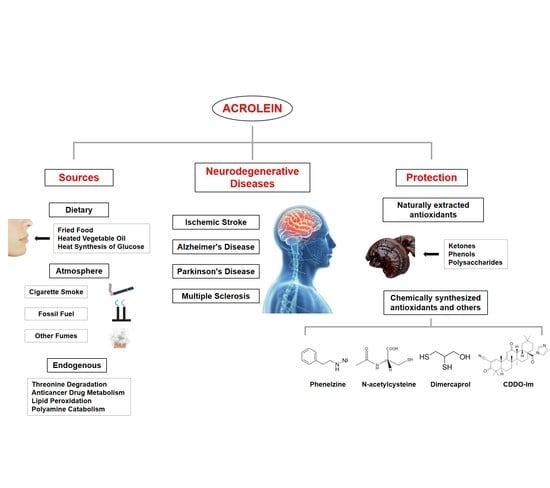
The Role of Acrolein in Neurodegenerative Diseases and Its Protective Strategy
Abstract
1. Introduction
2. Research Methodology
3. Acrolein Sources and Toxicokinetics
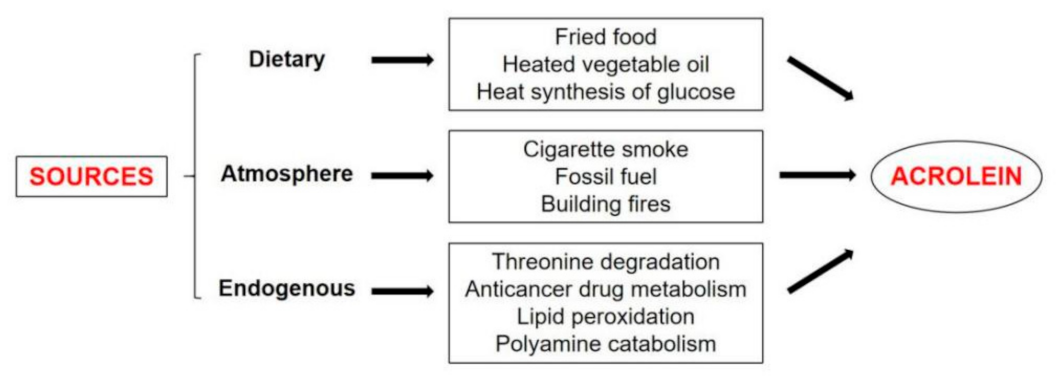
3.1. Source of Acrolein
3.1.1. Dietary
3.1.2. Atmosphere
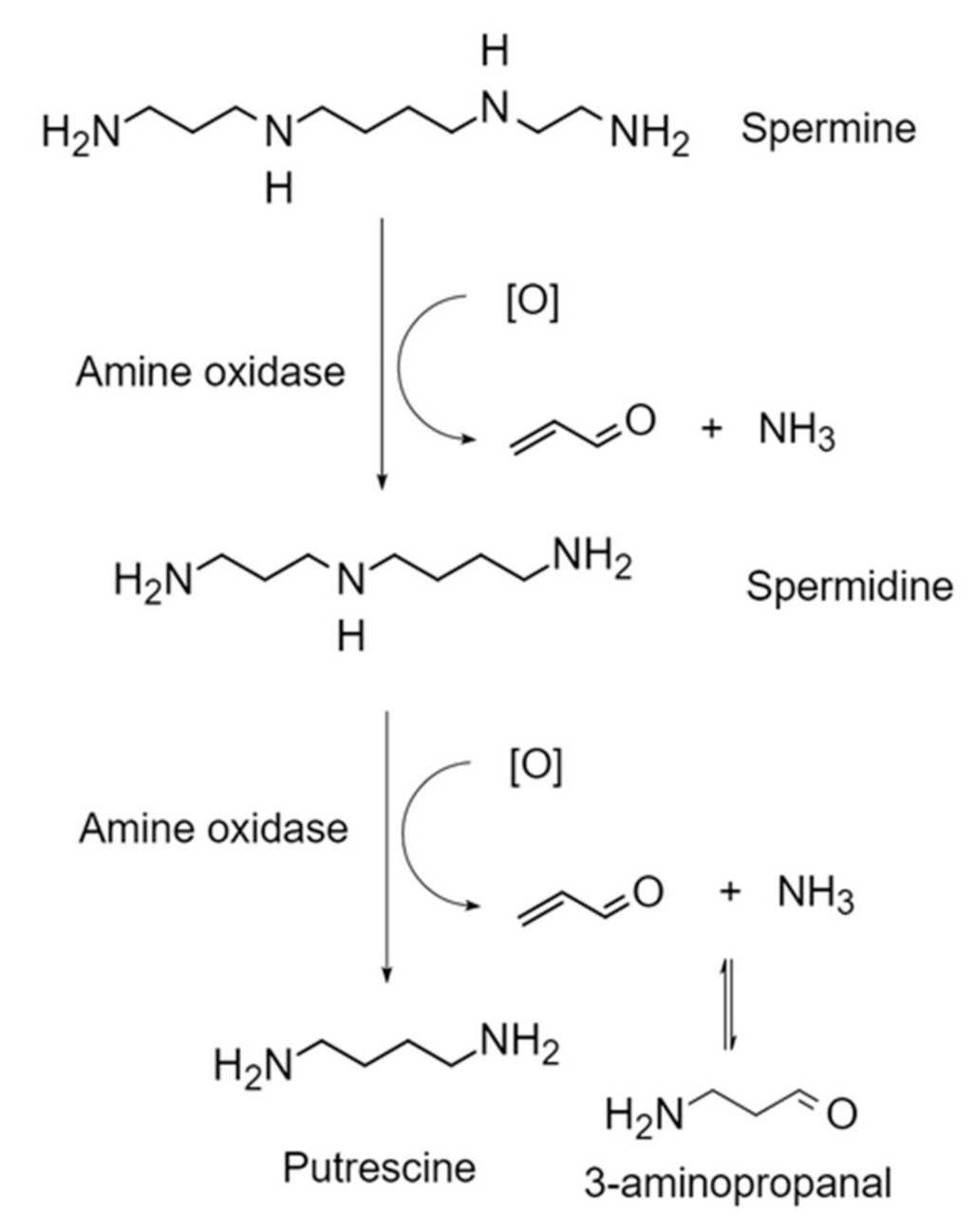
3.1.3. Endogenous Lipid Peroxidation
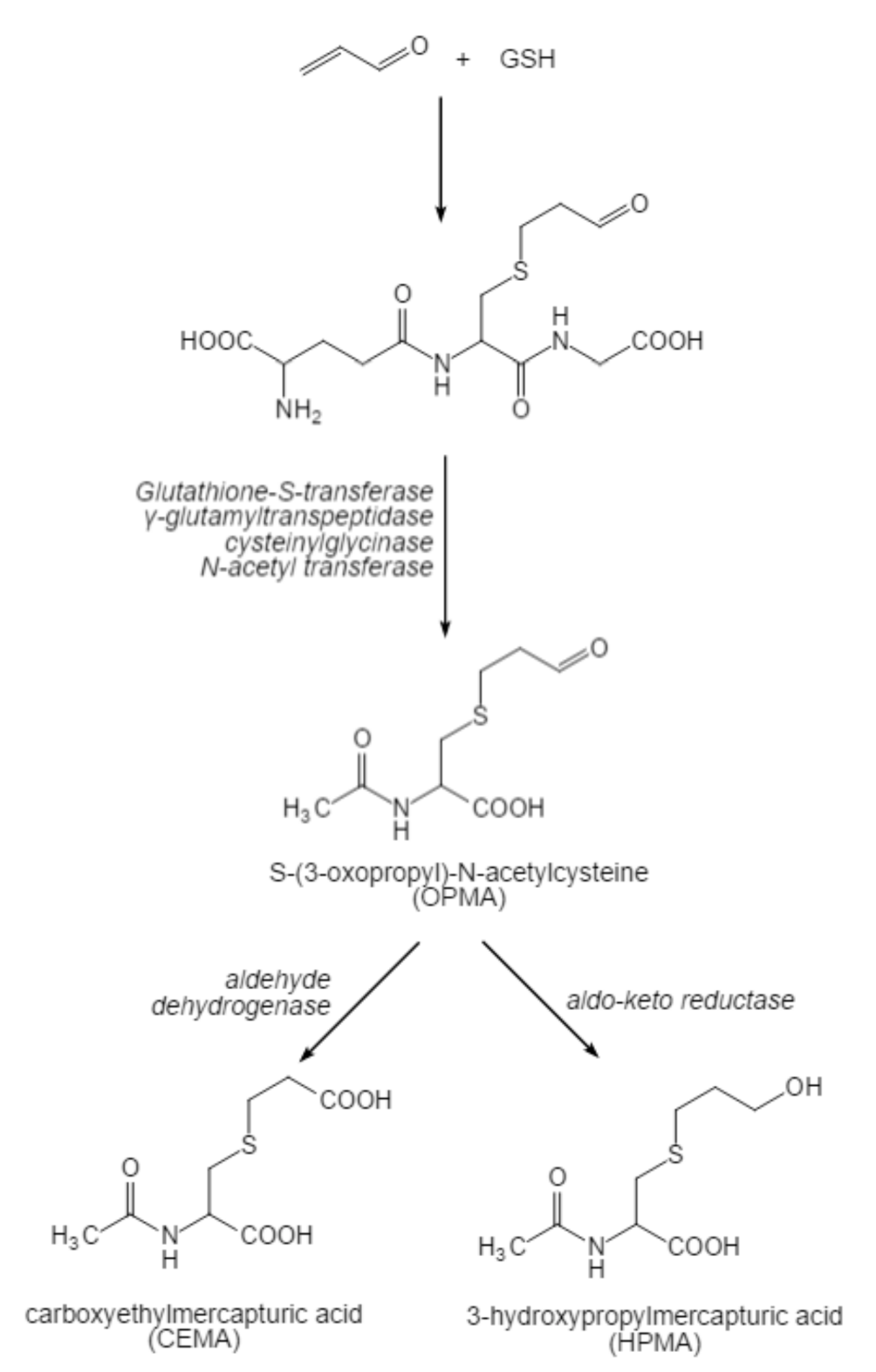
3.2. Toxic Kinetics of Acrolein
4. The Effect of Acrolein on Neurodegenerative Diseases
4.1. Ischemic Stroke
4.2. Alzheimer’s Disease (AD)
4.3. Parkinson’s Disease (PD)
4.4. Multiple Sclerosis (MS)
5. Protection
5.1. Naturally Extracted Antioxidants
5.2. Chemically Synthesized Antioxidants and Others
6. Future Perspectives
6.1. Optimization of Food Thermal Processing to Reduce Acrolein Production
6.2. Exploration of More Natural Products as Food Additives to Control Acrolein Levels
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Stevens, J.F.; Maier, C.S. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol. Nutr. Food Res. 2008, 52, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Vilanova, E. Acrolein. Encycl. Toxicol. 2014, 162, 63–68. [Google Scholar]
- Wang, G.W.; Guo, Y.; Vondriska, T.M.; Zhang, J.; Zhang, S.; Tsai, L.L.; Zong, N.C.; Bolli, R.; Bhatnagar, A.; Prabhu, S.D. Acrolein consumption exacerbates myocardial ischemic injury and blocks nitric oxide-induced PKCepsilon signaling and cardioprotection. J. Mol. Cell Cardiol. 2008, 44, 1016–1022. [Google Scholar] [CrossRef]
- Boleti, A.; Flores, T.; Moreno, S.E.; Anjos, L.D.; Migliolo, L. Neuroinflammation: An overview of neurodegenerative and metabolic diseases and of biotechnological studies. Neurochem. Int. 2020, 136, 104714. [Google Scholar] [CrossRef]
- Zhao, W.Z.; Wang, H.T.; Huang, H.J.; Lo, Y.L.; Lin, M.Y. Neuroprotective Effects of Baicalein on Acrolein-induced Neurotoxicity in the Nigrostriatal Dopaminergic System of Rat Brain. Mol. Neurobiol. 2017, 51, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Saiki, R.; Park, H.; Ishii, I.; Yoshida, M.; Nishimura, K.; Toida, T.; Tatsukawa, H.; Kojima, S.; Ikeguchi, Y.; Pegg, A.E.; et al. Brain infarction correlates more closely with acrolein than with reactive oxygen species. Biochem. Biophys. Res. Commun. 2011, 404, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Tully, M.; Tang, J.; Zheng, L.X.; Acosta, G.; Tian, R.; Hayward, L.; Race, N.; Mattson, D.; Shi, R.Y. Systemic Acrolein Elevations in Mice With Experimental Autoimmune Encephalomyelitis and Patients with Multiple Sclerosis. Front. Neurol. 2018, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Andres, S.; Palavinskas, R.; Berg, K.; Appel, K.E.; Lampen, A. Toxicology and risk assessment of acrolein in food. Mol. Nutr. Food Res. 2011, 55, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Moghe, A.; Ghare, S.; Lamoreau, B.; Mohammad, M.; Barve, S.; McClain, C.; Joshi-Barve, S. Molecular mechanisms of acrolein toxicity: Relevance to human disease. Toxicol. Sci. 2015, 143, 242–255. [Google Scholar] [CrossRef]
- Kachele, M.; Monakhova, Y.B.; Kuballa, T.; Lachenmeier, D.W. Nmr Investigation of Acrolein Stability in Hydroalcoholic Solution as a Foundation for the Valid Hs-Spme/Gc-Ms Quantification of the Unsaturated Aldehyde in Beverages. Anal. Chim. Acta 2014, 820, 112–118. [Google Scholar] [CrossRef]
- Yasuhara, A.; Shibamoto, T. Determination of Acrolein Evolved from Heated Vegetable Oil by N-Methylhydrazine Conversion. Agric. Biol. Chem. 1991, 55, 2639–2640. [Google Scholar]
- Jiang, K.; Huang, C.; Liu, F.; Zheng, J.; Ou, J.; Zhao, D.; Ou, S. Origin and Fate of Acrolein in Foods. Foods 2022, 11, 1976. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.M.; Beland, F.A.; Lachenmeier, D.W.; Phillips, D.H.; Chung, F.L.; Dorman, D.C.; Elmore, S.E.; Hammond, S.K.; Krstev, S.; Linhart, I.; et al. Carcinogenicity of Acrolein, Crotonaldehyde, and Arecoline. Lancet Oncol. 2021, 22, 19–20. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Kistler, K.A.; Pennington, A.; Spyrou, A.; Kouretas, D.; Gillman, G. Aldehyde levels in e-cigarette aerosol: Findings from a replication study and from use of a new-generation device. Food Chem. Toxicol. 2018, 111, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Tayyarah, R.; Long, G.A. Comparison of select analytes in aerosol from e-cigarettes with smoke from conventional cigarettes and with ambient air. Regul. Toxicol. Pharm. 2014, 70, 704–710. [Google Scholar] [CrossRef]
- Carmella, S.G.; Chen, M.L.; Zhang, Y.; Zhang, S.Y.; Hatsukami, D.K.; Hecht, S.S. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: Effects of cigarette smoking. Chem. Res. Toxicol. 2007, 20, 986–990. [Google Scholar] [CrossRef] [PubMed]
- Kehrer, J.P.; Biswal, S.S. The molecular effects of acrolein. Toxicol. Sci. 2000, 57, 6–15. [Google Scholar] [CrossRef]
- Burcham, P.C. Acrolein and Human Disease: Untangling the Knotty Exposure Scenarios Accompanying Several Diverse Disorders. Chem. Res. Toxicol. 2017, 30, 145–161. [Google Scholar] [CrossRef]
- Zirak, M.R.; Mehri, S.; Karimani, A.; Zeinali, M.; Hayes, A.W.; Karimi, G. Mechanisms behind the atherothrombotic effects of acrolein, a review. Food Chem. Toxicol. 2019, 129, 38–53. [Google Scholar] [CrossRef]
- Yoshida, M.; Tomitori, H.; Machi, Y.; Hagihara, M.; Higashi, K.; Goda, H.; Ohya, T.; Niitsu, M.; Kashiwagi, K.; Igarashi, K. Acrolein toxicity: Comparison with reactive oxygen species. Biochem. Biophys. Res. Commun. 2009, 378, 313–318. [Google Scholar] [CrossRef]
- Kwak, M.-K.; Kensler, T.W.; Casero, R.A. Induction of phase 2 enzymes by serum oxidized polyamines through activation of Nrf2: Effect of the polyamine metabolite acrolein. Biochem. Biophys. Res. Commun. 2003, 305, 662–670. [Google Scholar] [CrossRef]
- Halagowder, A.D.; Niranjali, S.D. Detection of Acrolein–Lysine Adducts in Plasma Low-Density Lipoprotein and in Aorta of Cyclophosphamide-Administered Rats. Arch. Toxicol. 2004, 78, 397–401. [Google Scholar]
- Yoshida, M.; Tomitori, H.; Machi, Y.; Katagiri, D.; Ueda, S.; Horiguchi, K.; Kobayashi, E.; Saeki, N.; Nishimura, K.; Ishii, I.; et al. Acrolein, IL-6 and CRP as markers of silent brain infarction. Atherosclerosis 2009, 203, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Jin, M.H.; Pi, R.B.; Zhang, J.J.; Ouyang, Y.; Chao, X.J.; Chen, M.H.; Liu, P.Q.; Yu, J.C.; Ramassamy, C.; et al. Acrolein induces Alzheimer’s disease-like pathologies in vitro and in vivo. Toxicol. Lett. 2013, 217, 184–191. [Google Scholar] [CrossRef]
- Park, Y.S.; Misonou, Y.; Fujiwara, N.; Takahashi, M.; Miyamoto, Y.; Koh, Y.H.; Suzuki, K.; Taniguchi, N. Induction of thioredoxin reductase as an adaptive response to acrolein in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2005, 327, 1058–1065. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Pocernich, C.B.; Butterfield, D.A. Acrolein inhibits NADH-linked mitochondrial enzyme activity: Implications for Alzheimer’s disease. Neurotox. Res. 2003, 5, 515–519. [Google Scholar] [CrossRef]
- Srivastava, S.; Sithu, S.D.; Vladykovskaya, E.; Haberzettl, P.; Hoetker, D.J.; Siddiqui, M.A.; Conklin, D.J.; D’Souza, S.E.; Bhatnagar, A. Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis 2011, 215, 301–308. [Google Scholar] [CrossRef]
- Huang, Y.J.; Zhang, L.; Shi, L.Y.; Wang, Y.Y.; Yang, Y.B.; Ke, B.; Zhang, T.Y.; Qin, J. Caloric restriction ameliorates acrolein-induced neurotoxicity in rats. Neurotoxicology 2018, 65, 44–51. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, T.W.; Lin, Y.Y.; Ho, W.C.; Tsai, H.C.; Chen, S.P.; Lin, A.M.; Liu, T.Y.; Wang, H.T. Acrolein is involved in ischemic stroke-induced neurotoxicity through spermidine/spermine-N1-acetyltransferase activation. Exp. Neurol. 2020, 323, 113066. [Google Scholar] [CrossRef]
- Pegg, A.E. Mammalian polyamine metabolism and function. IUBMB Life 2009, 61, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xie, C.S.; Markesbery, W.R. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging 2001, 22, 187–194. [Google Scholar] [CrossRef]
- Yoshida, M.; Higashi, K.; Kobayashi, E.; Saeki, N.; Wakui, K.; Kusaka, T.; Takizawa, H.; Kashiwado, K.; Suzuki, N.; Fukuda, K.; et al. Correlation between images of silent brain infarction, carotid atherosclerosis and white matter hyperintensity, and plasma levels of acrolein, IL-6 and CRP. Atherosclerosis 2010, 211, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Kato, N.; Uemura, T.; Mizoi, M.; Nakamura, M.; Saiki, R.; Hatano, K.; Sato, K.; Kakizaki, S.; Nakamura, A.; et al. Time dependent transition of the levels of protein-conjugated acrolein (PC-Acro), IL-6 and CRP in plasma during stroke. Eneurologicalsci 2017, 7, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Choi, J.Y.; Jo, C.; Koh, Y.H. Involvement of ADAM10 in acrolein-induced astrocytic inflammation. Toxicol. Lett. 2020, 318, 44–49. [Google Scholar] [CrossRef]
- Tomitori, H.; Nakamura, M.; Sakamoto, A.; Terui, Y.; Yoshida, M.; Igarashi, K.; Kashiwagi, K. Augmented glutathione synthesis decreases acrolein toxicity. Biochem. Biophys. Res. Commun. 2012, 418, 110–115. [Google Scholar] [CrossRef]
- Nakamura, M.; Uemura, T.; Saiki, R.; Sakamoto, A.; Park, H.; Nishimura, K.; Terui, Y.; Toida, T.; Kashiwagi, K.; Igarashi, K. Toxic acrolein production due to Ca2+ influx by the NMDA receptor during stroke. Atherosclerosis 2016, 244, 131–137. [Google Scholar] [CrossRef]
- Yoshida, M.; Higashi, K.; Jin, L.; Machi, Y.; Suzuki, T.; Masuda, A.; Dohmae, N.; Suganami, A.; Tamura, Y.; Nishimura, K.; et al. Identification of acrolein-conjugated protein in plasma of patients with brain infarction. Biochem. Biophys. Res. Commun. 2010, 391, 1234–1239. [Google Scholar] [CrossRef]
- Sakata, K.; Kashiwagi, K.; Sharmin, S.; Ueda, S.; Irie, Y.; Murotani, N.; Igarashi, K. Increase in putrescine, amine oxidase, and acrolein in plasma of renal failure patients. Biochem. Biophys. Res. Commun. 2003, 305, 143–149. [Google Scholar] [CrossRef]
- Boor, P.J.; Sanduja, R.; Nelson, T.J.; Ansari, G.A. In vivo metabolism of the cardiovascular toxin, allylamine. Biochem. Pharmacol. 1987, 36, 4347–4353. [Google Scholar] [CrossRef]
- Saiki, R.; Nishimura, K.; Ishii, I.; Omura, T.; Okuyama, S.; Kashiwagi, K.; Igarashi, K. Intense correlation between brain infarction and protein-conjugated acrolein. Stroke 2009, 40, 3356–3361. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Watanabe, K.; Ishibashi, M.; Saiki, R.; Kuni, K.; Nishimura, K.; Toida, T.; Kashiwagi, K.; Igarashi, K. Aggravation of brain infarction through an increase in acrolein production and a decrease in glutathione with aging. Biochem. Biophys. Res. Commun. 2016, 473, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Halagappa, V.K.M.; Guo, Z.H.; Pearson, M.; Matsuoka, Y.; Cutler, R.G.; LaFerla, F.M.; Mattson, M.P. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2007, 26, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, R.; Laferla, F.M. Astrocytes: Conductors of the Alzheimer disease neuroinflammatory symphony. Exp. Neurol. 2013, 239, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Castegna, A.; Lauderback, C.M.; Mohmmad-Abdul, H.; Butterfield, D.A. Modulation of phospholipid asymmetry in synaptosomal membranes by the lipid peroxidation products, 4-hydroxynonenal and acrolein: Implications for Alzheimer’s disease. Brain Res. 2004, 1004, 193–197. [Google Scholar] [CrossRef]
- Seidler, N.W.; Yeargans, G.S. Albumin-bound polyacrolein: Implications for Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2004, 320, 213–217. [Google Scholar] [CrossRef]
- Markesbery, W.R.; Lovell, M.A. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer’s disease. Neurobiol. Aging 1998, 19, 33–36. [Google Scholar] [CrossRef]
- Mello, C.F.; Sultana, R.; Piroddi, M.; Cai, J.; Pierce, W.M.; Klein, J.B.; Butterfield, D.A. Acrolein induces selective protein carbonylation in synaptosomes. Neuroscience 2007, 147, 674–679. [Google Scholar] [CrossRef]
- Hensley, K.; Hall, N.; Subramaniam, R.; Cole, P.; Harris, M.; Aksenov, M.; Aksenova, M.; Gabbita, S.P.; Wu, J.F.; Carney, J.M. Brain regional correspondence between Alzheimer’s disease histopathology and biomarkers of protein oxidation. J. Neurochem. 2010, 65, 2146–2156. [Google Scholar] [CrossRef]
- Williams, T.I.; Lynn, B.C.; Markesbery, W.R.; Lovell, M.A. Increased levels of 4-hydroxynonenal and acrolein, neurotoxic markers of lipid peroxidation, in the brain in Mild Cognitive Impairment and early Alzheimer’s disease. Neurobiol. Aging 2006, 27, 1094–1099. [Google Scholar] [CrossRef]
- Luo, J.; Robinson, J.P.; Shi, R. Acrolein-induced cell death in PC12 cells: Role of mitochondria-mediated oxidative stress. Neurochem. Int. 2005, 47, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Pocernich, C.B.; Cardin, A.L.; Racine, C.L.; Lauderback, C.M.; Butterfield, D.A. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: Relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem. Int. 2001, 39, 141–149. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xie, C.; Markesbery, W.R. Decreased glutathione transferase activity in brain and ventricular fluid in Alzheimer’s disease. Neurology 1998, 51, 1562–1566. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.R.; Lovell, M.A.; Yatin, M.; Dhillon, H.; Markesbery, W.R. Regional membrane phospholipid alterations in Alzheimer’s disease. Neurochem. Res. 1998, 23, 81–88. [Google Scholar] [CrossRef]
- Rochet, J.C.; Hay, B.A.; Guo, M. Molecular insights into Parkinson’s disease. Prog. Mol. Biol. Transl. Sci. 2012, 107, 125–188. [Google Scholar]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Acosta, G.; Race, N.; Herr, S.; Fernandez, J.; Tang, J.; Rogers, E.; Shi, R. Acrolein-mediated alpha-synuclein pathology involvement in the early post-injury pathogenesis of mild blast-induced Parkinsonian neurodegeneration. Mol. Cell Neurosci. 2019, 98, 140–154. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhuang, X.; Lu, J. Neuroprotective effects of baicalein in animal models of Parkinson’s disease: A systematic review of experimental studies. Phytomedicine 2019, 55, 302–309. [Google Scholar] [CrossRef]
- Speen, A.; Jones, C.; Patel, R.; Shah, H.; Nallasamy, P.; Brooke, E.A.; Zhu, H.; Li, Y.R.; Jia, Z. Mechanisms of CDDO-imidazolide-mediated cytoprotection against acrolein-induced neurocytotoxicity in SH-SY5Y cells and primary human astrocytes. Toxicol. Lett. 2015, 238, 32–42. [Google Scholar] [CrossRef]
- Henchcliffe, C.; Beal, M.F. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat. Clin. Pract. Neurol. 2008, 4, 600–609. [Google Scholar] [CrossRef]
- Takano, K.; Ogura, M.; Yoneda, Y.; Nakamura, Y. Oxidative metabolites are involved in polyamine-induced microglial cell death. Neuroscience 2005, 134, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Schain, M.; Kreisl, W.C. Neuroinflammation in Neurodegenerative Disorders—A Review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Masuda, T.; Sankowski, R.; Staszewski, O.; Bottcher, C.; Amann, L.; Sagar; Scheiwe, C.; Nessler, S.; Kunz, P.; van Loo, G.; et al. Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature 2019, 566, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Lin, H.C.; Zhao, W.Z.; Huang, H.J.; Lo, Y.L.; Wang, H.T.; Lin, A.M. Acrolein acts as a neurotoxin in the nigrostriatal dopaminergic system of rat: Involvement of alpha-synuclein aggregation and programmed cell death. Sci. Rep. 2017, 7, 45741. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Kalia, S.K.; McLean, P.J.; Lozano, A.M.; Lang, A.E. alpha-Synuclein oligomers and clinical implications for Parkinson disease. Ann. Neurol. 2013, 73, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Ambaw, A.; Zheng, L.; Tambe, M.A.; Strathearn, K.E.; Acosta, G.; Hubers, S.A.; Liu, F.; Herr, S.A.; Tang, J.; Truong, A.; et al. Acrolein-mediated neuronal cell death and alpha-synuclein aggregation: Implications for Parkinson’s disease. Mol. Cell Neurosci. 2018, 88, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Stefanis, L. α-Synuclein in Parkinson’s Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009399. [Google Scholar] [CrossRef]
- Castellani, R.; Smith, M.A.; Richey, P.L.; Perry, G. Glycoxidation and oxidative stress in Parkinson disease and diffuse Lewy body disease. Brain Res. 1996, 737, 195–200. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Goldman, S.M.; Kamel, F.; Ross, G.W.; Jewell, S.A.; Bhudhikanok, G.S.; Umbach, D.; Marras, C.; Hauser, R.A.; Jankovic, J.; Factor, S.A.; et al. Head injury, alpha-synuclein Rep1, and Parkinson’s disease. Ann. Neurol. 2012, 71, 40–48. [Google Scholar] [CrossRef]
- Tully, M.; Shi, R.Y. New Insights in the Pathogenesis of Multiple Sclerosis-Role of Acrolein in Neuronal and Myelin Damage. Int. J. Mol. Sci. 2013, 14, 20037–20047. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Page, J.C.; Tully, M. Molecular mechanisms of acrolein-mediated myelin destruction in CNS trauma and disease. Free Radic. Res. 2015, 49, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Tully, M.; Zheng, L.X.; Shi, R.Y. Acrolein detection: Potential theranostic utility in multiple sclerosis and spinal cord injury. Expert Rev. Neurother. 2014, 14, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Leung, G.; Sun, W.; Zheng, L.; Brookes, S.; Tully, M.; Shi, R. Anti-acrolein treatment improves behavioral outcome and alleviates myelin damage in experimental autoimmune enchephalomyelitis mouse. Neuroscience 2011, 173, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Uchida, K.; Shi, R.Y. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem. Res. 2005, 30, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Liu-Snyder, P.; McNally, H.; Shi, R.; Borgens, R.B. Acrolein-mediated mechanisms of neuronal death. J. Neurosci. Res. 2006, 84, 209–218. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsuoka, Y.; Sakai, K.; Fujiya, N.; Mano, J. Catechins in green tea powder (matcha) are heat-stable scavengers of acrolein, a lipid peroxide-derived reactive carbonyl species. Food Chem. 2021, 355, 129403. [Google Scholar] [CrossRef]
- Yin, Z.; Jiang, K.Y.; Shi, L.; Fei, J.; Zheng, J.; Ou, S.Y.; Ou, J.Y. Formation of di-cysteine acrolein adduct decreases cytotoxicity of acrolein by ROS alleviation and apoptosis intervention. J. Hazard. Mater. 2020, 387, 11. [Google Scholar] [CrossRef]
- Leuci, R.; Brunetti, L.; Poliseno, V.; Laghezza, A.; Loiodice, F.; Tortorella, P.; Piemontese, L. Natural Compounds for the Prevention and Treatment of Cardiovascular and Neurodegenerative Diseases. Foods 2021, 10, 17. [Google Scholar] [CrossRef]
- Zhang, D.M.; Jiang, X.Y.; Xiao, L.B.; Lu, Y.L.; Sang, S.M.; Lv, L.S.; Dong, W.J. Mechanistic Studies of inhibition on Acrolein by Myricetin. Food Chem. 2020, 323, 126788. [Google Scholar] [CrossRef]
- Fan, D.; Yassar, A.; Liu, K.; Michael, M.A.; Paul, H.; Margaret, B.; John, D.A.; Tim, P.; Oliver, M.; Andrew, L. Supplementation of Blackcurrant Anthocyanins Increased Cyclic Glycine-Proline in the Cerebrospinal Fluid of Parkinson Patients: Potential Treatment to Improve Insulin-Like Growth Factor-1 Function. Nutrients 2018, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Kim, T.; Rehman, S.U.; Khan, M.S.; Amin, F.U.; Khan, M.; Ikram, M.; Kim, M.O. Natural Dietary Supplementation of Anthocyanins via PI3K/Akt/Nrf2/HO-1 Pathways Mitigate Oxidative Stress, Neurodegeneration, and Memory Impairment in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 55, 6076–6093. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.L.; Buz’Zard, A.R.; Lau, B.H.S. Pycnogenol (R) protects neurons from amyloid-beta peptide-induced apoptosis. Mol Brain Res. 2002, 104, 55–65. [Google Scholar] [CrossRef]
- Selley, M.L. (E)-4-hydroxy-2-nonenal may be involved in the pathogenesis of Parkinson’s disease. Free Radic. Biol. Med. 1998, 25, 169–174. [Google Scholar] [CrossRef]
- Kobayashi M, S.; Han, D.; Packer, L. Antioxidants and Herbal Extracts Protect Ht-4 Neuronal Cells against Glutamate-Induced Cytotoxicity. Free Radic. Res. 2000, 32, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Keller, J.N.; Scheff, S.W. Protective effect of Pycnogenol in human neuroblastoma SH-SY5Y cells following acrolein-induced cytotoxicity. Free Radic. Biol. Med. 2008, 45, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, X.; Cao, K.; Xu, J.; Yue, T.; Dai, F.; Zhou, B.; Lu, W.; Feng, Z.; Liu, J. Curcumin analog 1,5-bis (2-trifluoromethylphenyl)-1,4-pentadien-3-one exhibits enhanced ability on Nrf2 activation and protection against acrolein-induced ARPE-19 cell toxicity. Toxicol. Appl. Pharmacol. 2013, 272, 726–735. [Google Scholar] [CrossRef]
- Wang, Y.D.; Chang, X.X.; Zheng, B.; Chen, Y.; Xie, J.H.; Shan, J.L.; Hu, X.Y.; Ding, X.M.; Hu, X.B.; Yu, Q. Protective Effect of Ganoderma atrum Polysaccharide on Acrolein-Induced Apoptosis and Autophagic Flux in IEC-6 Cells. Foods 2022, 11, 14. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, N.Q.; Lau, C.F.; Chao, J.; Sun, Z.; Chang, R.C.; Chen, F.; Wang, M. In vitro attenuation of acrolein-induced toxicity by phloretin, a phenolic compound from apple. Food Chem. 2012, 135, 1762–1768. [Google Scholar] [CrossRef]
- Suabjakyong, P.; Saiki, R.; Van Griensven, L.J.; Higashi, K.; Nishimura, K.; Igarashi, K.; Toida, T. Polyphenol extract from Phellinus igniarius protects against acrolein toxicity in vitro and provides protection in a mouse stroke model. PLoS ONE 2015, 10, e0122733. [Google Scholar] [CrossRef]
- Gu, Y.P.; Yang, X.M.; Luo, P.; Li, Y.Q.; Tao, Y.X.; Duan, Z.H.; Xiao, W.; Zhang, D.Y.; Liu, H.Z. Inhibition of acrolein-induced autophagy and apoptosis by a glycosaminoglycan from Sepia esculenta ink in mouse Leydig cells. Carbohydr. Polym. 2017, 163, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Park, J.; Butler, B.; Acosta, G.; Vega-Alvarez, S.; Zheng, L.; Tang, J.; McCain, R.; Zhang, W.; Ouyang, Z.; et al. Mitigation of sensory and motor deficits by acrolein scavenger phenelzine in a rat model of spinal cord contusive injury. J. Neurochem. 2016, 138, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.; Sekhon, C.; Khan, M.; Patel, S.J.; Singh, I.; Singh, A.K. N-Acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res. 2003, 971, 1–8. [Google Scholar] [CrossRef]
- Tian, R.; Shi, R. Dimercaprol is an acrolein scavenger that mitigates acrolein-mediated PC-12 cells toxicity and reduces acrolein in rat following spinal cord injury. J. Neurochem. 2017, 141, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sun, Z.; Jiang, Y.; Chen, F.; Wang, M.F. Acrolein Scavengers: Reactivity, Mechanism and Impact on Health. Mol. Nutr. Food Res. 2011, 55, 1375–1390. [Google Scholar] [CrossRef]
- Bispo, V.S.; Campos, I.; Di Mascio, P.; Medeiros, M.H.G. Structural Elucidation of a Carnosine-Acrolein Adduct and Its Quantification in Human Urine Samples. Sci. Rep. 2016, 6, 19348. [Google Scholar] [CrossRef]
| Food | Acrolein Content (mg/kg or mg/L) | Daily Consumption | |
|---|---|---|---|
| Food (g or mL/day) | Acrolein (μg/day) | ||
| Fruits | 0.05 | 337 | 15–17 |
| Vegetable oil | 2.80 × 10−3–10.20 × 10−3 | 50 | 0.14–0.51 |
| Vegetables | 0.50 | 260–500 | 200–250 |
| Potatoes | 0.60 | 250 | 150 |
| Oil | 0.20 | 50 | 10 |
| Frying fats and oils | 0.276 | 50 | 13.8 |
| Fried fish coating | 0.10 | NA | |
| Cheese | 1.00 | 40 | 40 |
| French fries | 1.97–4.85 | NA | |
| Donuts | 0.90 | 60–400 | 54–360 |
| Codfish fillet | 0.10 | 100 | 10 |
| Alcoholic beverages | 0.247 | 84–493 | 20.74–121.77 |
| Wine | 3.80 | 43–400 | 163–1520 |
| Brandy/cognac | 1.50 | 3 | 2–33 |
| Lager beer | 0.002 | 142 | 0.2 |
| Tequila | 0.404 | NA | |
| whiskey | 0.252 | Up to 180 | Up to 45.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, X.; Wang, Y.; Zheng, B.; Chen, Y.; Xie, J.; Song, Y.; Ding, X.; Hu, X.; Hu, X.; Yu, Q. The Role of Acrolein in Neurodegenerative Diseases and Its Protective Strategy. Foods 2022, 11, 3203. https://doi.org/10.3390/foods11203203
Chang X, Wang Y, Zheng B, Chen Y, Xie J, Song Y, Ding X, Hu X, Hu X, Yu Q. The Role of Acrolein in Neurodegenerative Diseases and Its Protective Strategy. Foods. 2022; 11(20):3203. https://doi.org/10.3390/foods11203203
Chicago/Turabian StyleChang, Xinxin, Yudan Wang, Bing Zheng, Yi Chen, Jianhua Xie, Yiming Song, Xiaomeng Ding, Xiaoyi Hu, Xiaobo Hu, and Qiang Yu. 2022. "The Role of Acrolein in Neurodegenerative Diseases and Its Protective Strategy" Foods 11, no. 20: 3203. https://doi.org/10.3390/foods11203203
APA StyleChang, X., Wang, Y., Zheng, B., Chen, Y., Xie, J., Song, Y., Ding, X., Hu, X., Hu, X., & Yu, Q. (2022). The Role of Acrolein in Neurodegenerative Diseases and Its Protective Strategy. Foods, 11(20), 3203. https://doi.org/10.3390/foods11203203