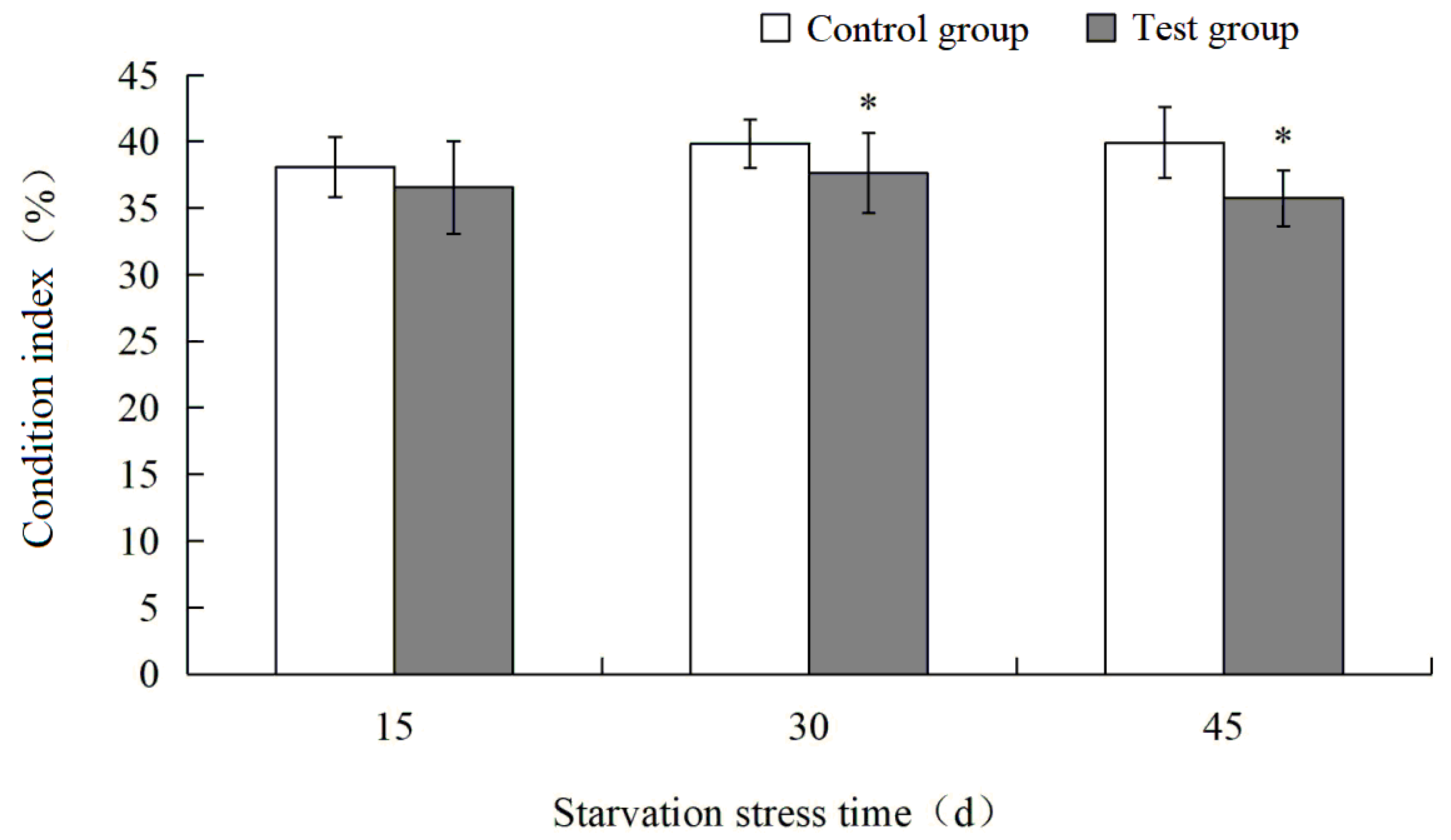
3.1. Effects of Starvation Stress on Condition Index of M. yessoensis
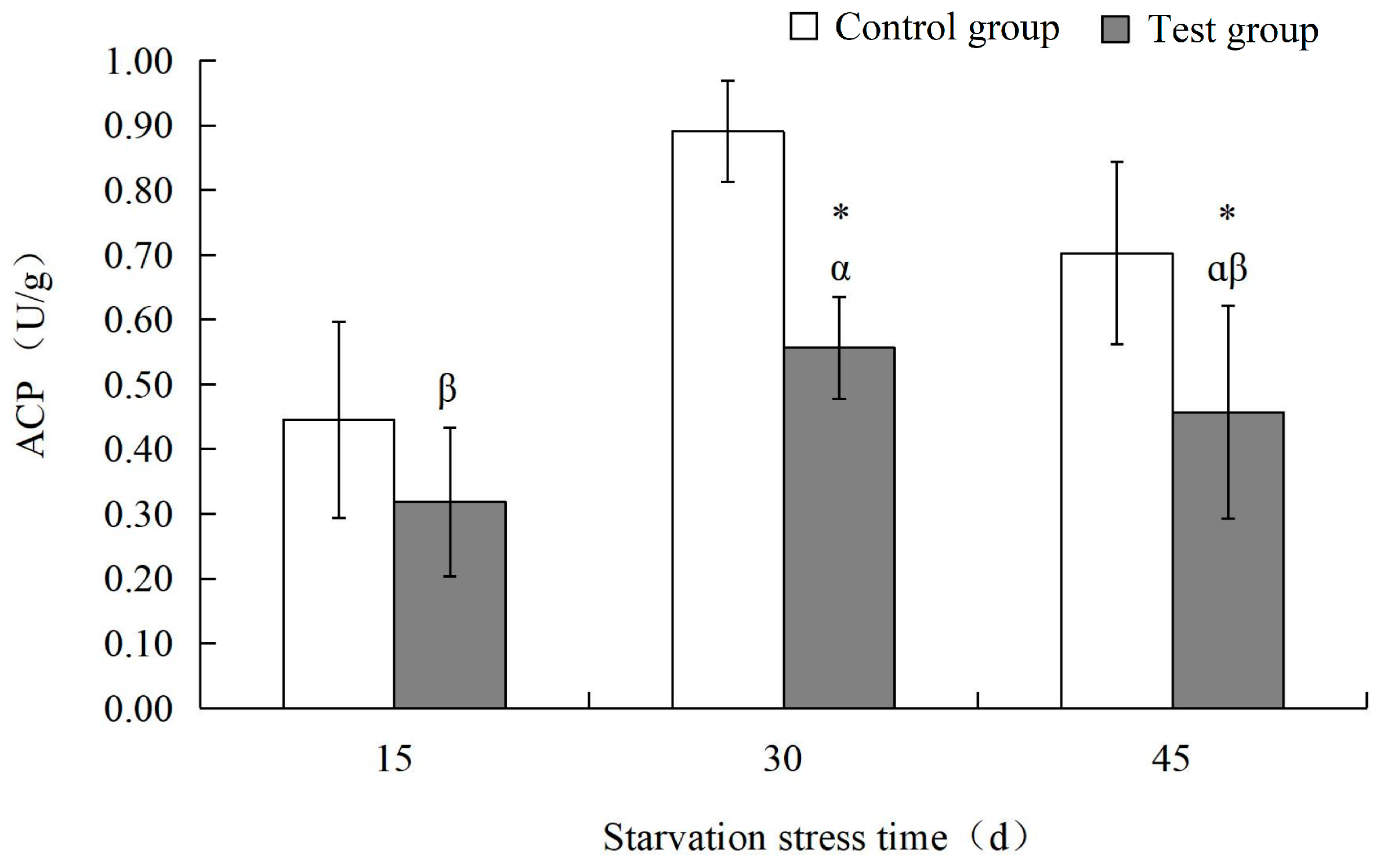
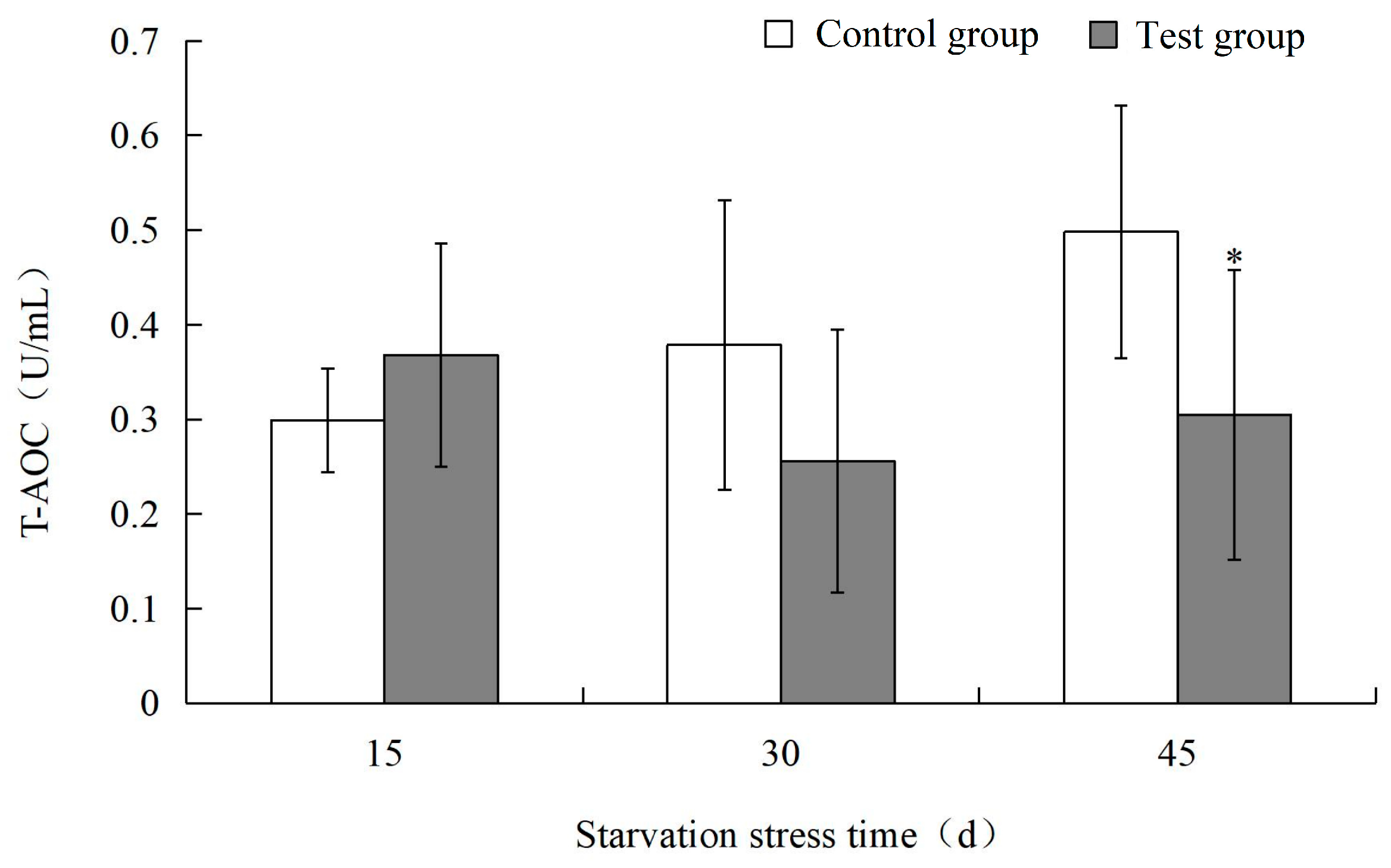
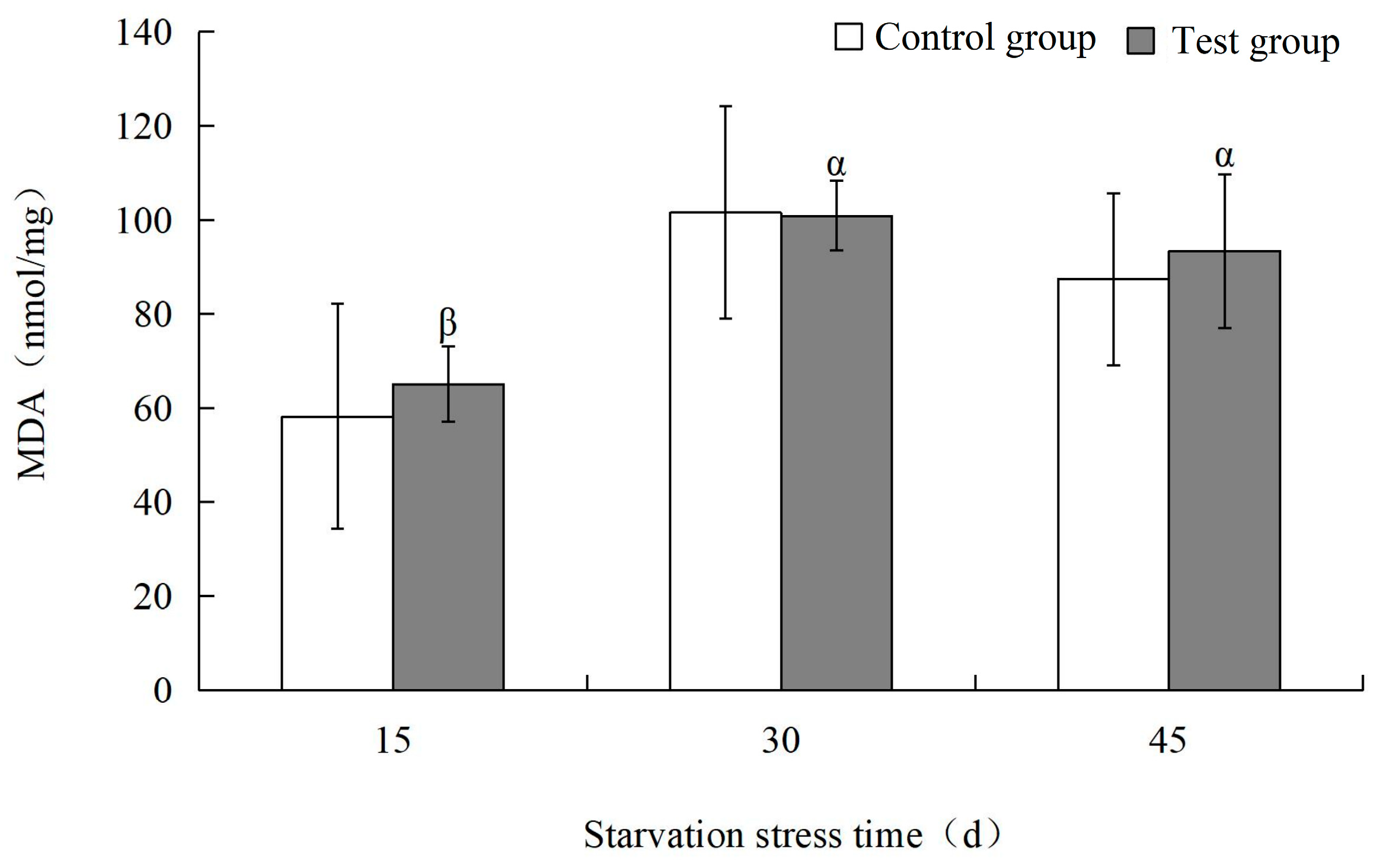
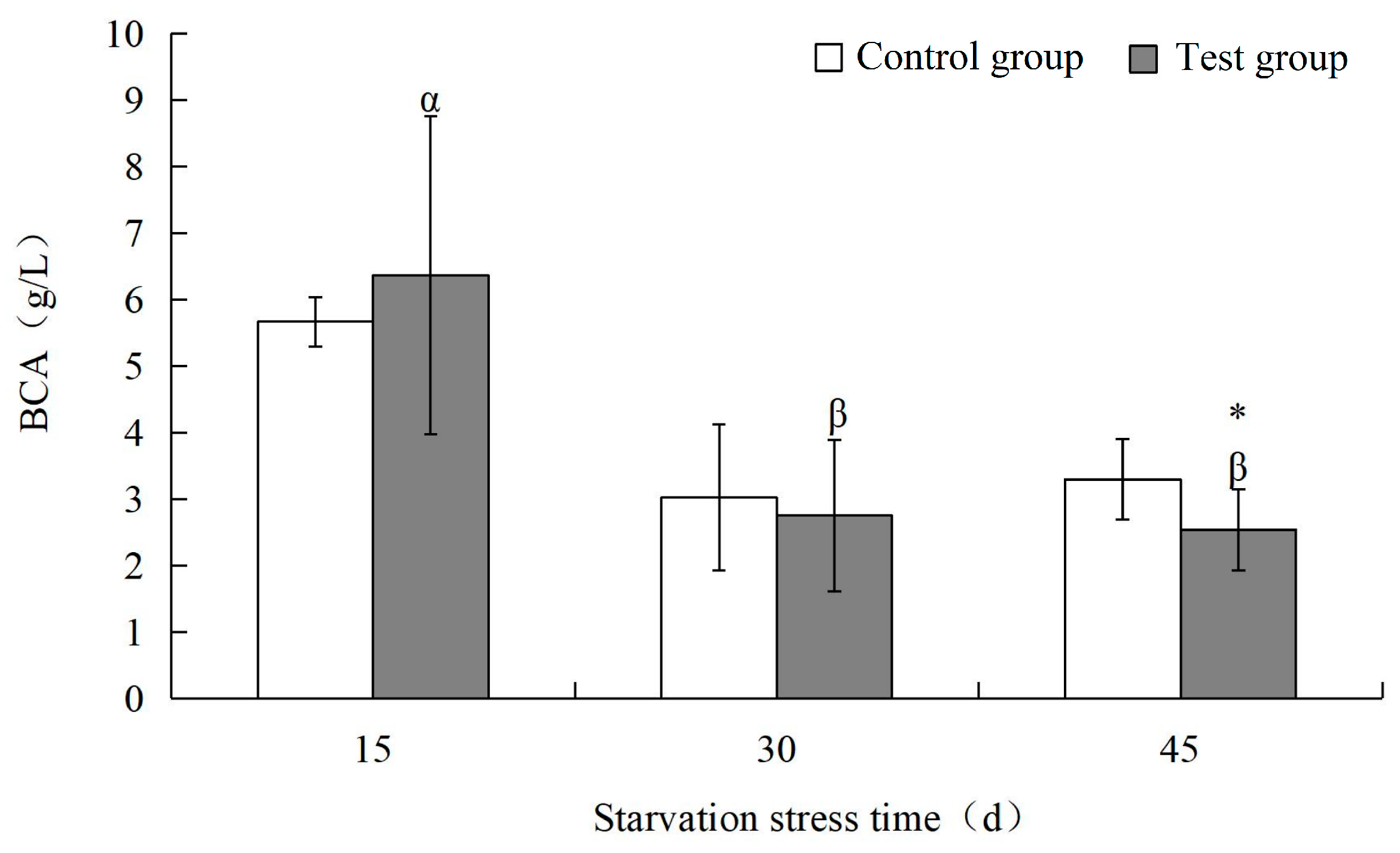
3.2. Effects of Starvation Stress on Biochemical Indexes of M. yessoensis
3.3. Sequence Analysis
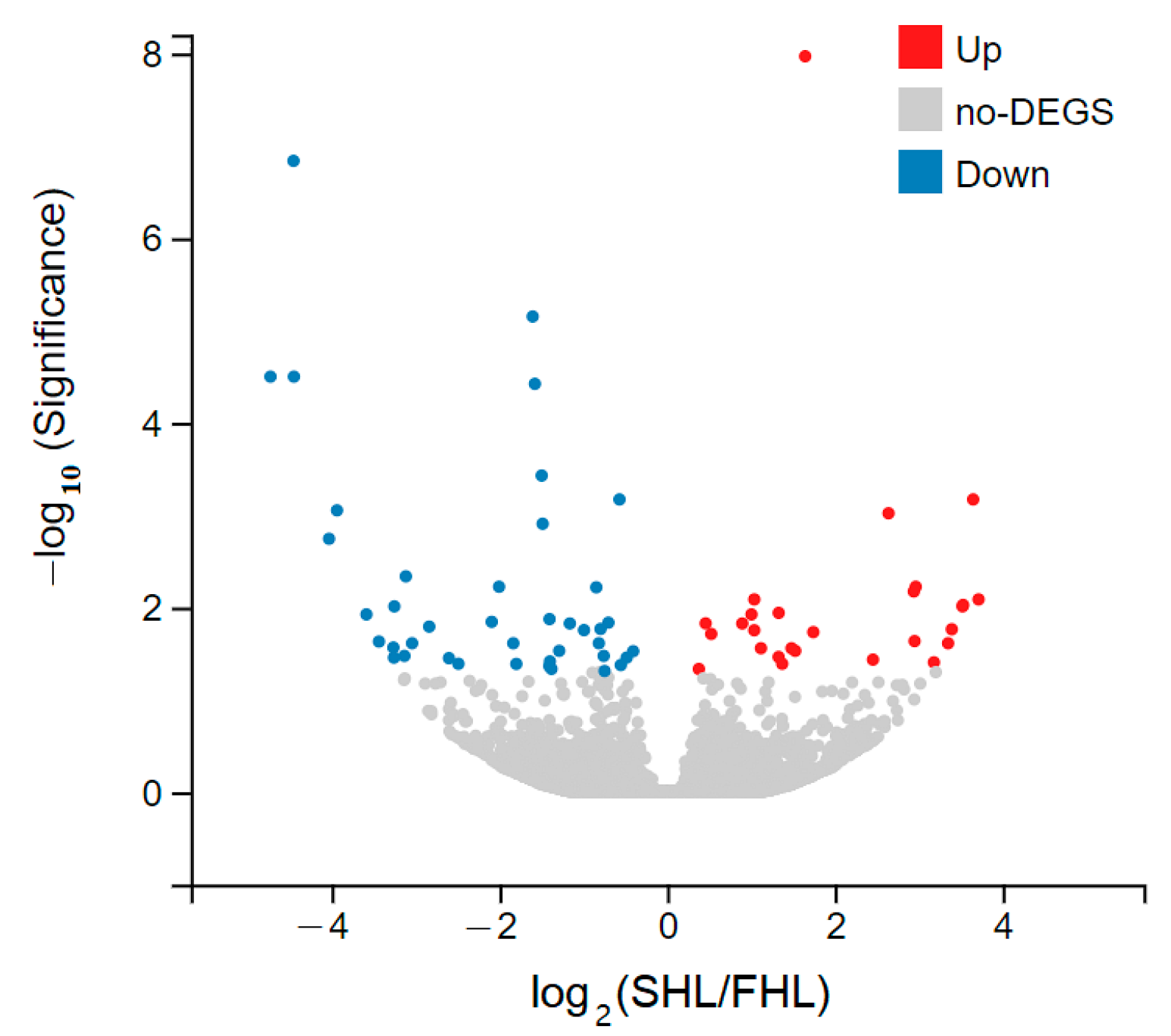
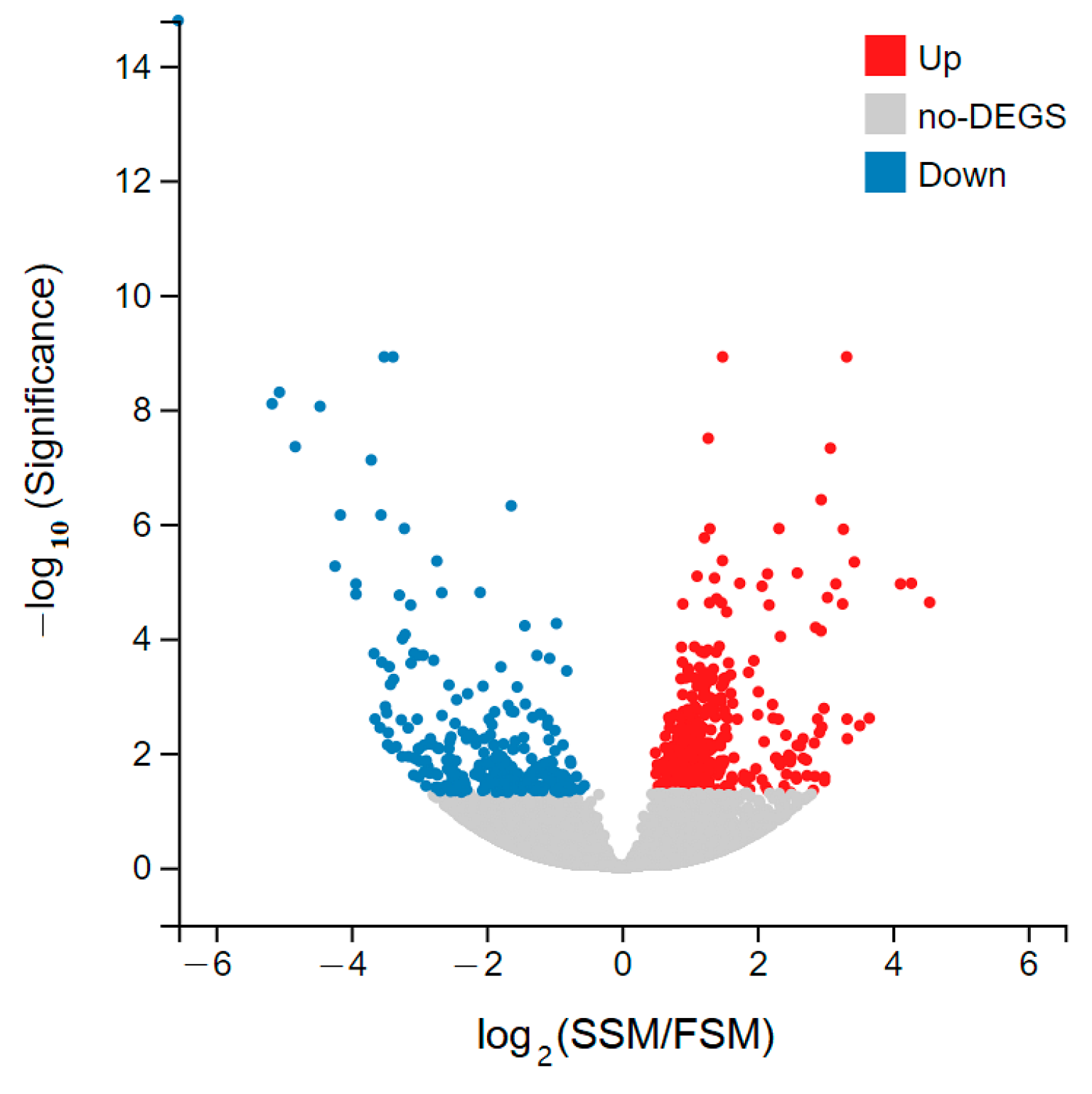
3.4. Differentially Expressed Genes
3.5. GO Functional Analysis of DEGs
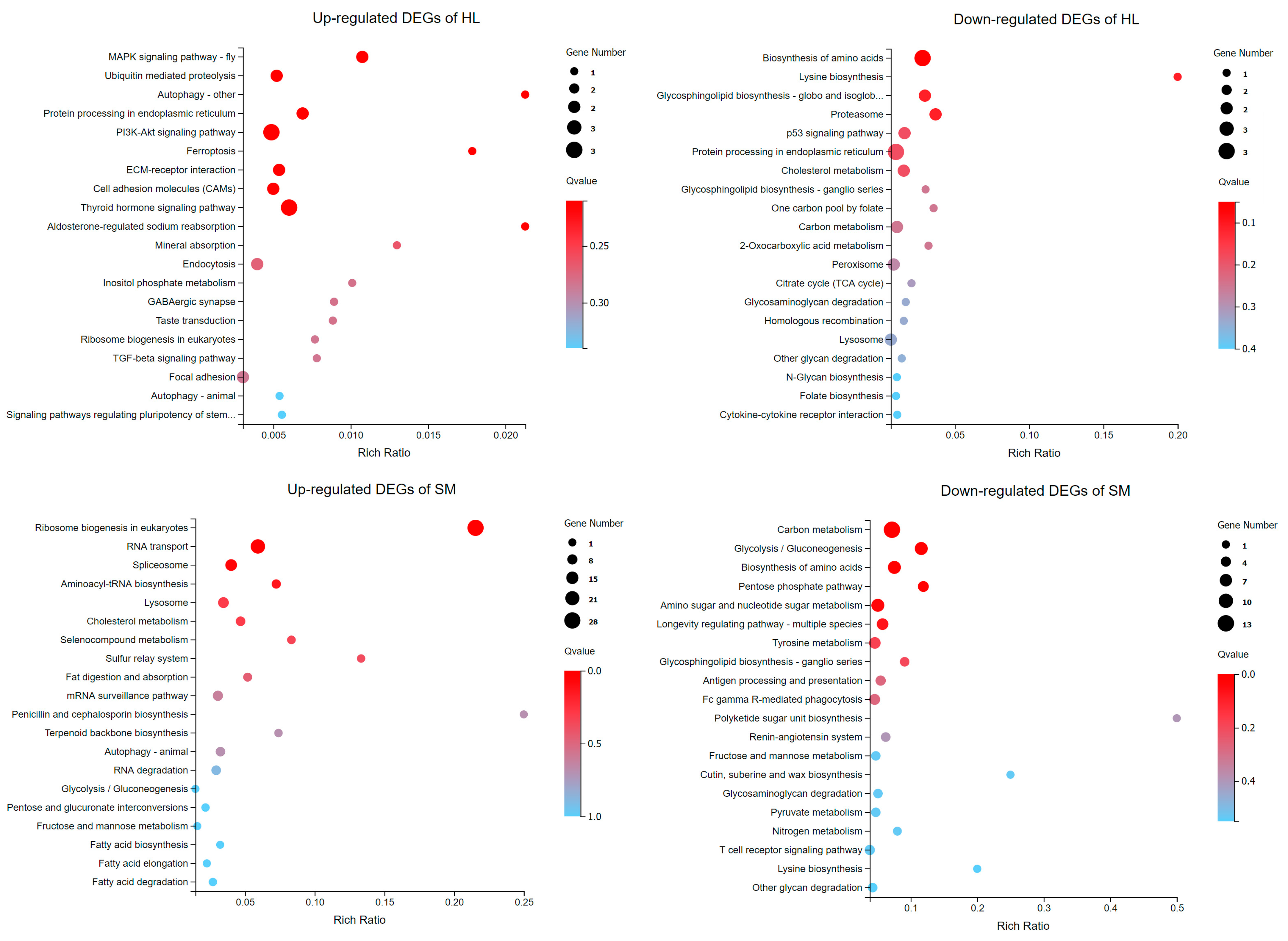
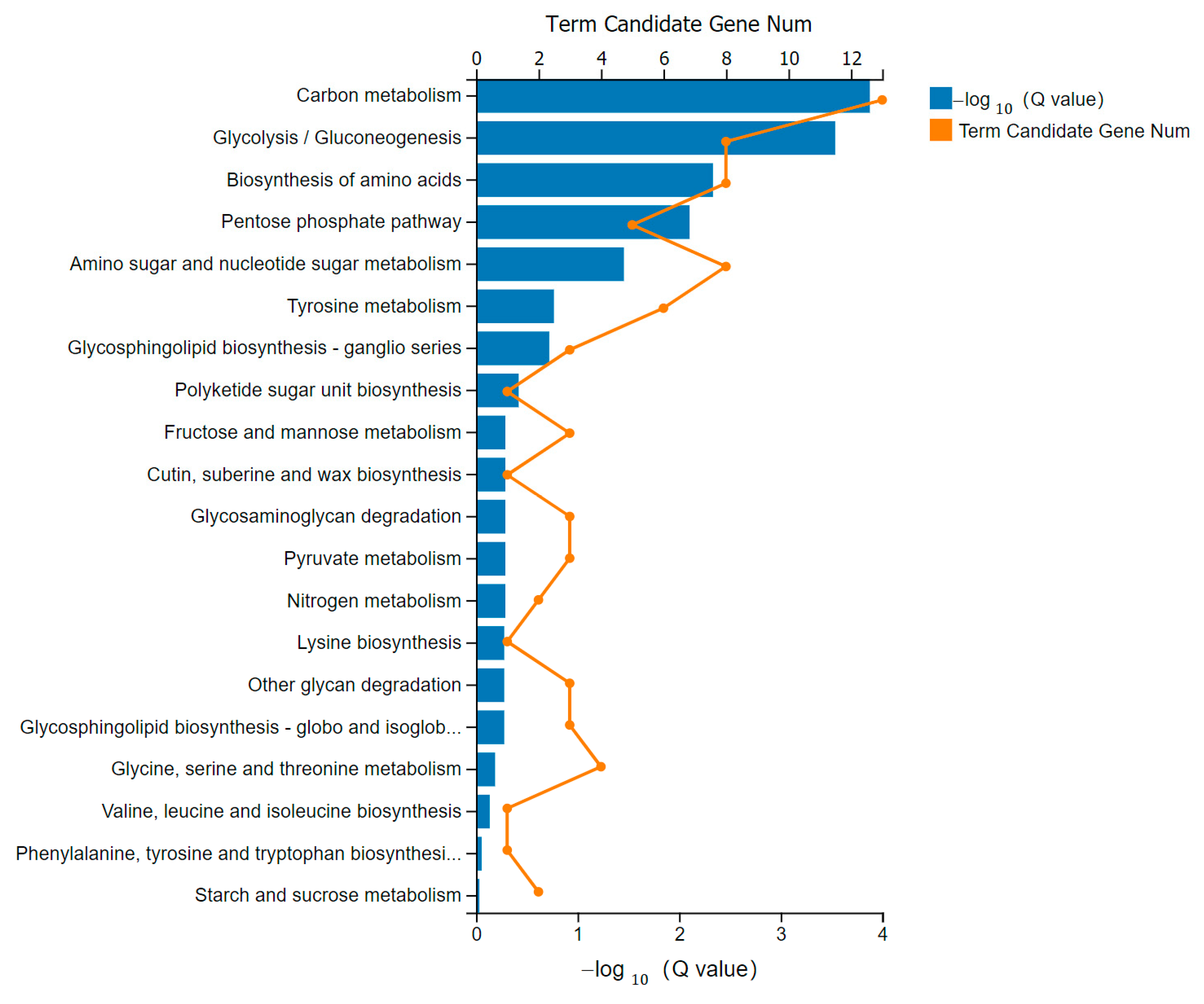
3.6. KEGG Pathway Analysis of DEGs
3.7. Metabolism Pathway Enrichment and Representative Pathway in Striated Muscle
4. Discussion
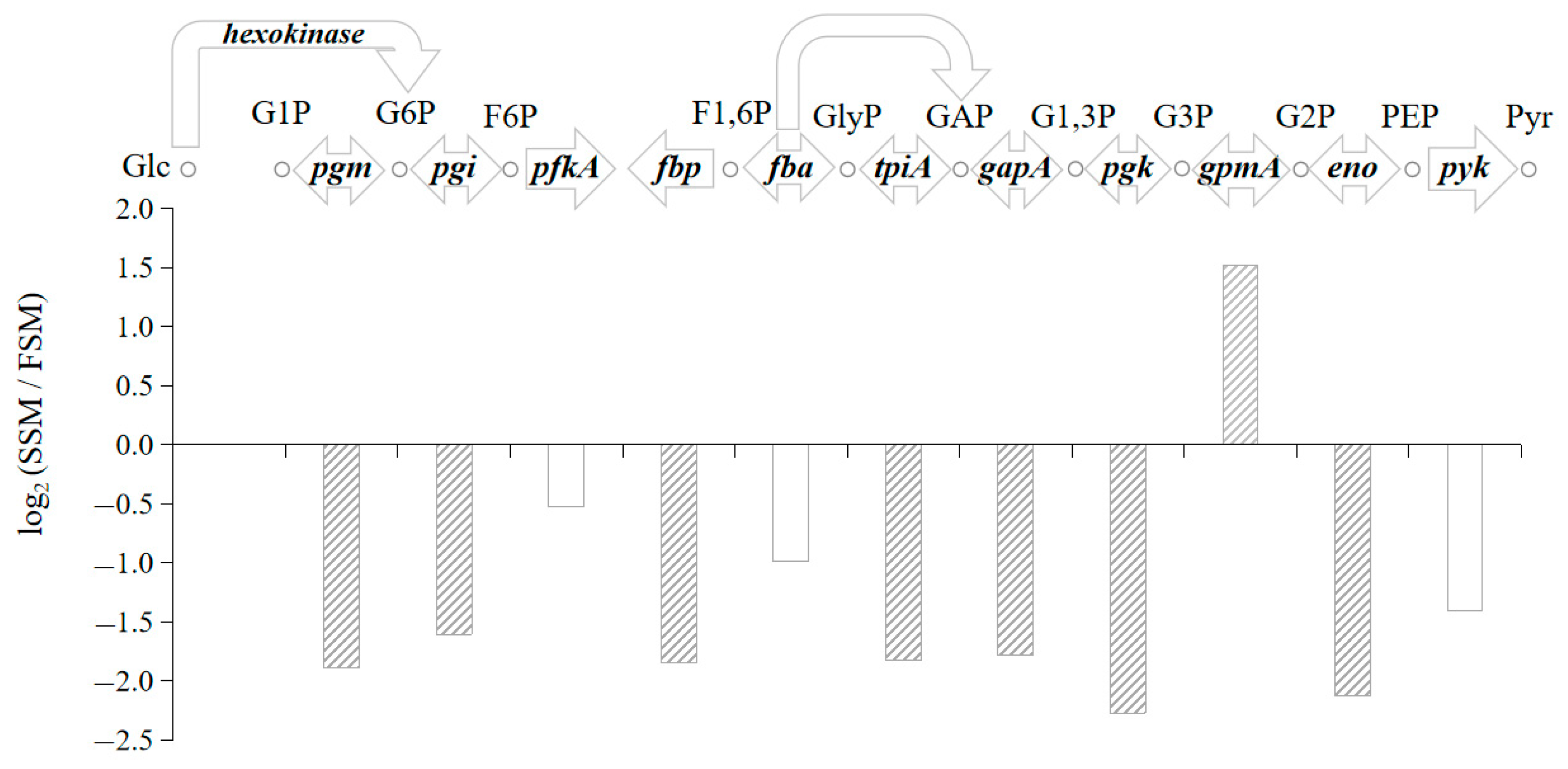
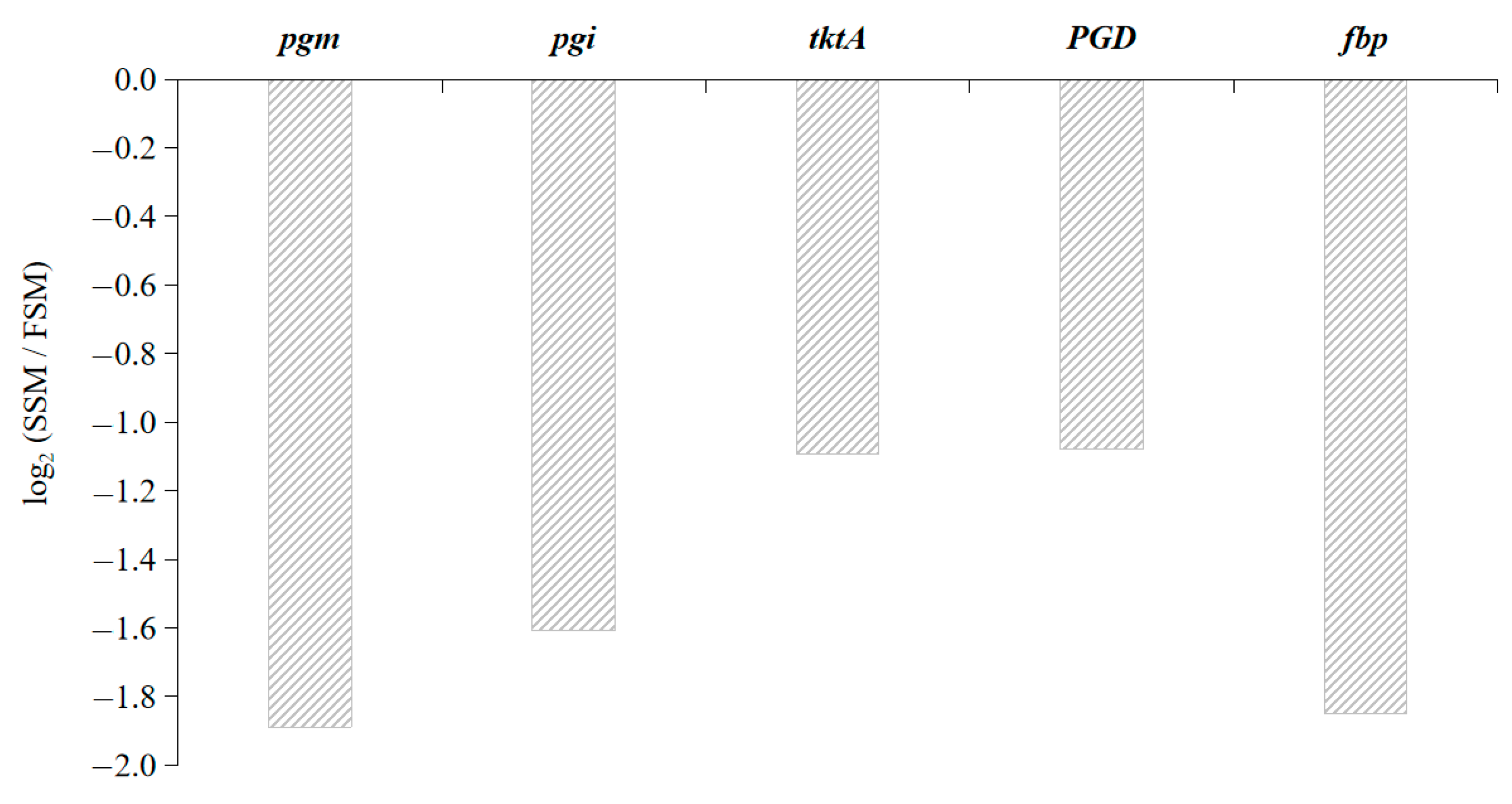
4.1. DEGs in Pathway of Glycolysis/Gluconeogenesis
4.2. Judgement of Regulation Direction
4.3. DEGs Involved in Pentose Phosphate Pathway
4.4. Genes Encoding Filament Protein of Striated Adductor Muscle Structure
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagashima, K.; Sato, M.; Kawamata, K.; Nakamura, A.; Ohta, T. Genetic Structure of Japanese Scallop Population in Hokkaido, Analyzed by Mitochondrial Haplotype Distribution. Mar. Biotechnic. 2005, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.A.; Li, D.C.; Wang, X.Y.; Wang, Q.Z.; Li, H.L.; Teng, W.M.; Liu, X.F.; Zhou, Z.C. Reason of Massive of Japanese Scallop Pationopecten yessoenisis in Raft Cultivation in Coastal Changhai County. Ocean. Sci. J. 2019, 38, 420–427. [Google Scholar] [CrossRef]
- Song, L.; Wu, J.; Du, J.; Li, N.; Wang, K.; Wang, P. Comparison of Two Methods to Assess the Size Structure of Phytoplankton Community Assemblages, in Liaodong Bay, China. J. Ocean Univ. China 2019, 18, 1207–1215. [Google Scholar] [CrossRef]
- Song, L.; Bi, X.D.; Fu, J.; Song, G.J.; Wu, J.H.; Liu, Y.; Liu, S.X. Characteristics of eukaryotic microalgae and their environmental correlation in the northern Yellow Sea. China Environ. Sci. 2021, 41, 1336–1344. [Google Scholar] [CrossRef]
- Song, L.; Bi, X.D.; Song, G.J.; Du, J.; Wu, J.H.; Wang, Z.S.; Hu, C.K. Size-fractionated eukaryotic microalgae and its influencing factors Dachangshan Island and its adjacent waters. China Environ. Sci. 2020, 40, 2627–2634. [Google Scholar] [CrossRef]
- Chauvaud, L.; Thouzeau, G.; Paulet, Y.-M. Effects of environmental factors on the daily growth rate of Pecten maximus juveniles in the Bay of Brest (France). J. Exp. Mar. Biol. Ecol. 1998, 227, 83–111. [Google Scholar] [CrossRef]
- Liu, Y.; Saitoh, S.-I.; Maekawa, K.; Mochizuki, K.-I.; Tian, Y. Impact of short-term climate effects and sea ice coverage variation on Japanese scallop aquaculture in Saroma Lake, Japan. Aquac. Res. 2018, 49, 1752–1767. [Google Scholar] [CrossRef]
- Xu, B.; Chen, M.; Yang, H.; Zhao, S. Starvation-induced Changes of Hemocyte Parameters in the Zhikong Scallop Chlamys farreri. J. Shellfish Res. 2008, 27, 1195–1200. [Google Scholar] [CrossRef]
- Butt, D.; Aladaileh, S.; O’Connor, W.; Raftos, D.A. Effect of starvation on biological factors related to immunological defence in the Sydney rock oyster (Saccostrea glomerata). Aquaculture 2007, 264, 82–91. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Chen, J.-C.; Man, S.N.C.; Morni, W.Z.W.; Suhaili, A.S.N.; Cheng, S.-Y.; Hsu, C.-H. Modulation of innate immunity and gene expressions in white shrimp Litopenaeus vannamei following long-term starvation and re-feeding. Results Immunol. 2012, 2, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Cuzon, G.; Cahu, C.; Aldrin, J.F.; Messager, J.L.; Stéphan, G.; Mével, M. Starvation effect on metabolism of Penaeus japonicus. Proc. World Maric. Soc. 2009, 11, 410–423. [Google Scholar] [CrossRef]
- Speers-Roesch, B.; Callaghan, N.I.; MacCormack, T.J.; Lamarre, S.G.; Sykes, A.V.; Driedzic, W.R. Enzymatic capacities of metabolic fuel use in cuttlefish (Sepia officinalis) and responses to food deprivation: Insight into the metabolic organization and starvation survival strategy of cephalopods. J. Comp. Physiol. B 2016, 186, 711–725. [Google Scholar] [CrossRef]
- Moran, A.; Manahan, D. Physiological recovery from prolonged ‘starvation’ in larvae of the Pacific oyster Crassostrea gigas. J. Exp. Mar. Biol. Ecol. 2004, 306, 17–36. [Google Scholar] [CrossRef]
- Liu, W.; Li, Q.; Gao, F.; Kong, L. Effect of starvation on biochemical composition and gametogenesis in the Pacific oyster Crassostrea gigas. Fish. Sci. 2010, 76, 737–745. [Google Scholar] [CrossRef]
- Telahigue, K.; Hajji, T.; Rabeh, I.; El Cafsi, M. The Effect of Starvation on the Biochemical Composition of the Digestive Gland, the Gonads and the Adductor Muscle of the Scallop Flexopecten glaber. Food Nutr. Sci. 2013, 4, 405–413. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, Y.; He, M. Molecular identification of an insulin growth factor binding protein (IGFBP) and its potential role in an insulin-like peptide system of the pearl oyster, Pinctada fucata. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2017, 214, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, X.; Zhihui, Y.; Xun, X.; Zhang, J.; Guo, X.; Jiao, W.; Zhang, L.; Liu, W.; Wang, J.; et al. Scallop genome reveals molecular adaptations to semi-sessile life and neurotoxins. Nat. Commun. 2017, 8, 1721. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.; Zhang, W.; Zhang, X.; Tian, Y.; Wang, X.; Hao, Z.; Chang, Y. Transcriptional changes in the Japanese scallop (Mizuhopecten yessoensis) shellinfested by Polydora provide insights into the molecular mechanism of shell formation and immunomodulation. Sci. Rep. 2018, 8, 17664. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Liu, Z.; Dong, Y.; Sun, X.; Wu, B.; Yu, T.; Zheng, Y.; Yang, A.; Zhao, Q.; Zhao, D. Transcriptomics analysis revealing candidate genes and networks for sex differentiation of yesso scallop (Patinopecten yessoensis). BMC Genom. 2019, 20, 671. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Li, Y.; Lou, J.; Yang, Z.; Liao, H.; Fu, Q.; Guo, Z.; Lian, S.; Hu, X.; Bao, Z. Transcriptomic Profiling Provides Insights into Inbreeding Depression in Yesso Scallop Patinopecten yessoensis. Mar. Biotechnol. 2019, 21, 623–633. [Google Scholar] [CrossRef]
- Karayücel, S.; Çelik, M.Y.; Karayücel, I.; Öztürk, R.; Eyüboğlu, B. Effects of stocking density on survival, growth and biochemical composition of cultured mussels (Mytilus galloprovincialis, Lamarck 1819) from an offshore submerged longline system. Aquac. Res. 2013, 46, 1369–1383. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Li, W.J.; Fu, C.D.; Yu, Z.A.; Teng Wm Liu, Z.Y. Immune response of Japanese scallop Patinopecten yessoensis to starvation. Fish. Sci. 2011, 30, 441–444. [Google Scholar] [CrossRef]
- Chantler, P.D. Chapter 4 Scallop adductor muscles: Structure and function. Dev. Aquacult. Fish. Sci. 2006, 35, 229–316. [Google Scholar] [CrossRef]
- Grün, E.; Heyne, D. Perinatal development of glucose-6-phosphatase and fructose-1,6-diphosphatase activities in pig liver. Acta Biol. Med. Ger. 1982, 41, 593–600. [Google Scholar] [PubMed]
- Lozinska-Gabska, M.; Dziewulska-Szwajkowska, D.; Szalagan, E. Isolation of fructose-1,6-bisphosphatase from the foot muscle of a snail Helix aspersa maxima. Zool. Pol. 2003, 48, 81–91. [Google Scholar]
- Gleeson, T.T. Post-Exercise Lactate Metabolism: A Comparative Review of Sites, Pathways, and Regulation. Annu. Rev. Physiol. 1996, 58, 565–581. [Google Scholar] [CrossRef]
- Lai, Z.X.; Liu, Y.; Wang, Q.H.; Zheng, Z.; Deng, Y.W. cDNA cloning of FBP Gene in Pinctada fucata martensii and its response to temperature stress. Prog. Fish. Sci. 2019, 40, 106–114. [Google Scholar]
- Storey, K.; Baldwin, J.; Hochachka, P. Squid muscle fructose diphosphatase and its role in the control of F6P-FDP cycling. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1975, 52, 165–168. [Google Scholar] [CrossRef]
- Behrisch, H.W. On the subunit structure of seasonal isoenzymes of fructose 1,6-diphosphatase from muscle of the Alaskan king crab Paralithodes camtschatica. Comp. Biochem. Physiol. Part B: Comp. Biochem. 1975, 51, 317–321. [Google Scholar] [CrossRef]
- Storey, K. Purification and properties of fructose diphosphatase from bumblebee flight muscle Role of the enzyme in control of substrate cycling. Biochim. Biophys. Acta (BBA) Enzym. 1978, 523, 443–453. [Google Scholar] [CrossRef]
- O’Doherty, P.; Feltham, L. Thermal regulation of gluconeogenesis in the giant scallop, Placopecten magellanicus (Gmelin). Comp. Biochem. Physiol. Part B: Comp. Biochem. 1971, 39, 163–165. [Google Scholar] [CrossRef]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Dastoor, Z.; Dreyer, J.L. Potential role of nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase in apoptosis and oxidative stress. J. Cell Sci. 2001, 114, 1643–1653. [Google Scholar] [CrossRef] [PubMed]
- Sirover, M.A. Emerging new functions of the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase, in mammalian cells. Life Sci. 1996, 58, 2271–2277. [Google Scholar] [CrossRef]
- Sirover, M.A. On the functional diversity of glyceraldehyde-3-phosphate dehydrogenase: Biochemical mechanisms and regulatory control. Biochim. Biophys. Acta (BBA) Gen. Subj. 2011, 1810, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.W.; Cash, P.; Ellefsen, S.; Nilsson, G.E. Proteomic changes in the crucian carp brain during exposure to anoxia. Proteomics 2009, 9, 2217–2229. [Google Scholar] [CrossRef]
- Jayasundara, N.; Tomanek, L.; Dowd, W.W.; Somero, G.N. Proteomic analysis of cardiac response to thermal acclimation in the eurythermal goby fish Gillichthys mirabilis. J. Exp. Biol. 2015, 218, 1359–1372. [Google Scholar] [CrossRef]
- Varó, I.; Cardenete, G.; Hontoria, F.; Monroig, Ó.; Iglesias, J.; Otero, J.J.; Almansa, E.; Navarro, J.C. Dietary Effect on the Proteome of the Common Octopus (Octopus vulgaris) Paralarvae. Front. Physiol. 2017, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Jiménez, L.; Leyva-Carrillo, L.; Peregrino-Uriarte, A.B.; Duarte-Gutiérrez, J.L.; Tresguerres, M.; Yepiz-Plascencia, G. Regulation of glyceraldehyde-3-phosphate dehydrogenase by hypoxia inducible factor 1 in the white shrimp Litopenaeus vannamei during hypoxia and reoxygenation. Comp. Biochem. Physiol. Part A: Mol. Integr. Physiol. 2019, 235, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Baumgarner, B.L.; Bharadwaj, A.S.; Inerowicz, D.; Goodman, A.S.; Brown, P.B. Proteomic analysis of rainbow trout (Oncorhynchus mykiss) intestinal epithelia: Physiological acclimation to short-term starvation. Comp. Biochem. Physiol. Part D: Genom. Proteom. 2013, 8, 58–64. [Google Scholar] [CrossRef]
- Liu KCFan, M.X.; Zhang, C.Y.; Cai, F.C.; Li, Q.Y.; Chen, M.M.; Jiang, Y.; Hu, W.B.; Lin, F. Phylogenetic analysis of enolase gene family in different species. Appl. Ecol. Environ. Res. 2019, 17, 11537–11550. [Google Scholar]
- Leu, J.-H.; Chang, C.-C.; Wu, J.-L.; Hsu, C.-W.; Hirono, I.; Aoki, T.; Juan, H.-F.; Lo, C.-F.; Kou, G.-H.; Huang, H.-C. Comparative analysis of differentially expressed genes in normal and white spot syndrome virus infected Penaeus monodon. BMC Genom. 2007, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Shen-Miller, J.; Lindner, P.; Xie, Y.; Villa, S.; Wooding, K.; Clarke, S.G.; Loo, R.R.O.; Loo, J.A. Thermal-Stable Proteins of Fruit of Long-Living Sacred Lotus Nelumbo nucifera Gaertn var. China Antique. Trop. Plant Biol. 2013, 6, 69–84. [Google Scholar] [CrossRef]
- Li, X.; Meng, X.; Luo, K.; Luan, S.; Cao, B.; Kong, J. cDNA cloning and expression analysis of a phosphopyruvate hydratase gene from the chinese shrimp Fenneropenaeus chinensis. Fish Shellfish Immunol. 2017, 63, 173–180. [Google Scholar] [CrossRef]
- Rojas-Pirela, M.; Andrade-Alviárez, D.; Rojas, V.; Kemmerling, U.; Cáceres, A.J.; Michels, P.A.; Concepción, J.L.; Quiñones, W. Phosphoglycerate kinase: Structural aspects and functions, with special emphasis on the enzyme from Kinetoplastea. Open Biol. 2020, 10, 200302. [Google Scholar] [CrossRef]
- Colgan, D. Phylogenetic Studies of Marsupials Based on Phosphoglycerate Kinase DNA Sequences. Mol. Phylogenetics Evol. 1999, 11, 13–26. [Google Scholar] [CrossRef]
- Kvam, C.; Olsvik, E.S.; McKinley-McKee, J.; Saether, O. Studies on recombinant Acetobacter xylinum alpha-phosphoglucomutase. Biochem. J. 1997, 326, 197–203. [Google Scholar] [CrossRef]
- Gao, H.B.; Pei-Yi, Y.U.; Sun, Y.J.; Jiang, L.J. Analysis of carbon metabolism and differential expression of related enzyme genes in castor leaves under Pb and Zn stress. Plant Physiol. J. 2019, 55, 483–492. [Google Scholar]
- Pan, B.-Y.; Liu, Y.-K.; Wu, H.-K.; Pang, X.-Q.; Wang, S.-G.; Tang, B.; Xu, C.-D. Role of phosphoglucomutase in regulating trehalose metabolism in Nilaparvata lugens. 3 Biotech 2020, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J.; Bahnson, B.J.; Chien, W.; Ringe, A.D.; Petsko, G.A. Crystal Structure of Rabbit Phosphoglucose Isomerase, a Glycolytic Enzyme That Moonlights as Neuroleukin, Autocrine Motility Factor, and Differentiation Mediator. Biochemistry 2000, 39, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Angira, D.; Natarajan, N.; Dedania, S.R.; Patel, D.H.; Thiruvenkatam, V. Characterization of P. aeruginosa Glucose 6- Phosphate Isomerase: A Functional Insight via In-Vitro Activity Study. Curr. Top. Med. Chem. 2020, 20, 2651–2661. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.G.; Sun, X.R.; Jiang, J.L.; Sun, M.L.; Jie, G.U.; Jiang, Z.Q.; Lin, Y.E. The effect of acute low salinity stress on gpi gene in Takifugu rubripes. Acta Hydrobiol. Sin. 2019, 43, 771–777. [Google Scholar]
- Stincone, A.; Prigione, A.; Cramer, T.; Wamelink, M.M.C.; Campbell, K.; Cheung, E.; Olin-Sandoval, V.; Grüning, N.-M.; Krüger, A.; Tauqeer Alam, M.; et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. 2015, 90, 927–963. [Google Scholar] [CrossRef]
- Tyshenko, M.G.; Walker, V.K. Towards a reconciliation of the introns early or late views: Triosephosphate isomerase genes from insects. Biochim. Biophys Acta. 1997, 1353, 131–136. [Google Scholar] [CrossRef]
- Anuar, T.S.; Azreen, S.N.; Salleh, F.; Moktar, N. Molecular epidemiology of giardiasis among Orang Asli in Malaysia: Application of the triosephosphate isomerase gene. BMC Infect. Dis. 2014, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, S.; Liu, G.; Li, F. Triosephosphate isomerase (TPI) facilitates the replication of WSSV in Exopalaemon carinicauda. Dev. Comp. Immunol. 2017, 71, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Wang, C.; Wang, B.; Qin, H.; Hu, S.; Wang, P.; Sun, C.; Zhang, S. Comparative transcriptome analysis of Litopenaeus vannamei reveals that triosephosphate isomerase-like genes play an important role during decapod iridescent virus 1 infection. Front. Immunol. 2020, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Walton, M.J. Metabolic effects of feeding a high protein/low carbohydrate diet as compared to a low protein/high carbohydrate diet to rainbow troutSalmo gairdneri. Fish Physiol. Biochem. 1986, 1, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Lin, H.-Z.; Jiang, S.-G.; Chen, X.; Wu, K.-C.; Tian, L.-X.; Liu, Y.-J. Effect of seven carbohydrate sources on juvenile Penaeus monodon growth performance, nutrient utilization efficiency and hepatopancreas enzyme activities of 6-phosphogluconate dehydrogenase, hexokinase and amylase. Anim. Feed Sci. Technol. 2012, 174, 86–95. [Google Scholar] [CrossRef]
- McCue, M.D. Starvation physiology: Reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 156, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Feng, Y.; Duan, J.; Xiong, G.; Fan, W.; Liu, S.; Zhong, L.; Wang, K.; Geng, Y.; Ouyang, P.; et al. Antistarvation Strategies of E. Sinensis: Regulatory Networks under Hepatopancreas Consumption. Oxidative Med. Cell. Longev. 2020, 2020, 6085343. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Bao, X.; Liu, Y.; Liu, W.; Zhao, S.; Liu, S. Effect of Heat Starvation Stress on Physiological Immunity and Metabolism of Mizuhopecten yessoensis. Sustainability 2022, 14, 13217. https://doi.org/10.3390/su142013217
Song L, Bao X, Liu Y, Liu W, Zhao S, Liu S. Effect of Heat Starvation Stress on Physiological Immunity and Metabolism of Mizuhopecten yessoensis. Sustainability. 2022; 14(20):13217. https://doi.org/10.3390/su142013217
Chicago/Turabian StyleSong, Lun, Xiangbo Bao, Yin Liu, Weidong Liu, Sufang Zhao, and Suxuan Liu. 2022. "Effect of Heat Starvation Stress on Physiological Immunity and Metabolism of Mizuhopecten yessoensis" Sustainability 14, no. 20: 13217. https://doi.org/10.3390/su142013217
APA StyleSong, L., Bao, X., Liu, Y., Liu, W., Zhao, S., & Liu, S. (2022). Effect of Heat Starvation Stress on Physiological Immunity and Metabolism of Mizuhopecten yessoensis. Sustainability, 14(20), 13217. https://doi.org/10.3390/su142013217



