Characterization of the Biological Activities of a New Polyphenol-Rich Extract from Cinnamon Bark on a Probiotic Consortium and Its Action after Enzymatic and Microbial Fermentation on Colorectal Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of Cinnamon Bark Bioactives
2.2. Qualitative and Quantitative Analysis of Cinnamon Bark Extract
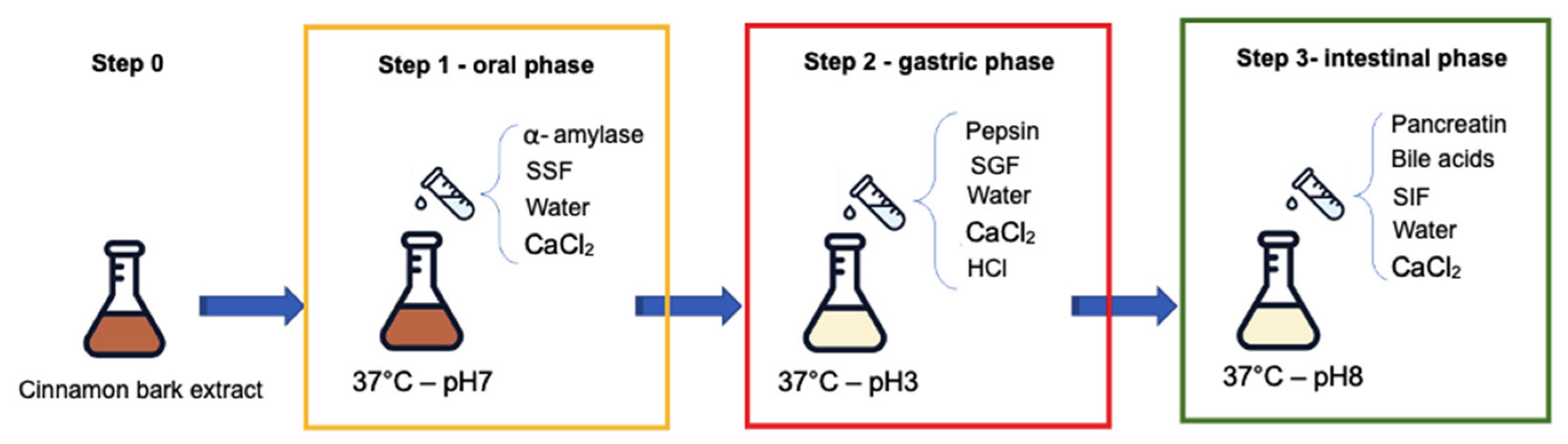
2.3. In Vitro Oral and Gastrointestinal Digestion Employing INFOGEST Protocol
2.4. Bacterial Strains and Culture Conditions
2.5. Screening of the Antimicrobial Activity of Polyphenol-Rich Cinnamon Bark Extract and In Vitro Digested Polyphenol-Rich Cinnamon Bark Extract
2.6. Growth Experiment with Single Probiotic Strains on Polyphenol-Rich Cinnamon Bark Extract and In Vitro Digested Polyphenol-Rich Cinnamon-Bark Extract
2.7. Assessment of Biotranforming Capacities of Single Probiotic Strains and Combined as a Probiotic Consortium
2.8. Maintenance of Cell Lines
2.9. Cell Viability Assay
2.10. Statistical Analysis
3. Results
3.1. Selection of Extraction Method from Cinnamon Bark
3.2. Preliminary Characterization of the Polyphenol-Rich Cinnamon Bark Extract
3.3. In Vitro Enzymatic Digestion of Polyphenol-Rich Cinnamon Bark Extract and Characterization of the Digest
3.4. Antimicrobial Activity of Polyphenol-Rich and Digested Cinnamon Bark Extract
3.5. Growth Experiments of Probiotic Bacteria in Presence of the Polyphenol-Rich and Digested Cinnamon Bark Extract
3.6. Extraction and Characterization of Lactobacillus and Bifidobacterium Secondary Metabolites in Presence of Cinnamic Acid, Polyphenol-Rich, and Digested Cinnamon Bark Extract
3.6.1. Analysis of the Metabolites Produced after Biotransformation of trans-Cinnamic Acid
3.6.2. Analysis of the Metabolites Produced after Biotransformation of Polyphenol-Rich Cinnamon Bark Extract
3.6.3. Analysis of the Metabolites Produced after Biotransformation of the In Vitro Enzymatic Digestion of the Polyphenol-Rich Cinnamon Bark Extract
3.7. Evaluation of the Effects of Polyphenol-Rich and In Vitro Digested Polyphenol-Rich Cinnamon Bark Extract on Healthy and Tumor Colorectal Cell Lines
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, S.; Xu, H.; Sui, Y.; Mei, X.; Shi, J.; Cai, S.; Xiong, T.; Carrillo, C.; Castagnini, J.M.; Zhu, Z.; et al. Comparing the LC-MS phenolic acids profiles of seven different varieties of brown rice (Oryza sativa L.). Foods 2022, 11, 1552. [Google Scholar] [CrossRef]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, M.; Berrada, H.; Zhu, Z.; Grimi, N.; Barba, F.J. Pulsed electric fields (PEF), pressurized liquid extraction (PLE) and combined PEF + PLE process evaluation: Effects on Spirulina microstructure, biomolecules recovery and Triple TOF-LC-MS-MS polyphenol composition. Innov. Food Sci. Emerg. Technol. 2022, 77, 102989. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sanchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.R.; de Vas Gunawardana, N.; Katulanda, P. Efficacy and safety of “true” cinnamon (Cinnamon zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis. Diabet. Med. 2012, 29, 1480–1492. [Google Scholar] [CrossRef]
- Ravindran, P.; Shylaja, M.; Babu, N.; Krishnamoorthy, B. Botany and crop improvement of Cinnamon and Cassia. In Cinnamon and Cassia—The Genus Cinnamomum; Ravindran, P.N., Babu, K.N., Eds.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Cheng, D.M.; Kuhn, P.; Poulev, A.; Rojo, L.E.; Lila, M.A.; Raskin, I. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012, 135, 2994–3002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Bioaccessibility of polyphenols and cinnamaldehyde in cinnamon beverages subjected to in vitro gastro-pancreatic digestion. J. Funct. Foods 2014, 7, 506–516. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macia, A.; Motilva, M.J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [Green Version]
- Clifford, M.N. Diet-derived phenols in plasma and tissues and their implications for health. Planta Med. 2004, 70, 1103–1114. [Google Scholar] [CrossRef] [Green Version]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, V.; Bartolomè, B. A Survey of modulation of gut microbiota by dietary polyphenols. BioMed Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Marin, L.; Miguelez, E.M.; Villar, C.J.; Lombò, F. Bioavailability of dietary phenols and gut microbiota metabolism: Antimicrobial properties. BioMed Res. Int. 2015, 2015, 905215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, G.; Clifford, M.N. Colonic metabolites of berry polyphenols: The missing link to biological activity? Br. J. Nutr. 2010, 104, S48–S66. [Google Scholar] [CrossRef] [Green Version]
- Gaur, G.; Oh, J.H.; Filannino, P.; Gobbetti, M.; van Pijkeren, J.P.; Ganzle, M.G. Genetic determinants of hydroxycinnamic acid metabolism in heterofermentative lactobacilli. Appl. Environ. Microbiol. 2020, 86, e02461-19. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Reveron, I.; Mancheno, J.M.; de Las Rivas, B.; Munoz, R. Characterization of a feruloyl esterase from Lactobacillus plantarum. Appl. Environ. Microbiol. 2013, 79, 5130–5136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esteban-Torres, M.; Landete, J.M.; Reveron, I.; Santamaria, L.; de Las Rivas, B.; Munoz, R. A Lactobacillus plantarum esterase active on a broad range of phenolic esters. Appl. Environ. Microbiol. 2015, 81, 3235–3242. [Google Scholar] [CrossRef] [Green Version]
- Cavin, J.F.; Barthelmebs, L.; Divies, C. Molecular characterization of an inducible p-coumaric acid decarboxylase from Lactobacillus plantarum: Gene cloning, transcriptional analysis, overexpression in Escherichia coli, purification, and characterization. Appl. Environ. Microbiol. 1997, 63, 1939–1944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, H.; Landete, J.M.; Curiel, J.A.; de Las Rivas, B.; Mancheno, J.M.; Munoz, R. Characterization of the p-coumaric acid decarboxylase from Lactobacillus plantarum CECT 748T. J. Agric. Food Chem. 2008, 56, 3068–3072. [Google Scholar] [CrossRef] [PubMed]
- Hervert-Hernandez, D.; Goni, I. Dietary polyphenols and human gut microbiota: A review. Food Rev. Int. 2011, 27, 154–169. [Google Scholar] [CrossRef]
- Chait, Y.A.; Gunenc, A.; Bendali, F.; Hosseinian, F. Simulated gastrointestinal digestion and in vitro colonic fermentation of carob polyphenols: Bioaccessibility and bioactivity. LWT 2020, 117, 108623. [Google Scholar] [CrossRef]
- Gong, L.; Chi, J.; Zhang, Y.; Wang, J.; Sun, B. In vitro evaluation of the bioaccessibility of phenolic acids in different whole wheats as potential prebiotics. LWT 2019, 100, 435–443. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Viuda-Martos, M.; Perez-Alvarez, J.A.; Fernandez-Lopez, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113. [Google Scholar] [CrossRef] [Green Version]
- Nissen, L.; Casciano, F.; Gianotti, A. Intestinal fermentation in vitro models to study food-induced gut microbiota shift: An updated review. FEMS Microbiol. Lett. 2020, 367, fnaa097. [Google Scholar] [CrossRef]
- Campone, L.; Celano, R.; Rizzo, S.; Piccinelli, A.L.; Rstrelli, L.; Russo, M. Development of an enriched polyphenol (natural antioxidant) extract from orange juice (Citrus sinensis) by adsorption on macroporous resins. J. Food Qual. 2020, 2020, 1251957. [Google Scholar] [CrossRef] [Green Version]
- Zhi-shen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- McMurrough, I.; McDowell, J. Chromatographic separation and automated analysis of flavanols. Anal. Biochem. 1978, 91, 92–100. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Balance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Presti, I.; D’orazio, G.; Labra, M.; La Ferla, B.; Mezzasalma, V.; Bizzaro, G.; Giardina, S.; Michelotti, A.; Tursi, F.; Vassallo, M.; et al. Evaluation of the probiotic properties of new Lactobacillus and Bifidobacterium strains and their in vitro effect. Appl. Microbiol. Biotechnol. 2015, 99, 5613–5626. [Google Scholar] [CrossRef]
- Zeaiter, Z.; Regonesi, M.E.; Cavini, S.; Labra, M.; Sello, G.; Di Gennaro, P. Extraction and characterization of inulin-type fructans from artichoke wastes and their effect on the growth of intestinal bacteria associated with health. Biomed Res. Int. 2019, 2019, 1083952. [Google Scholar] [CrossRef] [Green Version]
- De Giani, A.; Bovio, F.; Forcella, M.E.; Lasagni, M.; Fusi, P.; Di Gennaro, P. Prebiotic effect of Maitake extract on a probiotic consortium and its action after microbial fermentation on colorectal cell lines. Foods 2021, 10, 2536. [Google Scholar] [CrossRef]
- Watson, D.; O’Connel Motherway, M.; Schoterman, M.H.; van Neerven, R.J.; Nauta, A.; van Sinderen, D. Selective carbohydrate utilization by lactobacilli and bifidobacterial. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef]
- Lennox, E.S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1955, 1, 190–206. [Google Scholar] [CrossRef]
- Santini, C.; Baffoni, L.; Gaggia, F.; Granata, M.; Gasbarri, R.; Di Gioia, D.; Biavati, B. Characterization of probiotic strains: An application as feed additives in poultry against Campylobacter jejuni. Int. J. Food Microbiol. 2010, 141, S98–S108. [Google Scholar] [CrossRef]
- Ivanov, S.A.; Nomura, K.; Malfanov, I.L.; Sklyar, I.V.; Ptitsyn, L.R. Isolation of a novel catechin from Bergenia rhizomes that has pronounced lipase-inhibiting and antioxidative properties. Fitoterapia 2011, 82, 212–218. [Google Scholar] [CrossRef]
- Lee, J.; Lee, D.G.; Park, J.Y.; Chae, S.; Lee, S. Analysis of the trans-cinnamic acid content in Cinnamomum spp. and commercial cinnamon powder using HPLC. J. Agric. Chem. Environ. 2015, 4, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Rao, P.V.; Gan, S.H. Cinnamon: A multifaceted medicinal plant. Evid. Based Complement. Alternat. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakasha, G.K.; Mandadi, K.K.; Poulose, S.M.; Jadegoud, Y.; Nagana Gowda, G.A.; Patil, B.S. Inhibition of colon cancer cell growth and antioxidant activity of bioactive compounds from Poncirus trifoliata (L.) Raf. Bioorg. Med. Chem. 2007, 15, 4923–4932. [Google Scholar] [CrossRef]
- Shafizadeh, F. Branched-chain sugars of natural occurrence. Adv. Carbohydr. Chem. 1956, 48, 263–283. [Google Scholar] [CrossRef]
- Koppikar, S.J.; Choudhari, A.S.; Suryavanshi, S.A.; Kumari, S.; Chattopadhyay, S.; Kaul-Ghanekar, R. Aqueous cinnamon extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer 2010, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Gilani, S.; Ghasem, N. Evaluation of the extraction process parameters on bioactive compounds of cinnamon bark: A comparative study. Process Biochem. 2022, 114, 93–101. [Google Scholar] [CrossRef]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Nayme, K.; Timinouni, M.; Lyoussi, B.; Abdellaoui, A. Antibacterial activity of cinnamon essential oils and their synergistic potential with antibiotics. J. Adv. Pharm. Technol. Res. 2019, 10, 63–67. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Muchuweti, M.; Kativu, E.; Mupure, C.H.; Chidewe, C.; Ndhlala, A.R.; Benhura, M.A.N. Phenolic composition and antioxidant properties of some species. Am. J. Food Technol. 2007, 2, 414–420. [Google Scholar] [CrossRef]
- Ferron, L.; Colombo, R.; Mannucci, B.; Papetti, A. A new italian purple corn variety (Morandyn) byproduct extract: Antiglycative and hypoglycemic in vitro activities and preliminary bioaccessibility studies. Molecules 2020, 25, 1958. [Google Scholar] [CrossRef] [Green Version]
- Keskin, D.; Toroglu, S. Studies on antimicrobial activities of solvent extracts of different spices. J. Environ Biol. 2011, 32, 251–256. [Google Scholar]
- Gorniak, I.; Bartoszewski, R.; Kroliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–271. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, R.K. Therapeutic and pharmaceutical potential of Cinnamomum tamala. J. Pharm. Pharm. Sci. 2017, 6, 18–28. [Google Scholar]
- Feniman, C.M.; Rall, V.L.; Doyama, J.T.; Júnior, A.F. Cell enumeration and visualisation by transmission electron microscopy of Lactobacillus rhamnosus treated with cinnamon (Cinnamomum zeylanicum B.) essential oil. Nat. Prod. Res. 2012, 26, 1721–1723. [Google Scholar] [CrossRef]
- Sanchez-Maldonado, A.F.; Schieber, A.; Ganzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Whiting, G.C.; Carr, J.G. Metabolism of cinnamic acid and hydroxy-cinnamic acids by Lactobacillus pastorianus var. quinicus. Nature 1959, 184, 1427–1428. [Google Scholar] [CrossRef] [PubMed]
- Known, H.K.; Hwang, J.S.; So, J.S.; Lee, C.G.; Sahoo, A.; Ryu, J.H.; Jeon, W.K.; Ko, B.S.; Lee, S.H.; Park, Z.Y.; et al. Cinnamon extract induces tumor cell death through inhibition of NFkB and AP1. BMC Cancer 2010, 10, 392. [Google Scholar] [CrossRef]
- Lv, J.; Huang, H.; Yu, L.; Whent, M.; Niu, Y.; Shi, H.; Wang, T.T.Y.; Luthria, D.; Charles, D.; Yu, L.L. Phenolic composition and nutraceutical properties of organic and conventional cinnamon and peppermint. Food Chem. 2012, 132, 1442–1450. [Google Scholar] [CrossRef]







| Molecule | Aqueous Extract | Digested Extract |
|---|---|---|
| Total polyphenols (µgGAEeq/mg of powder extract) | 520.05 ± 17.43 | 222.86 ± 13.71 |
| Flavonoids (µgCATeq/mg of powder extract) | 294.77 ± 19.83 | 3.01 ± 0.64 |
| Catechins (µgCATeq/mg of powder extract) | 77.92 ± 3.39 | 0.35 ± 1.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Giani, A.; Pagliari, S.; Zampolli, J.; Forcella, M.; Fusi, P.; Bruni, I.; Campone, L.; Di Gennaro, P. Characterization of the Biological Activities of a New Polyphenol-Rich Extract from Cinnamon Bark on a Probiotic Consortium and Its Action after Enzymatic and Microbial Fermentation on Colorectal Cell Lines. Foods 2022, 11, 3202. https://doi.org/10.3390/foods11203202
De Giani A, Pagliari S, Zampolli J, Forcella M, Fusi P, Bruni I, Campone L, Di Gennaro P. Characterization of the Biological Activities of a New Polyphenol-Rich Extract from Cinnamon Bark on a Probiotic Consortium and Its Action after Enzymatic and Microbial Fermentation on Colorectal Cell Lines. Foods. 2022; 11(20):3202. https://doi.org/10.3390/foods11203202
Chicago/Turabian StyleDe Giani, Alessandra, Stefania Pagliari, Jessica Zampolli, Matilde Forcella, Paola Fusi, Ilaria Bruni, Luca Campone, and Patrizia Di Gennaro. 2022. "Characterization of the Biological Activities of a New Polyphenol-Rich Extract from Cinnamon Bark on a Probiotic Consortium and Its Action after Enzymatic and Microbial Fermentation on Colorectal Cell Lines" Foods 11, no. 20: 3202. https://doi.org/10.3390/foods11203202






