Polymicrobial Infection (Gram-Positive and Gram-Negative) Exacerbates Systemic Inflammatory Response Syndrome in a Conscious Swine Extremity Trauma Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Analgesics and Anesthesia
2.3. Surgical Instrumentation
2.4. Blood Draw Analysis and Culturing
2.5. Establishment of SIRS Criteria for Sinclair Swine
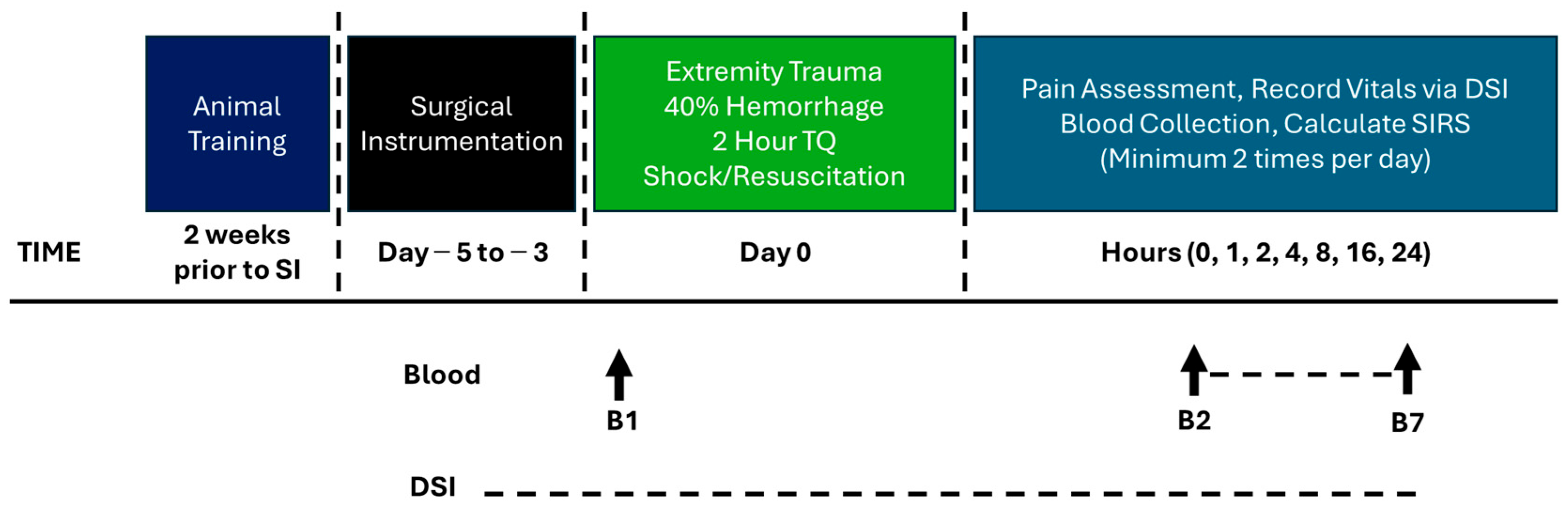
2.6. Model Development Phase 1: Complex Extremity Trauma Injury (CETI)
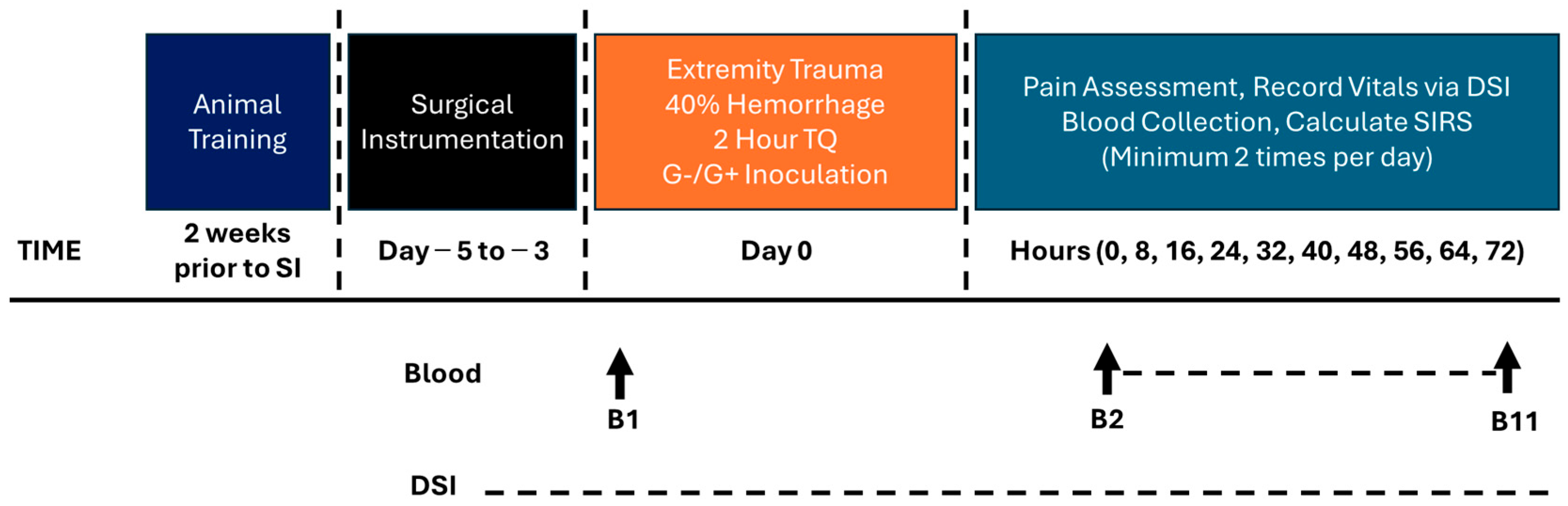
2.7. Model Development Phase 2: Bacterial Administration and Extended Monitoring
2.8. Tissue Plating
2.9. Statistical Analysis
3. Results
3.1. Final SIRS Criteria for Sinclair Swine
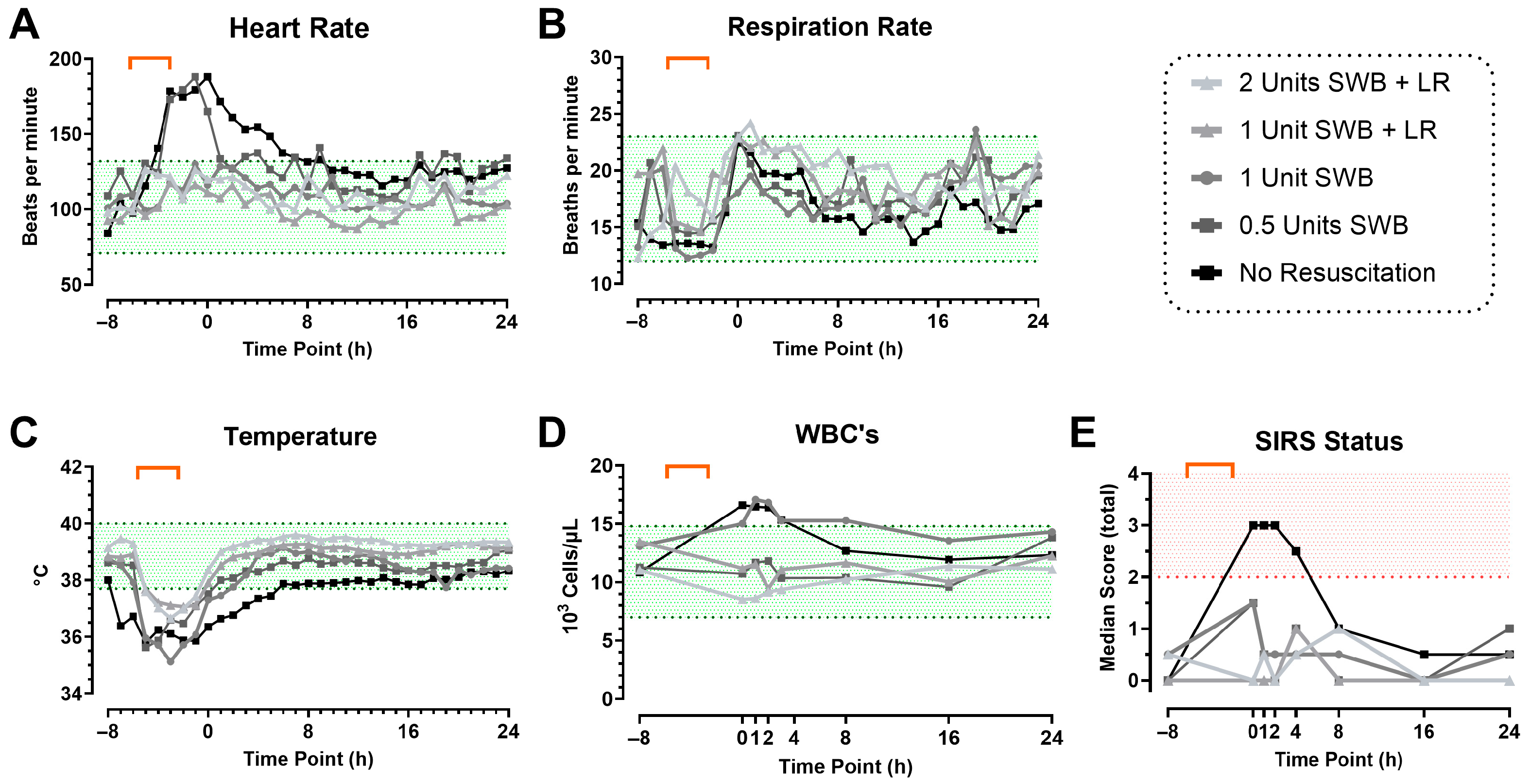
3.2. Model Development Phase 1: CETI
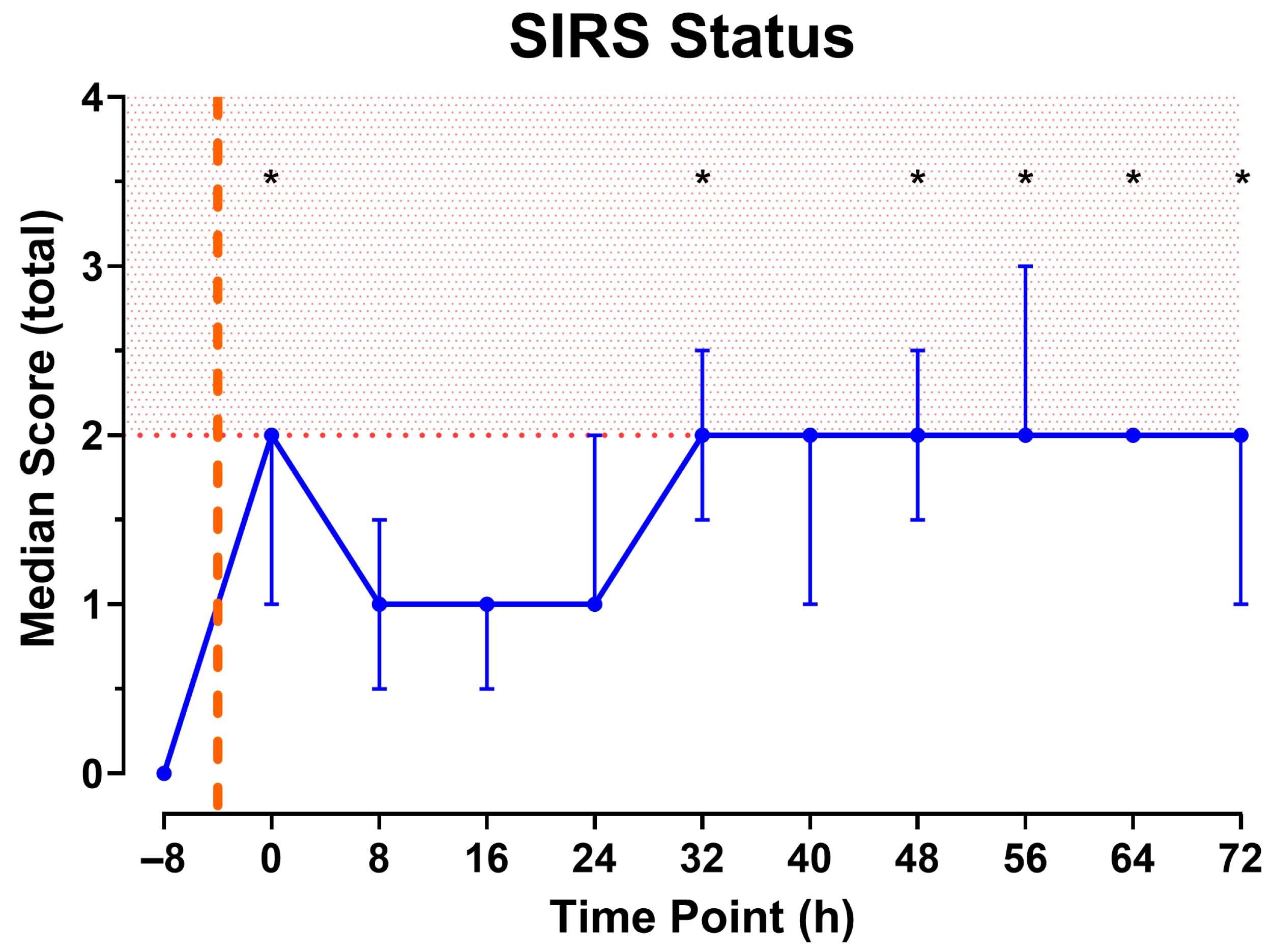
3.3. Model Development Phase 2: Inoculation with Bacteria and Prolonged Monitoring
3.4. Local Wound Infection
3.5. Blood Biochemistry Laboratory and Organ Dysfunction
| Analyte | Baseline | 0 h | 24 h | 48 h | 72 h |
|---|---|---|---|---|---|
| Lactate (mM) | 1.4 ± 0.4 | 7.2 ± 4.0 * | 2.8 ± 2.5 | 2.6 ± 2.5 | 2.1 ± 1.5 |
| Glucose (mg/dL) | 97 ± 8 | 181 ± 43 * | 112 ± 17 | 101 ± 22 | 100 ± 20 |
| ALT (U/L) | 57 ± 8 | 56 ± 12 | 96 ± 49 | 97 ± 52 | 89 ± 50 |
| AST (U/L) | 41 ± 20 | 159 ± 157 | 248 ± 213 | 139 ± 121 | 66 ± 59 |
| ALP (IU/L) | 61 ± 14 | 62 ± 13 | 65 ± 16 | 74 ± 79 | 71 ± 96 |
| GGT (U/L) | 42 ± 8 | 40 ± 6 | 39 ± 6 | 40 ± 5 | 41 ± 6 |
| Albumin (mM) | 1.1 ± 0.1 | 1.0 ± 0.1 * | 1.0 ± 0.1 * | 0.9 ± 0.1 * | 0.9 ± 0.1 * |
| INR | 1.25 ± 0.20 | 1.27 ± 0.16 | 1.32 ± 0.13 | 1.29 ± 0.09 | 1.81 ± 1.61 |
| Fibrinogen (mg/dL) | 347 ± 67 | 250 ± 65 * | 513 ± 58 * | 622 ± 64 * | 669 ± 88 * |
| D-Dimer (µg/mL) | 0.24 ± 0.11 | 0.22 ± 0.11 | 0.17 ± 0.08 | 0.22 ± 0.09 | 0.19 ± 0.09 |
| PT (sec) | 16.1 ± 2.0 | 16.3 ± 1.6 | 16.9 ± 1.3 | 16.6 ± 1.0 | 20.6 ± 12.4 |
| a PTT (sec) | 57.1 ± 24.2 | 43.8 ± 17.5 | 77.3 ± 38.5 | 57.2 ± 32.8 | 65.6 ± 70.8 |
| BUN (mg/dL) | 7.7 ± 2.2 | 19.3 ± 3.2 * | 13.9 ± 4.3 * | 9.8 ± 6.9 | 10.0 ± 9.0 |
| Creatinine (mg/dL) | 0.95 ± 0.13 | 1.87 ± 0.41 * | 0.99 ± 0.18 | 0.95 ± 0.30 | 0.96 ± 0.32 |
| Lymphocytes (103 cells/µL) | 5.82 ± 0.77 1 | 2.93 ± 0.53 *,1 | 7.09 ± 2.85 2 | 6.53 ± 2.73 1 | 5.96 ± 2.73 1 |
| Neutrophils (103 cells/µL) | 5.37 ± 2.53 1 | 9.57 ± 2.66 *,1 | 9.71 ± 6.60 2 | 14.38 ± 5.55 *,1 | 13.10 ± 5.61 *,1 |
| Platelets (103 cells/µL) | 356 ± 70 | 240 ± 46 * | 276 ± 61 * | 320 ± 127 | 500 ± 164 * |
| Red Blood Cells (106 cells/µL) | 5.52 ± 0.36 1 | 5.24 ± 0.58 1 | 3.87 ± 0.43 * | 3.61 ± 0.37 * | 3.71 ± 0.43 * |
| Hemoglobin (g/dL) | 11.98 ± 1.04 | 11.46 ± 1.37 | 8.46 ± 1.36 * | 7.53 ± 0.95 * | 7.71 ± 1.05 * |
| Hematocrit (%) | 35.84 ± 3.52 | 34.40 ± 4.68 | 25.57 ± 4.32 * | 23.20 ± 3.66 * | 23.96 ± 3.64 * |
4. Discussion
Limitations of This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALT | Alanine Transaminase |
| ALP | Alkaline Phosphatase |
| AST | Aspartate Transaminase |
| BP | Blood Pressure |
| bpm | Beats per Minute |
| brpm | Breaths per Minute |
| BUN | Blood Urea Nitrogen |
| CETI | Complex Extremity Trauma Injury |
| CFU | Colony Forming Unit |
| DSI | Data Sciences International |
| GGT | Gamma-glutamyl Transferase |
| Hb | Hemoglobin |
| HCT | Hematocrit |
| HR | Heart Rate |
| IM | Intramuscular |
| INR | International Normalized Ratio |
| IQR | Interquartile Range |
| LR | Lactated Ringer’s Solution |
| MAP | Mean Arterial Pressure |
| MDR | Multidrug resistant |
| RBCs | Red Blood Cells |
| RR | Respiration Rate |
| SIRS | Systemic Inflammatory Response Syndrome |
| SD | Standard Deviation |
| SWB | Shed Whole Blood |
| Temp | Temperature |
| TQ | Tourniquet |
| WBC | White Blood Cell |
References
- Belmont, P.J., Jr.; McCriskin, B.J.; Hsiao, M.S.; Burks, R.; Nelson, K.J.; Schoenfeld, A.J. The nature and incidence of musculoskeletal combat wounds in Iraq and Afghanistan (2005–2009). J. Orthop. Trauma. 2013, 27, e107–e113. [Google Scholar] [CrossRef]
- Belmont, P.J., Jr.; McCriskin, B.J.; Sieg, R.N.; Burks, R.; Schoenfeld, A.J. Combat wounds in Iraq and Afghanistan from 2005 to 2009. J. Trauma Acute Care Surg. 2012, 73, 3–12. [Google Scholar] [CrossRef]
- Lack, W.D.; Karunakar, M.A.; Angerame, M.R.; Seymour, R.B.; Sims, S.; Kellam, J.F.; Bosse, M.J. Type III open tibia fractures: Immediate antibiotic prophylaxis minimizes infection. J. Orthop. Trauma 2015, 29, 1–6. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Jang, J.H.; Choi, E.; Kim, T.; Yeo, H.J.; Jeon, D.; Kim, Y.S.; Cho, W.H. Navigating the Modern Landscape of Sepsis: Advances in Diagnosis and Treatment. Int. J. Mol. Sci. 2024, 25, 7396. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L. Current sepsis therapeutics. eBioMedicine 2022, 86, 104318. [Google Scholar] [CrossRef] [PubMed]
- Riha, G.M.; Kiraly, L.N.; Diggs, B.S.; Cho, S.D.; Fabricant, L.J.; Flaherty, S.F.; Kuehn, R.; Underwood, S.J.; Schreiber, M.A. Management of the open abdomen during the global war on terror. JAMA Surg. 2013, 148, 59–64. [Google Scholar] [CrossRef]
- Coleman, J.J.; Zarzaur, B.L. Surgical Management of Abdominal Trauma: Hollow Viscus Injury. Surg. Clin. N. Am. 2017, 97, 1107–1117. [Google Scholar] [CrossRef]
- Dewey, J. Systemic Inflammatory Response Syndrome (SIRS). In Salem Press Encyclopedia of Health; Salem Press: Hackensack, NJ, USA, 2023. [Google Scholar]
- Kaukonen, K.M.; Bailey, M.; Pilcher, D.; Cooper, D.J.; Bellomo, R. Systemic inflammatory response syndrome criteria in defining severe sepsis. N. Engl. J. Med. 2015, 372, 1629–1638. [Google Scholar] [CrossRef]
- Baddam, S.; Burns, B. Systemic Inflammatory Response Syndrome. 20 June 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK547669/ (accessed on 18 August 2025).
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Seymour, C.W.; Liu, V.X.; Iwashyna, T.J.; Brunkhorst, F.M.; Rea, T.D.; Scherag, A.; Rubenfeld, G.; Kahn, J.M.; Shankar-Hari, M.; Singer, M.; et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 762–774. [Google Scholar] [CrossRef]
- Lambden, S.; Laterre, P.F.; Levy, M.M.; Francois, B. The SOFA score-development, utility and challenges of accurate assessment in clinical trials. Crit. Care 2019, 23, 374. [Google Scholar] [CrossRef]
- Waterhouse, A.; Leslie, D.C.; Bolgen, D.E.; Lightbown, S.L.; Dimitrakakis, N.; Cartwright, M.J.; Seiler, B.T.; Lightbown, K.R.; Smith, K.P.; Lombardo, P.; et al. Modified Clinical Monitoring Assessment Criteria for Multi-Organ Failure during Bacteremia and Sepsis Progression in a Pig Model. Advan. Crit. Care Med. 2018, 1. [Google Scholar]
- Munley, J.A.; Kelly, L.S.; Gillies, G.S.; Pons, E.E.; Coldwell, P.S.; Kannan, K.B.; Whitley, E.M.; Bible, L.E.; Efron, P.A.; Mohr, A.M. Narrowing the gap: Preclinical trauma with postinjury sepsis model with increased clinical relevance. Shock 2023, 60, 272–279. [Google Scholar] [CrossRef]
- Störmann, P.; Wagner, N.; Köhler, K.; Auner, B.; Simon, T.P.; Pfeifer, R.; Horst, K.; Pape, H.C.; Hildebrand, F.; Wutzler, S.; et al. Monotrauma is associated with enhanced remote inflammatory response and organ damage, while polytrauma intensifies both in porcine trauma model. Eur. J. Trauma Emerg. Surg. 2020, 46, 31–42. [Google Scholar] [CrossRef]
- Hildebrand, F.; Andruszkow, H.; Huber-Lang, M.; Pape, H.-C.; van Griensven, M. Combined Hemorrhage/Trauma Models in Pigs—Current State and Future Perspectives. Shock 2013, 40, 247–273. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.L.; Wakam, G.K.; Siddiqui, A.; Williams, A.M.; Graham, N.; Kemp, M.T.; Chtraklin, K.; Bhatti, U.F.; Shamshad, A.; Li, Y.; et al. Development of a large animal model of lethal polytrauma and intra-abdominal sepsis with bacteremia. Trauma Surg. Acute Care Open 2021, 6, e000636. [Google Scholar] [CrossRef] [PubMed]
- Pluschke, A.M.; Simmons, G.S.; Keates, H.L.; Cameron, R.D.A.; Zhang, D.; Wright, J.D.; Williams, B.A. An updated method for the jugular catheterization of grower pigs for repeated blood sampling following an oral glucose tolerance test. Lab. Anim. 2017, 51, 397–404. [Google Scholar] [CrossRef]
- Gaeth, C.; Madaris, T.R.; Duarte, J.; Rodriguez, A.; Wegner, M.D.; Powers, A.; Stone, R. Modeling Sepsis: Establishment and Validation of a 72-Hour Swine Model of Penetrating Abdominal Trauma. Medicina 2025, 61, 1523. [Google Scholar] [CrossRef]
- Burmeister, D.M.; McIntyre, M.K.; Baker, B.A.; Rizzo, J.A.; Brown, A.; Natesan, S.; Chung, K.K.; Christy, R.J. Impact of Isolated Burns on Major Organs: A Large Animal Model Characterized. Shock 2016, 46 (Suppl. 1), 137–147. [Google Scholar] [PubMed]
- Tennent, D.J.; Shiels, S.M.; Jennings, J.A.; Haggard, W.O.; Wenke, J.C. Local control of polymicrobial infections via a dual antibiotic delivery system. J. Orthop. Surg. Res. 2018, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- Stone, R., 2nd; Saathoff, E.C.; Larson, D.A.; Wall, J.T.; Wienandt, N.A.; Magnusson, S.; Kjartansson, H.; Natesan, S.; Christy, R.J. Accelerated Wound Closure of Deep Partial Thickness Burns with Acellular Fish Skin Graft. Int. J. Mol. Sci. 2021, 22, 1590. [Google Scholar] [CrossRef] [PubMed]
- Shiels, S.M.; Sgromolo, N.M.; Wenke, J.C. Negative pressure wound therapy does not diminish efficacy of topical antibiotic powder in a preclinical contaminated wound model: An animal study. Bone Jt. Res. 2021, 10, 149–155. [Google Scholar] [CrossRef]
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Mongodin, E.F.; et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367, 731–739. [Google Scholar] [CrossRef]
- Jacobs, A.C.; Thompson, M.G.; Black, C.C.; Kessler, J.L.; Clark, L.P.; McQueary, C.N.; Gancz, H.Y.; Corey, B.W.; Moon, J.K.; Si, Y.; et al. AB5075, a Highly Virulent Isolate of Acinetobacter baumannii, as a Model Strain for the Evaluation of Pathogenesis and Antimicrobial Treatments. mBio 2014, 5, e01076-14. [Google Scholar] [CrossRef]
- Holloway, B.W. Genetic recombination in Pseudomonas aeruginosa. J. Gen. Microbiol. 1955, 13, 572–581. [Google Scholar] [CrossRef]
- Frykberg, E.R.; Tepas, J.J., 3rd. Terrorist bombings. Lessons learned from Belfast to Beirut. Ann. Surg. 1988, 208, 569–576. [Google Scholar] [CrossRef]
- Weil, Y.A.; Peleg, K.; Givon, A.; Mosheiff, R. Musculoskeletal injuries in terrorist attacks—A comparison between the injuries sustained and those related to motor vehicle accidents, based on a national registry database. Injury 2008, 39, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Xacur-Trabulce, A.; Casas-Fuentes, G.; Ruiz-Vasconcelos, V.; Reitz, M.M.; Henry, S.M.; Scalea, T.M.; Ribeiro, M.A.F., Jr. Tourniquet-related complications in extremity injuries: A scoping review of the literature. World J. Emerg. Surg. 2025, 20, 57. [Google Scholar] [CrossRef]
- Butler, F.; Holcomb, J.B.; Dorlac, W.; Gurney, J.; Inaba, K.; Jacobs, L.; Mabry, B.; Meoli, M.; Montgomery, H.; Otten, M.; et al. Who needs a tourniquet? And who does not? Lessons learned from a review of tourniquet use in the Russo-Ukrainian war. J. Trauma Acute Care Surg. 2024, 97 (Suppl. 1), S45–S54. [Google Scholar] [CrossRef]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.-F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef]
- Zhang, Q.; Raoof, M.; Chen, Y.; Sumi, Y.; Sursal, T.; Junger, W.; Brohi, K.; Itagaki, K.; Hauser, C.J. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 2010, 464, 104–107. [Google Scholar] [CrossRef]
- Parlato, M.; Cavaillon, J.M. Host response biomarkers in the diagnosis of sepsis: A general overview. Methods Mol. Biol. 2015, 1237, 149–211. [Google Scholar]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Hu, X.; Wang, Z.; Li, R.; Gan, L.; Zhang, M.; Wang, T. The role of mtDAMPs in the trauma-induced systemic inflammatory response syndrome. Front. Immunol. 2023, 14, 1164187. [Google Scholar] [CrossRef]
- de Azevedo, L.C.; Park, M.; Noritomi, D.T.; Maciel, A.T.; Brunialti, M.K.; Salomão, R. Characterization of an animal model of severe sepsis associated with respiratory dysfunction. Clinics 2007, 62, 491–498. [Google Scholar] [CrossRef]
- Zurek-Leffers, F.M.; Lehmann, F.; Brabenec, L.; Kintrup, S.; Hellenthal, K.E.M.; Mersjann, K.; Kneifel, F.; Hessler, M.; Arnemann, P.H.; Kampmeier, T.G.; et al. A model of porcine polymicrobial septic shock. Intensive Care Med. Exp. 2023, 11, 31. [Google Scholar] [CrossRef]
- Horst, K.; Simon, T.P.; Pfeifer, R.; Teuben, M.; Almahmoud, K.; Zhi, Q.; Santos, S.A.; Wembers, C.C.; Leonhardt, S.; Heussen, N.; et al. Characterization of blunt chest trauma in a long-term porcine model of severe multiple trauma. Sci. Rep. 2016, 6, 39659. [Google Scholar] [CrossRef] [PubMed]
- Rolland, T.J.; Hudson, E.R.; Graser, L.A.; Zahra, S.; Cucinotta, D.; Weil, B.R. Splenic modulation of the early inflammatory response to regional and global ischemia/reperfusion injury in swine. Am. J. Physiol. Heart Circ. Physiol. 2025, 329, H16–H31. [Google Scholar] [CrossRef] [PubMed]
- Soerensen, K.E.; Nielsen, O.L.; Birck, M.M.; Soerensen, D.B.; Leifsson, P.S.; Jensen, H.E.; Aalbaek, B.; Kristensen, A.T.; Wiinberg, B.; Kjelgaard-Hansen, M.; et al. The use of sequential organ failure assessment parameters in an awake porcine model of severe Staphylococcus aureus sepsis. Apmis 2012, 120, 909–921. [Google Scholar] [CrossRef]
- Nguyen, T.C.; Marini, J.C.; Guillory, B.; Valladolid-Brown, C.; Martinez-Vargas, M.; Subramanyam, D.; Cohen, D.; Cirlos, S.C.; Lam, F.; Stoll, B.; et al. Pediatric Swine Model of Methicillin-Resistant Staphylococcus aureus Sepsis-Induced Coagulopathy, Disseminated Microvascular Thrombosis, and Organ Injuries. Crit. Care Explor. 2023, 5, e0916. [Google Scholar] [CrossRef] [PubMed]
- Standl, T.; Annecke, T.; Cascorbi, I.; Heller, A.R.; Sabashnikov, A.; Teske, W. The Nomenclature, Definition and Distinction of Types of Shock. Dtsch. Arztebl. Int. 2018, 115, 757–768. [Google Scholar] [CrossRef]
- Guly, H.R.; Bouamra, O.; Spiers, M.; Dark, P.; Coats, T.; Lecky, F.E. Vital signs and estimated blood loss in patients with major trauma: Testing the validity of the ATLS classification of hypovolaemic shock. Resuscitation 2011, 82, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, P.C.; Sebat, F.; Kellett, J.G. Respiratory Rate: The Forgotten Vital Sign-Make It Count! Jt. Comm. J. Qual. Patient Saf. 2018, 44, 494–499. [Google Scholar] [CrossRef]
- van Veelen, M.J.; Brodmann Maeder, M. Hypothermia in Trauma. Int. J. Environ. Res. Public Health 2021, 18, 8719. [Google Scholar] [CrossRef] [PubMed]
- Andrianova, N.V.; Buyan, M.I.; Brezgunova, A.A.; Cherkesova, K.S.; Zorov, D.B.; Plotnikov, E.Y. Hemorrhagic Shock and Mitochondria: Pathophysiology and Therapeutic Approaches. Int. J. Mol. Sci. 2025, 26, 1843. [Google Scholar] [CrossRef]
- Sessler, D.I. Perioperative thermoregulation and heat balance. Lancet 2016, 387, 2655–2664. [Google Scholar] [CrossRef]
- Romanovsky, A.A.; Almeida, M.C.; Aronoff, D.M.; Ivanov, A.I.; Konsman, J.P.; Steiner, A.A.; Turek, V.F. Fever and hypothermia in systemic inflammation: Recent discoveries and revisions. Front. Biosci. 2005, 10, 2193–2216. [Google Scholar] [CrossRef]
- Lier, H.; Bernhard, M.; Hossfeld, B. Hypovolemic and hemorrhagic shock. Anaesthesist 2018, 67, 225–244. [Google Scholar] [CrossRef]
- Margraf, A.; Ludwig, N.; Zarbock, A.; Rossaint, J. Systemic Inflammatory Response Syndrome After Surgery: Mechanisms and Protection. Anesth. Analg. 2020, 131, 1693–1707. [Google Scholar] [CrossRef]
- Sikora, J.P.; Karawani, J.; Sobczak, J. Neutrophils and the Systemic Inflammatory Response Syndrome (SIRS). Int. J. Mol. Sci. 2023, 24, 13469. [Google Scholar] [CrossRef]
- Runciman, W.B.; Skowronski, G.A. Pathophysiology of haemorrhagic shock. Anaesth. Intensive Care 1984, 12, 193–205. [Google Scholar] [CrossRef]
- Harrois, A.; Soyer, B.; Gauss, T.; Hamada, S.; Raux, M.; Duranteau, J. Prevalence and risk factors for acute kidney injury among trauma patients: A multicenter cohort study. Crit. Care 2018, 22, 344. [Google Scholar] [CrossRef]
- Messerer, D.A.C.; Halbgebauer, R.; Nilsson, B.; Pavenstädt, H.; Radermacher, P.; Huber-Lang, M. Immunopathophysiology of trauma-related acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 91–111. [Google Scholar] [CrossRef]
- Liu, H.; Xiao, X.; Sun, C.; Sun, D.; Li, Y.; Yang, M. Systemic inflammation and multiple organ injury in traumatic hemorrhagic shock. Front. Biosci. 2015, 20, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Cralley, A.L.; Moore, E.E.; Kissau, D.; Coleman, J.R.; Vigneshwar, N.; DeBot, M.; Schaid, T.R., Jr.; Moore, H.B.; Cohen, M.J.; Hansen, K.; et al. A combat casualty relevant dismounted complex blast injury model in swine. J. Trauma Acute Care Surg. 2022, 93 (Suppl. 1), S110–S118. [Google Scholar] [CrossRef] [PubMed]
- Vang, M.; Østberg, M.; Steinmetz, J.; Rasmussen, L.S. Shock index as a predictor for mortality in trauma patients: A systematic review and meta-analysis. Eur. J. Trauma. Emerg. Surg. 2022, 48, 2559–2566. [Google Scholar] [CrossRef] [PubMed]
- Rady, M.Y.; Nightingale, P.; Little, R.A.; Edwards, J.D. Shock index: A re-evaluation in acute circulatory failure. Resuscitation 1992, 23, 227–234. [Google Scholar] [CrossRef]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef]
- Ogura, H.; Gando, S.; Iba, T.; Eguchi, Y.; Ohtomo, Y.; Okamoto, K.; Koseki, K.; Mayumi, T.; Murata, A.; Ikeda, T.; et al. SIRS-associated coagulopathy and organ dysfunction in critically ill patients with thrombocytopenia. Shock 2007, 28, 411–417. [Google Scholar] [CrossRef]
- BenÍtez, C.Y.; Ottolino, P.; Pereira, B.M.; Lima, D.S.; Guemes, A.; Khan, M.; Ribeiro Junior, M.A.F. Tourniquet use for civilian extremity hemorrhage: Systematic review of the literature. Rev. Col. Bras. Cir. 2021, 48, e20202783. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.B.; Dorlac, W.C.; Drew, B.G.; Butler, F.K.; Gurney, J.M.; Montgomery, H.R.; Shackelford, S.A.; Bank, E.A.; Kerby, J.D.; Kragh, J.F.; et al. Rethinking limb tourniquet conversion in the prehospital environment. J. Trauma. Acute Care Surg. 2023, 95, e54–e60. [Google Scholar] [CrossRef] [PubMed]
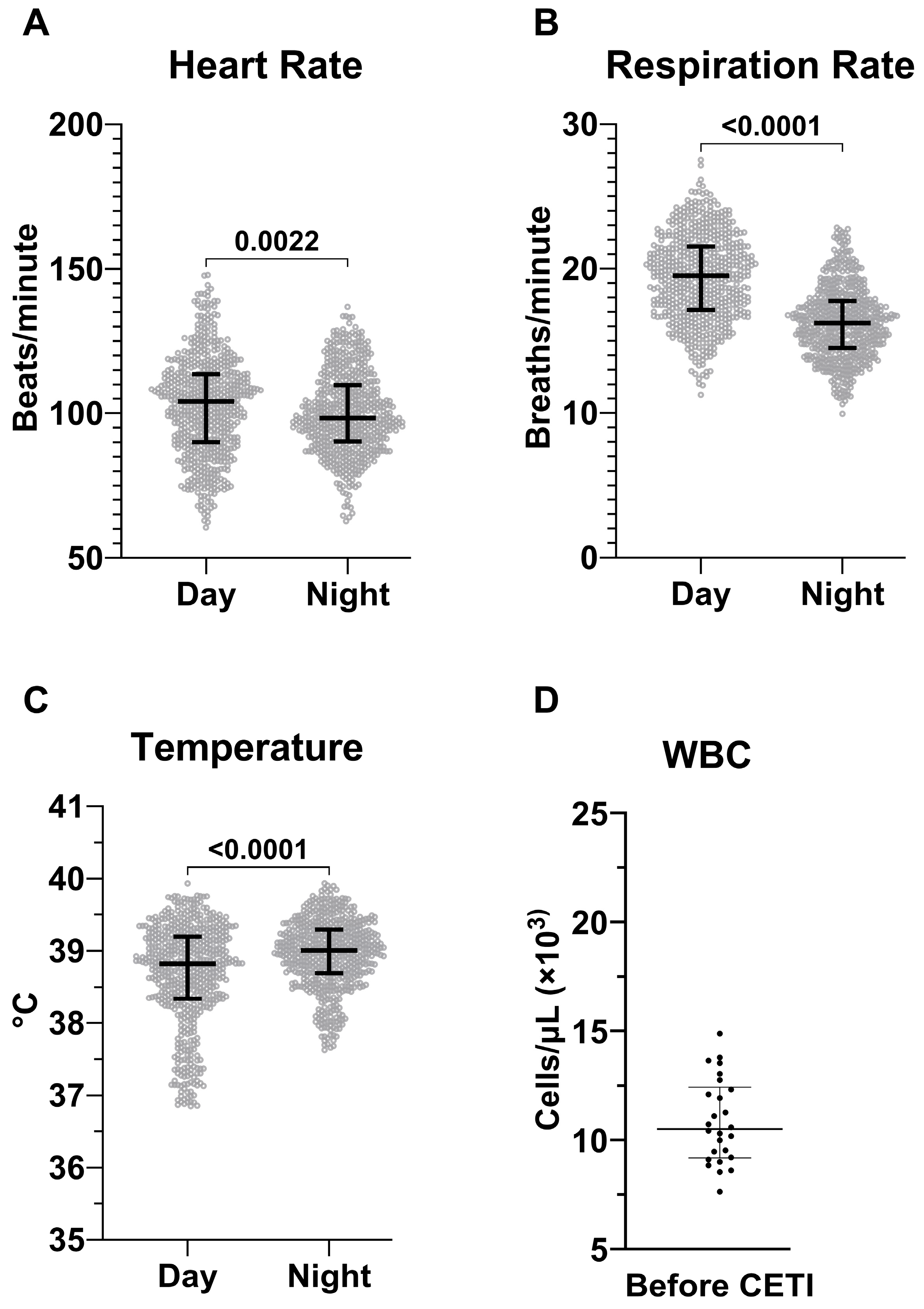

| Parameter | Time of Day | Average | SD | SIRS (+2 SD) | SIRS (−2 SD) |
|---|---|---|---|---|---|
| HR [bpm] | Day | 103 | 17 | 137 | 69 |
| Night | 100 | 14 | 128 | 72 | |
| RR [brpm] | Day | 19 | 3 | 25 | 13 |
| Night | 16 | 2.5 | 21 | 11 | |
| Temp [°C] | Day | 38.70 | 0.70 | 40.10 | 37.31 |
| Night | 39.00 | 0.47 | 39.94 | 38.06 | |
| WBC [103/µL] | 10.90 | 1.93 | 14.76 | 7.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaeth, C.C.; Madaris, T.R.; Duarte, J.M.; Powers, A.M.; Sandoval, C.M.; Shiels, S.M.; Stone, R., II. Polymicrobial Infection (Gram-Positive and Gram-Negative) Exacerbates Systemic Inflammatory Response Syndrome in a Conscious Swine Extremity Trauma Model. Pathophysiology 2025, 32, 59. https://doi.org/10.3390/pathophysiology32040059
Gaeth CC, Madaris TR, Duarte JM, Powers AM, Sandoval CM, Shiels SM, Stone R II. Polymicrobial Infection (Gram-Positive and Gram-Negative) Exacerbates Systemic Inflammatory Response Syndrome in a Conscious Swine Extremity Trauma Model. Pathophysiology. 2025; 32(4):59. https://doi.org/10.3390/pathophysiology32040059
Chicago/Turabian StyleGaeth, Catharina C., Travis R. Madaris, Jamila M. Duarte, Amber M. Powers, Christina M. Sandoval, Stefanie M. Shiels, and Randolph Stone, II. 2025. "Polymicrobial Infection (Gram-Positive and Gram-Negative) Exacerbates Systemic Inflammatory Response Syndrome in a Conscious Swine Extremity Trauma Model" Pathophysiology 32, no. 4: 59. https://doi.org/10.3390/pathophysiology32040059
APA StyleGaeth, C. C., Madaris, T. R., Duarte, J. M., Powers, A. M., Sandoval, C. M., Shiels, S. M., & Stone, R., II. (2025). Polymicrobial Infection (Gram-Positive and Gram-Negative) Exacerbates Systemic Inflammatory Response Syndrome in a Conscious Swine Extremity Trauma Model. Pathophysiology, 32(4), 59. https://doi.org/10.3390/pathophysiology32040059







