Functionality of Pea-Grass Carp Co-Precipitated Dual-Protein as Affected by Extraction pH
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
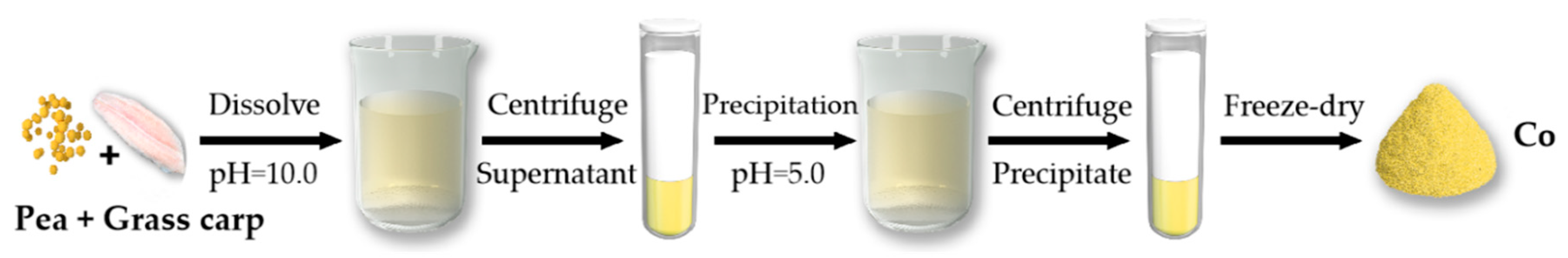
2.2. Preparation of Co
2.3. Solubility
2.4. Non-Reduced SDS-PAGE
2.5. Colour
2.6. Surface Hydrophobicity (H0)
2.7. Emulsifying Property
2.7.1. Preparation of Emulsion
2.7.2. Emulsifying Activity Index and Emulsifying Stability Index
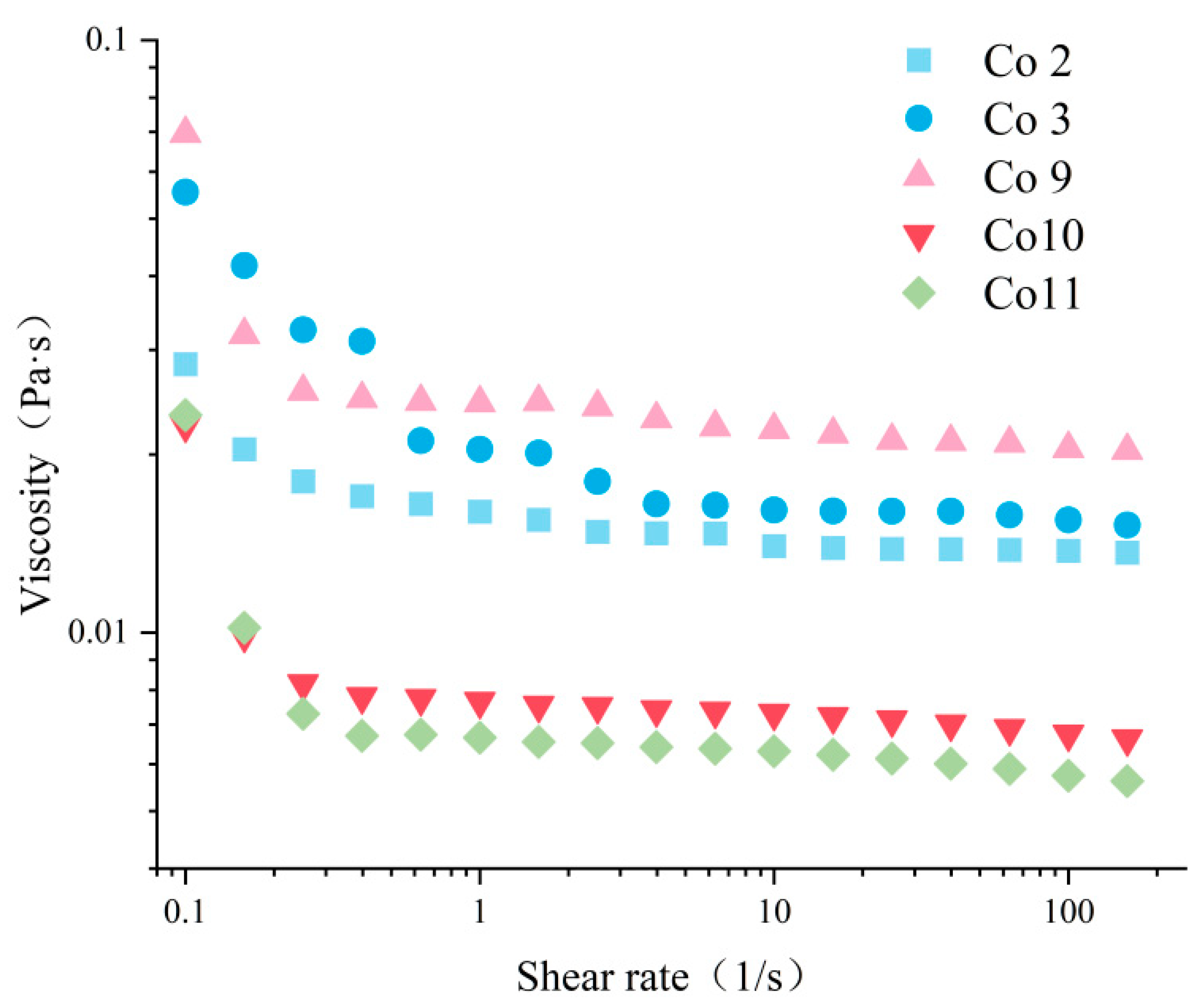
2.7.3. Rheological Properties of Emulsion
2.7.4. Microstructure of Emulsion
2.8. Gel Property
2.8.1. Preparation of Heat-Induced Gel
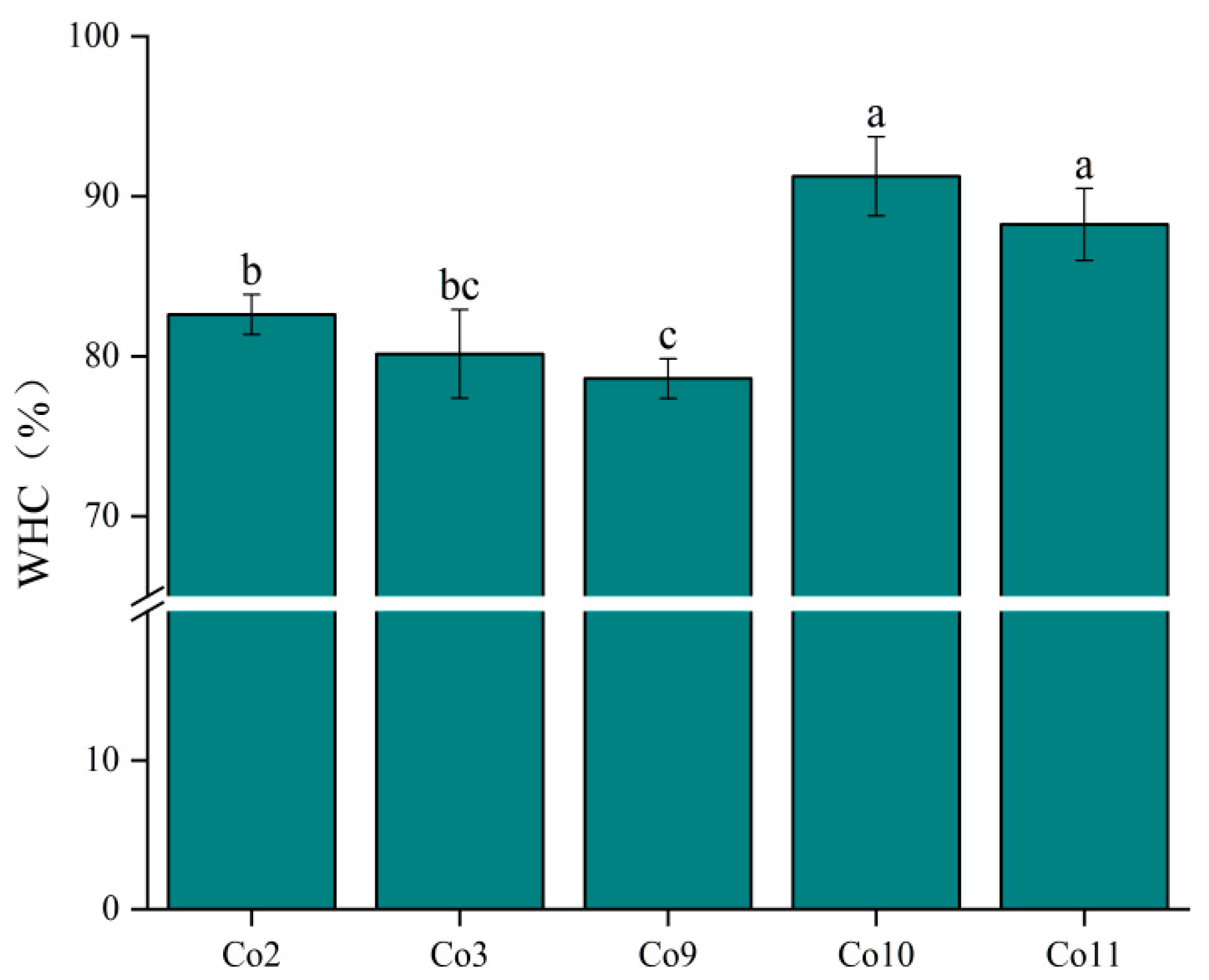
2.8.2. Water-Holding Capacity (WHC)
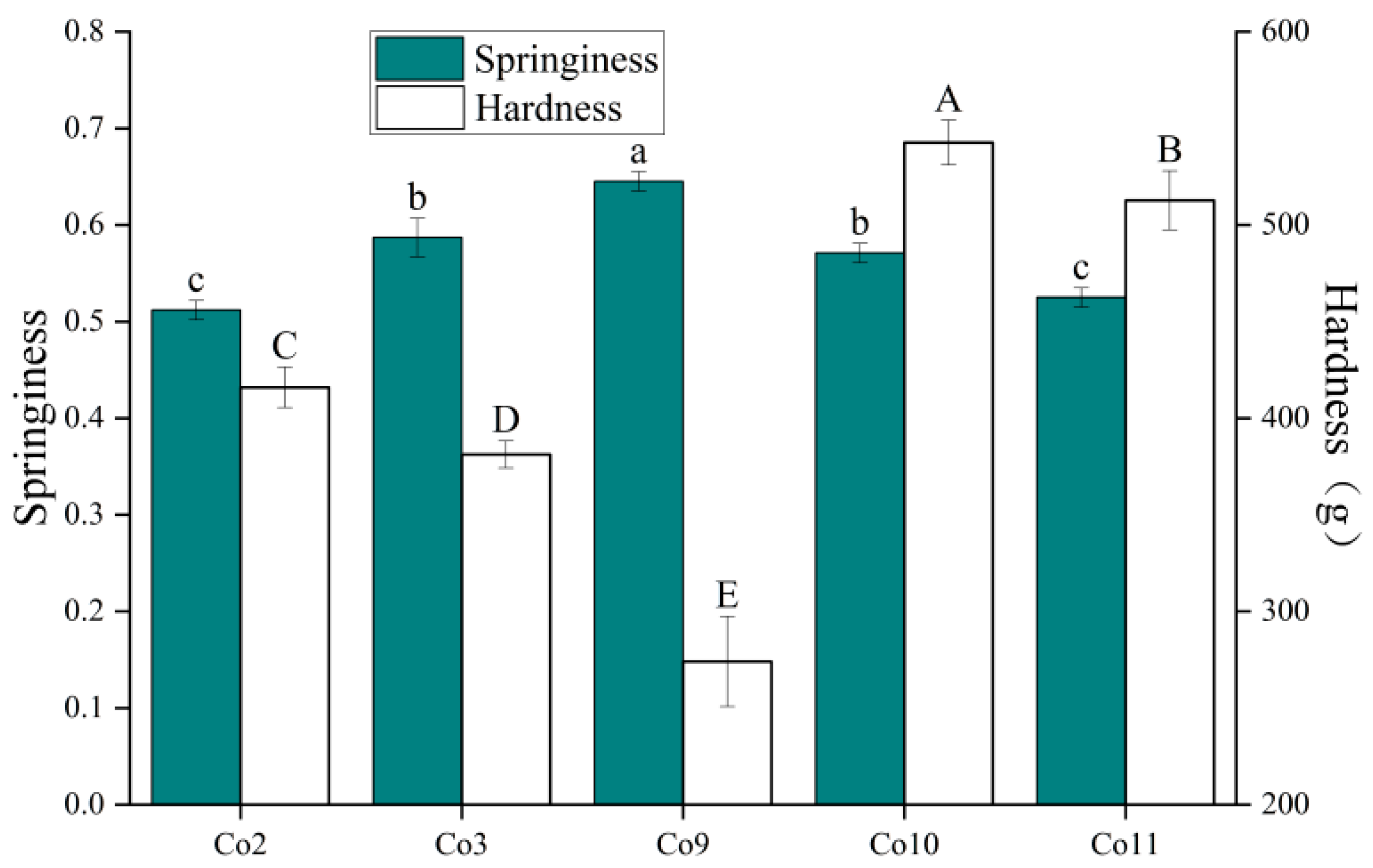
2.8.3. Hardness and Springiness
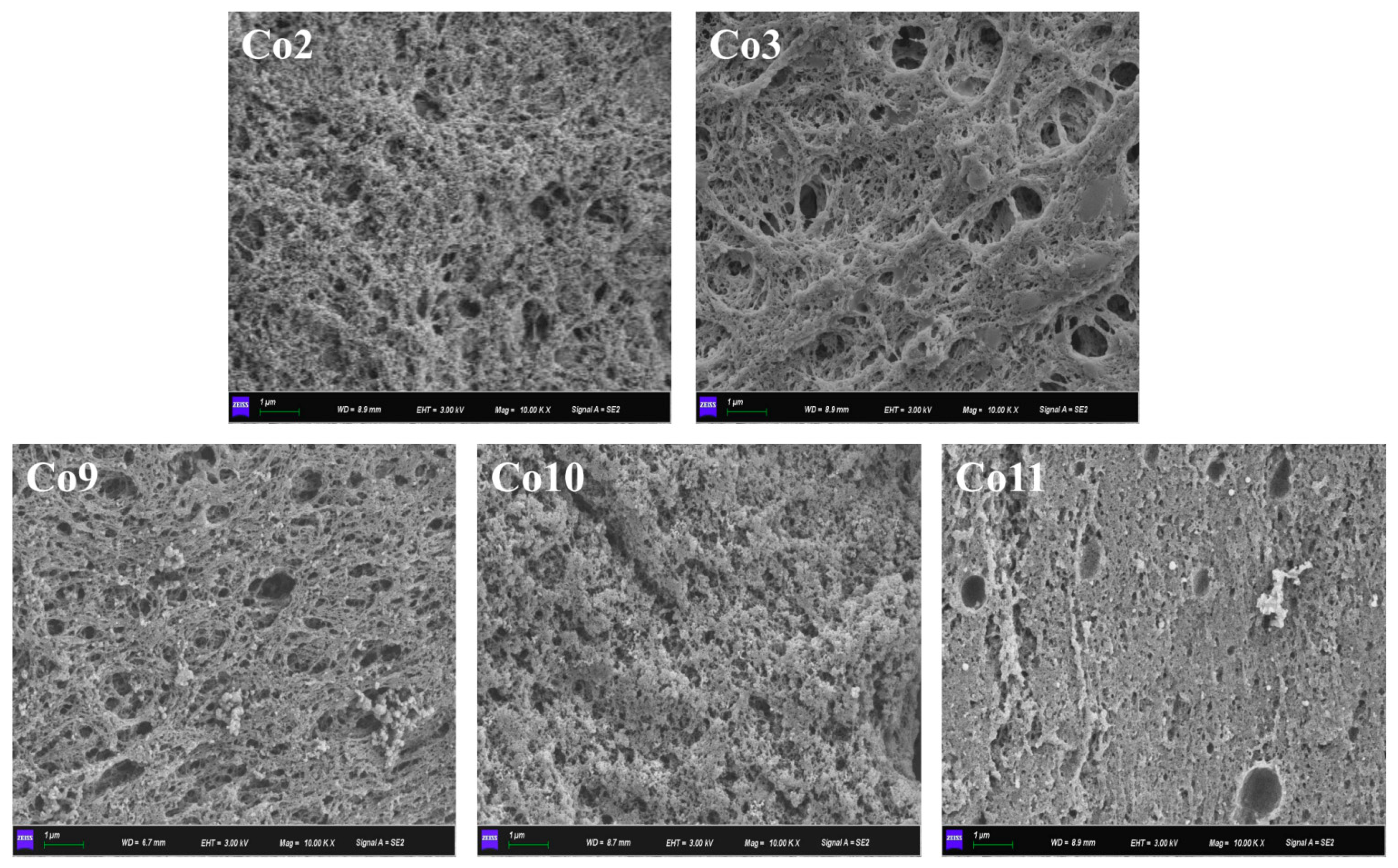
2.8.4. Microstructure of Gel
2.9. Statistical Analysis
3. Results and Discussion
3.1. Protein Composition Analysis
3.2. Colour
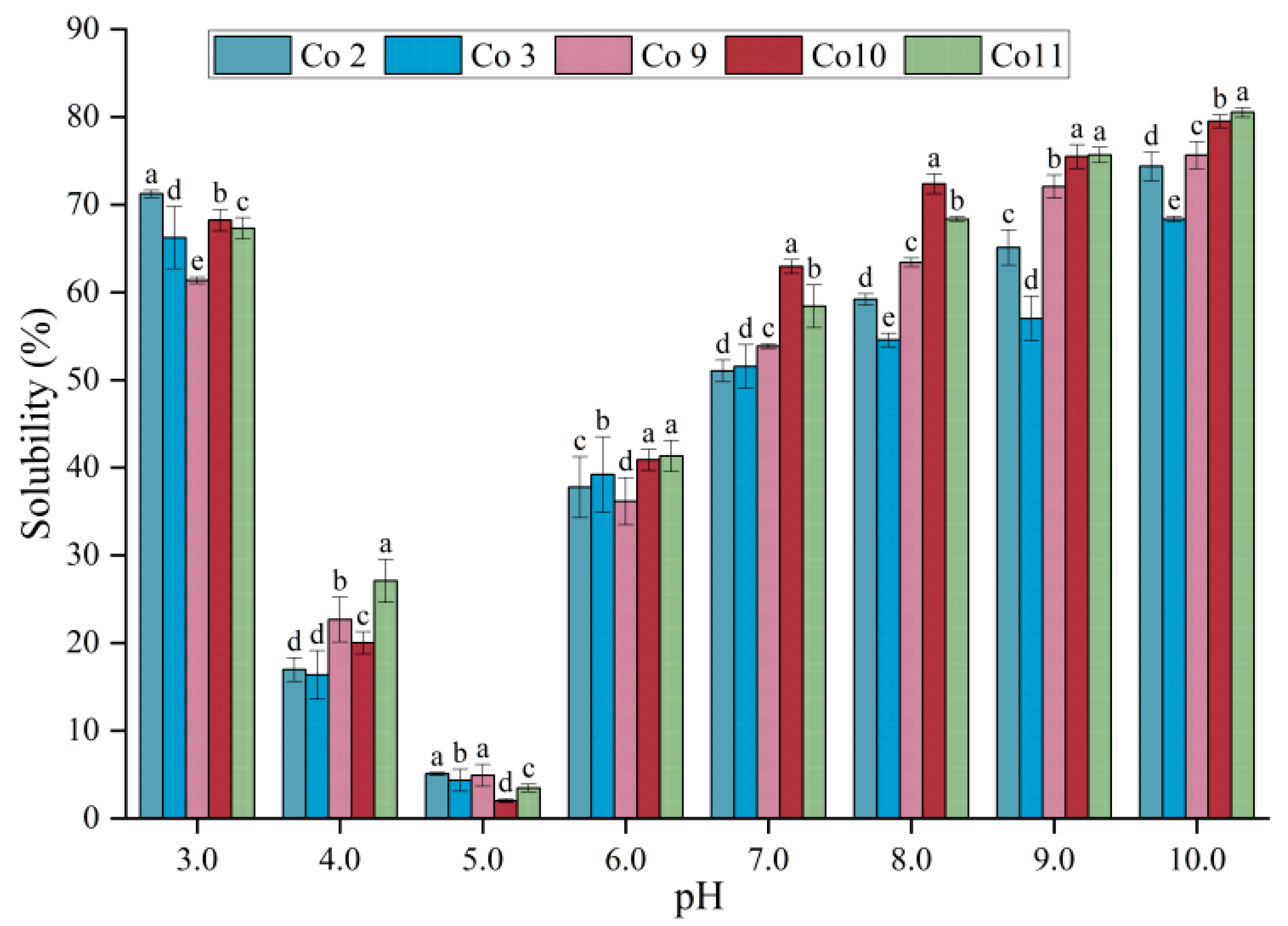
3.3. Solubility
3.4. Surface Hydrophobicity (H0)
3.5. Emulsifying Property
3.6. Gel Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Szenderák, J.; Fróna, D.; Rákos, M. Consumer Acceptance of Plant-Based Meat Substitutes: A Narrative Review. Foods 2022, 11, 1274. [Google Scholar] [CrossRef] [PubMed]
- Pointke, M.; Pawelzik, E. Plant-Based Alternative Products: Are They Healthy Alternatives? Micro- and Macronutrients and Nutritional Scoring. Nutrients 2022, 14, 601. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, I.C.; de Paula Ferreira, I.E.; Casanova, F.; Cavallieri, A.L.F.; Lima Nascimento, L.G.; de Carvalho, A.F.; Nogueira Silva, N.F. Colloidal and Acid Gelling Properties of Mixed Milk and Pea Protein Suspensions. Foods 2022, 11, 1383. [Google Scholar] [CrossRef]
- Wu, C.; Wang, T.; Ren, C.; Ma, W.; Wu, D.; Xu, X.; Wang, L.S.; Du, M. Advancement of food-derived mixed protein systems: Interactions, aggregations, and functional properties. Compr. Rev. Food Sci. Food Saf. 2021, 20, 627–651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, C.; Cao, W.; Zhou, C.; Zheng, H.; Zhao, L. A Comparative Functional Analysis of Pea Protein and Grass Carp Protein Mixture via Blending and Co-Precipitation. Foods 2021, 10, 3037. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Zhao, L.; Zhou, X.; Huang, Z.; Zhou, C.; Cao, W.; Zheng, H. Comparative Analysis of Heat-induced Gel Properties of Pea-grass Carp Dual-protein Prepared by Different Methods. J. Guangdong Ocean. Univ. 2022, 42, 79–86. [Google Scholar]
- Kristensen, H.T.; Christensen, M.; Hansen, M.S.; Hammershøj, M.; Dalsgaard, T.K. Protein-protein interactions of a whey-pea protein co-precipitate. Int. J. Food Sci. Technol. 2021, 56, 5777–5790. [Google Scholar] [CrossRef]
- Tahergorabi, R.; Beamer, S.K.; Matak, K.E.; Jaczynski, J. Functional food products made from fish protein isolate recovered with isoelectric solubilization/precipitation. LWT-Food Sci. Technol. 2012, 48, 89–95. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tou, J.C.; Jaczynski, J. Amino acid and mineral composition of protein and other components and their recovery yields from whole Antarctic krill (Euphausia superba) using isoelectric solubilization/precipitation. J. Food Sci. 2009, 74, 31–39. [Google Scholar] [CrossRef]
- Taskaya, L.; Chen, Y.C.; Beamer, S.; Tou, J.C.; Jaczynski, J. Compositional characteristics of materials recovered from whole gutted silver carp (Hypophthalmichthys molitrix) using isoelectric solubilization/precipitation. J. Agric. Food Chem. 2009, 57, 4259–4266. [Google Scholar] [CrossRef]
- Jafarpour, A.; Gorczyca, E.M. Alternative techniques for producing a quality surimi and kamaboko from common carp (Cyprinus carpio). J. Food Sci. 2008, 73, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Sun, C.X.; Corke, H.; Gul, K.; Gan, R.Y.; Fang, Y. The health benefits, functional properties, modifications, and applications of pea (Pisum sativum L.) protein: Current status, challenges, and perspectives. Compr. Rev. Food Sci. F 2020, 19, 1835–1876. [Google Scholar] [CrossRef]
- Food and Agriculture Organization Corporate Statistical Database (FAOSTAT). Production and Prices Statistics of the Food and Agriculture Organization of the United Nations. 2020. Available online: http://faostat.fao.org (accessed on 9 June 2022).
- Fischer, E.; Cachon, R.; Cayot, N. Pisum sativum vs Glycine max, a comparative review of nutritional, physicochemical, and sensory properties for food uses. Trends Food Sci. Tech. 2019, 95, 196–204. [Google Scholar] [CrossRef]
- Department of Fisheries and Fisheries Administration, Ministry of Agriculture and Rural Affairs. China Fishery Statistics Yearbook; China Agriculture Press: Beijing, China, 2021. [Google Scholar]
- Gilbert, J.A.; Bendsen, N.T.; Tremblay, A.; Astrup, A. Effect of proteins from different sources on body composition. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Tahergorabi, R.; Matak, K.E.; Jaczynski, J. Fish protein isolate: Development of functional foods with nutraceutical ingredients. J. Funct. Foods 2015, 18, 746–756. [Google Scholar] [CrossRef]
- Yang, Y.L.; Peng, X.B.; Dong, Z.; You, Y.; Xu, H.P. Study on emulsifying properties of salt-soluble proteins from grass carp. Sci. Technol. Food Ind. 2013, 34, 121–125. [Google Scholar]
- Tan, L.; Hong, P.; Yang, P.; Zhou, C.; Xiao, D.; Zhong, T. Correlation between the Water Solubility and Secondary Structure of Tilapia-Soybean Protein Co-Precipitates. Molecules 2019, 24, 4337. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Chen, H.; Lyu, F.; Lin, H.; Zhang, Q.; Ding, Y. Physicochemical properties and microstructure of fish myofibrillar protein-lipid composite gels: Effects of fat type and concentration. Food Hydrocoll. 2019, 90, 433–442. [Google Scholar] [CrossRef]
- Haskard, C.A.; Li-Chan, E.C. Hydrophobicity of bovine serum albumin and ovalbumin determined using uncharged (PRODAN) and anionic (ANS-) fluorescent probes. J. Agric. Food Chem. 1998, 46, 2671–2677. [Google Scholar] [CrossRef]
- Pearce, K.N.; Kinsella, J.E. Emulsifying properties of proteins: Evaluation of a turbidimetric technique. J. Agric. Food Chem. 1978, 26, 716–723. [Google Scholar] [CrossRef]
- Yong, X.X.; Rui, W.; Hong, L.Z.; Jia, M.Z.; Xue, P.L.; Shu, M.Y.; Jian, R.L.; Xiao, T.S. Binding of aldehydes to myofibrillar proteins as affected by two-step heat treatments. J. Sci. Food Agric. 2020, 100, 1195–1203. [Google Scholar]
- Xu, Y.; Xia, W.; Jiang, Q.; Rao, S. Acid-induced aggregation of actomyosin from silver carp (Hypophthalmichthys molitrix). Food Hydrocoll. 2012, 27, 309–315. [Google Scholar] [CrossRef]
- Mession, J.L.; Chihi, M.L.; Sok, N.; Saurel, R. Effect of globular pea proteins fractionation on their heat-induced aggregation and acid cold-set gelation. Food Hydrocoll. 2015, 46, 233–243. [Google Scholar] [CrossRef]
- Mession, J.L.; Assifaoui, A.; Cayot, P.; Saurel, R. Effect of pea proteins extraction and vicilin/legumin fractionation on the phase behavior in admixture with alginate. Food Hydrocoll. 2012, 29, 335–346. [Google Scholar] [CrossRef]
- Gehring, C.K.; Davenport, M.P.; Jaczynski, J. Functional and nutritional quality of protein and lipid recovered from fish processing by-products and underutilized aquatic species using isoelectric solubilization/precipitation. Curr. Nutr. Food Sci. 2009, 5, 17–39. [Google Scholar] [CrossRef]
- Ma, Z.; Boye, J.I.; Hu, X. In vitro digestibility, protein composition and techno-functional properties of Saskatchewan grown yellow field peas (Pisum sativum L.) as affected by processing. Food Res. Int. 2017, 92, 64–78. [Google Scholar] [CrossRef]
- Chen, G.X. Study on the Preparation and Properties of Grass Carp Surimi with Acidic and Alkallne Treatments; Wuhan Huazhong Agricultural University: Wuhan, China, 2013. [Google Scholar]
- Joshi, M.; Adhikari, B.; Aldred, P.; Panozzo, J.F.; Kasapis, S.; Barrow, C.J. Interfacial and emulsifying properties of lentil protein isolate. Food Chem. 2012, 134, 1343–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolai, T. Gelation of food protein-protein mixtures. Adv. Colloid Interface Sci. 2019, 270, 147–164. [Google Scholar] [CrossRef]
- Lam, A.C.Y.; Can Karaca, A.; Tyler, R.T.; Nickerson, M.T. Pea protein isolates: Structure, extraction, and functionality. Food Rev. Int. 2018, 34, 126–147. [Google Scholar] [CrossRef]
- Lu, B.Y.; Quillien, L.; Popineau, Y. Foaming and emulsifying properties of pea albumin fractions and partial characterisation of surface-active components. J. Sci. Food Agric. 2000, 80, 1964–1972. [Google Scholar] [CrossRef]
- Kimura, A.; Fukuda, T.; Zhang, M.; Motoyama, S.; Maruyama, N.; Utsumi, S. Comparison of Physicochemical Properties of 7S and 11S Globulins from Pea, Fava Bean, Cowpea, and French Bean with Those of Soybean French Bean 7S Globulin Exhibits Excellent Properties. J. Agric. Food Chem. 2008, 56, 10273–10279. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Theodore, A.E.; Demir, N.; Ingadottir, B. A comparative study between acid-and alkali-aided processing and surimi processing for the recovery of proteins from channel catfish muscle. J. Food Sci. 2005, 70, 298–306. [Google Scholar] [CrossRef]
- Du, M.; Xie, J.; Gong, B.; Xu, X.; Tang, W.; Li, X.; Li, C.; Xie, M. Extraction, physicochemical characteristics and functional properties of Mung bean protein. Food Hydrocoll. 2018, 76, 131–140. [Google Scholar] [CrossRef]
- Swanson, B.G. Pea and lentil protein extraction and functionality. J. Am. Oil Chem. Soc. 1990, 67, 276–280. [Google Scholar] [CrossRef]
- Chen, D.; Song, J.; Yang, H.; Xiong, S.; Liu, Y.; Liu, R.U. Effects of acid and alkali treatment on the properties of proteins recovered from whole gutted grass carp (ctenopharyngodon idellus) using isoelectric solubilization/precipitation. J. Food Qual. 2016, 39, 707–713. [Google Scholar] [CrossRef]
- Hong, W. Study on Unfolding and Subsequent Refloding Behaviors of Saurida Myosin Subjected to PH-Shifting; Guangdong Ocean University: Zhanjiang, China, 2014. [Google Scholar]
- QI, H.H. Preparation and Characterization of Co-Precipitated Protein from Tilapia Muscle and Soybean Meal; Guangdong Ocean University: Zhanjiang, China, 2018. [Google Scholar]
- Shand, P.J.; Ya, H.; Pietrasik, Z.; Wanasundara, P.K.J.P.D. Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chem. 2007, 102, 1119–1130. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Nakai, S. Structure-function relationships of food proteins: With an emphasis on the importance of protein hydrophobicity. J. Agric. Food Chem. 1983, 31, 676–683. [Google Scholar] [CrossRef]
- Jiang, S. Enhanced Physicochemical and Functional Properties of Pea (Pisum Sativum) Protein by pH-Shifting and Ultrasonication Combined Process; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2015. [Google Scholar]
- Liu, S.C. Study on Preparation and Properties of Protein Isolates from Tilapia; Guangdong Ocean University: Zhanjiang, China, 2011. [Google Scholar]
- Cui, L.; Bandillo, N.; Wang, Y.; Ohm, J.B.; Chen, B.; Rao, J. Functionality and structure of yellow pea protein isolate as affected by cultivars and extraction pH. Food Hydrocoll. 2020, 108, 106008. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and functional properties of seed proteins from six pea (Pisum sativum) genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Tang, C.H. Emulsifying properties of soy protein nanoparticles: Influence of the protein concentration and/or emulsification process. J. Agric. Food Chem. 2014, 62, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Coupland, J.N. The effect of surfactants on the solubility, zeta potential, and viscosity of soy protein isolates. Food Hydrocoll. 2004, 18, 101–108. [Google Scholar] [CrossRef]
- Zhang, M.X. Study on Emulsifying Properties of Mixture System for Tilapia Protein and Soybean Protein; Guangdong Ocean University: Zhanjiang, China, 2014. [Google Scholar]
- Dapčević-Hadnađev, T.; Dizdar, M.; Pojić, M.; Krstonošić, V.; Zychowski, L.M.; Hadnađev, M. Emulsifying properties of hemp proteins: Effect of isolation technique. Food Hydrocoll. 2019, 89, 912–920. [Google Scholar] [CrossRef]
- Chantrapornchai, W.; McClements, D.J. Influence of NaCl on optical properties, large-strain rheology and water holding capacity of heat-induced whey protein isolate gels. Food Hydrocoll. 2002, 16, 467–476. [Google Scholar] [CrossRef]
- Xiong, Y.L.; Blanehard, S.P.; Means, W.J. Properties of broiler myofibril gels containing emulsified lipids. Poult. Sci. 1992, 71, 1548–1555. [Google Scholar] [CrossRef]
- Shi, L. Composition and Functional Properties of Fish Protein Extracted Using Isoelectric Solubilization/Precipitation Method; Huazhong Agricultural University: Wuhan, China, 2016. [Google Scholar]
- Luo, J.; Li, L.; Luo, J.; Li, X.; Li, K.; Gao, Q. A high solid content bioadhesive derived from soybean meal and egg white: Preparation and properties. J. Polym. Environ. 2017, 25, 948–959. [Google Scholar] [CrossRef]
- O’Kane, F.E.; Happe, R.P.; Vereijken, J.M.; Gruppen, H.; van Boekel, M.A. Heat-induced gelation of pea legumin: Comparison with soybean glycinin. J. Agric. Food Chem. 2004, 52, 5071–5078. [Google Scholar] [CrossRef] [PubMed]
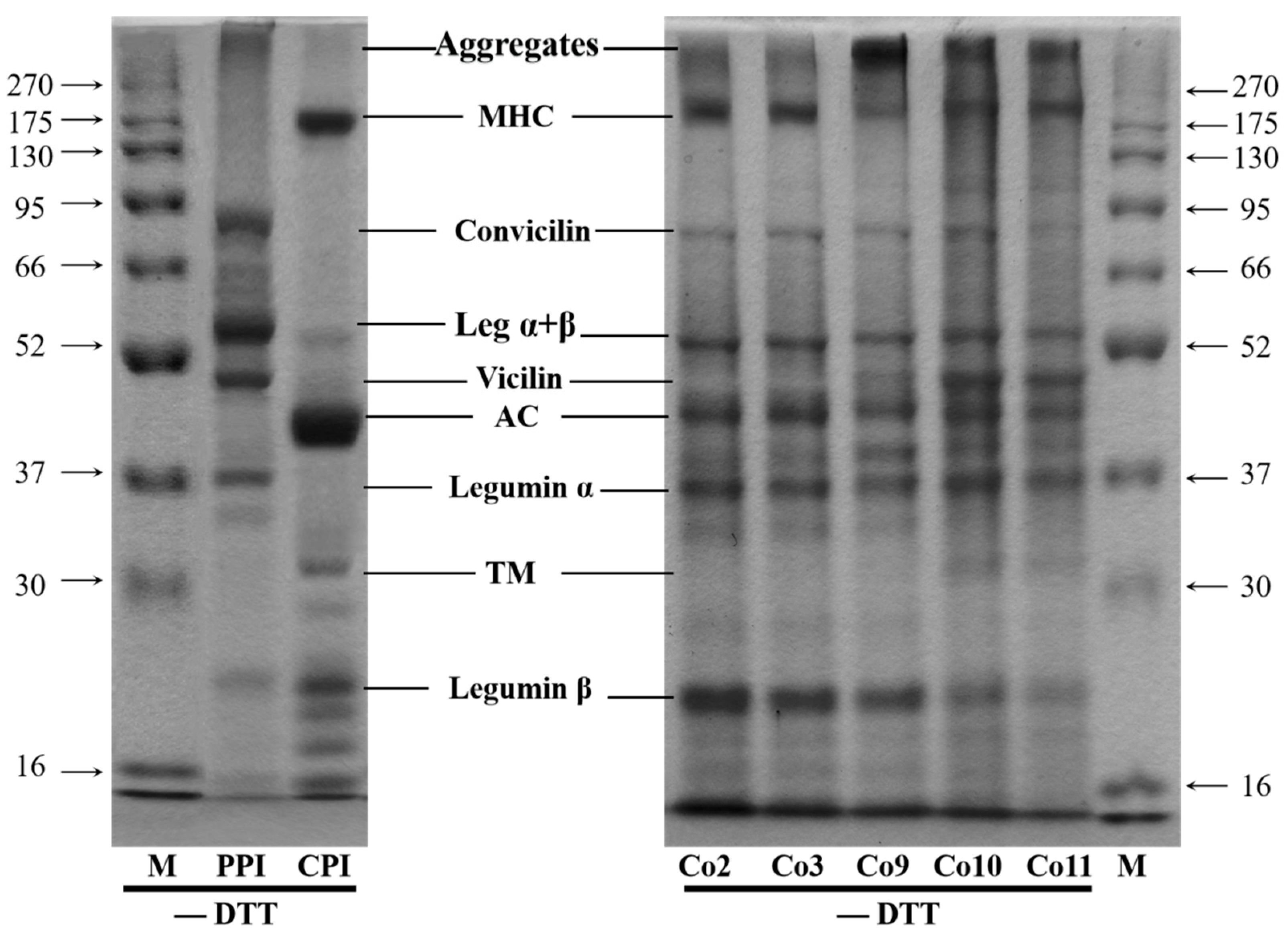
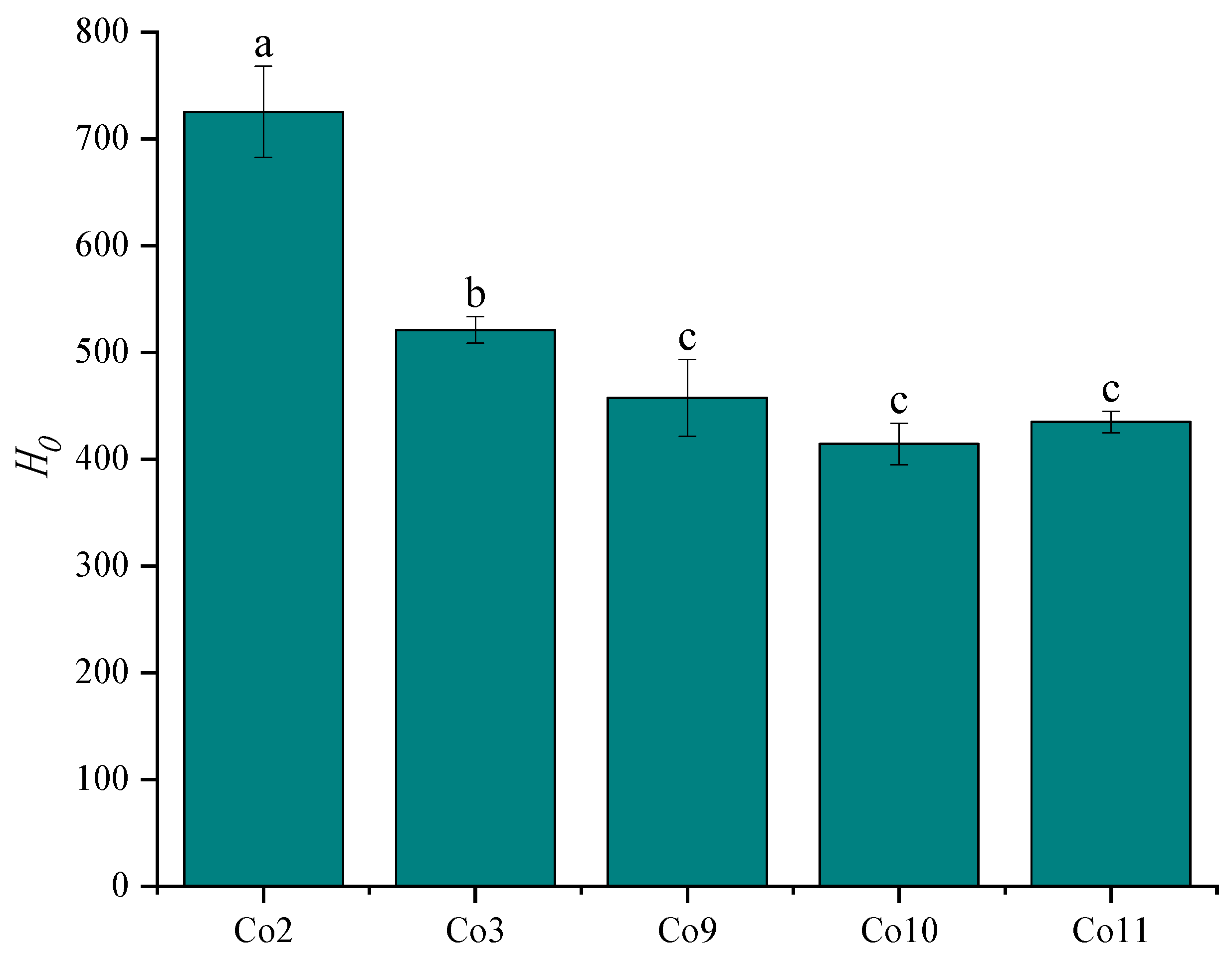


| Sample | Aggregates | MHC | Convicilin | Leg α + β | Vicilin | AC | Leg α | TM | leg β | Others | Vicilin/Leg α + β |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Co2 | 13.47 | 15.21 | 5.33 | 9.36 | 16.72 * | 13.33 | / | 21.50 | 5.07 | / | |
| Co3 | 11.92 | 19.36 | 6.28 | 8.34 | 18.47 * | 11.50 | / | 18.92 | 5.20 | / | |
| Co9 | 22.23 | 9.03 | 4.31 | 10.13 | 9.25 | 11.94 | 11.66 | 0.78 | 15.00 | 5.66 | 91.31 |
| Co10 | 17.45 | 13.75 | 4.01 | 5.74 | 15.22 | 15.87 | 12.54 | 4.60 | 6.01 | 4.80 | 265.16% |
| Co11 | 20.45 | 16.00 | 3.50 | 5.39 | 13.20 | 13.23 | 13.77 | 2.30 | 4.50 | 7.65 | 244.90 |
| Sample | L* | a* | b* | Whiteness |
|---|---|---|---|---|
| Co2 | 69.36 ± 0.32 c | −0.65 ± 0.01 d | 11.07 ± 0.33 c | 67.42 ± 1.07 b |
| Co3 | 67.41 ± 0.09 d | −0.66 ± 0.00 d | 12.96 ± 0.24 b | 64.92 ± 0.55 c |
| Co9 | 68.57 ± 0.24 c | −0.47 ± 0.01 b | 21.23 ± 0.36 a | 62.07 ± 1.24 d |
| Co10 | 71.23 ± 0.19 b | −0.58 ± 0.02 c | 11.34 ± 0.29 c | 69.07 ± 1.47 a |
| Co11 | 74.21 ± 0.24 a | −0.44 ± 0.02 a | 12.92 ± 0.19 b | 71.15 ± 0.88 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhang, C.; Zhao, L.; Cao, W.; Zhou, C.; Xie, X.; Chen, Y. Functionality of Pea-Grass Carp Co-Precipitated Dual-Protein as Affected by Extraction pH. Foods 2022, 11, 3136. https://doi.org/10.3390/foods11193136
Zhou X, Zhang C, Zhao L, Cao W, Zhou C, Xie X, Chen Y. Functionality of Pea-Grass Carp Co-Precipitated Dual-Protein as Affected by Extraction pH. Foods. 2022; 11(19):3136. https://doi.org/10.3390/foods11193136
Chicago/Turabian StyleZhou, Xiaohu, Chaohua Zhang, Liangzhong Zhao, Wenhong Cao, Chunxia Zhou, Xin Xie, and YuLian Chen. 2022. "Functionality of Pea-Grass Carp Co-Precipitated Dual-Protein as Affected by Extraction pH" Foods 11, no. 19: 3136. https://doi.org/10.3390/foods11193136
APA StyleZhou, X., Zhang, C., Zhao, L., Cao, W., Zhou, C., Xie, X., & Chen, Y. (2022). Functionality of Pea-Grass Carp Co-Precipitated Dual-Protein as Affected by Extraction pH. Foods, 11(19), 3136. https://doi.org/10.3390/foods11193136







