Pulsed-Field Ablation Is Associated with Lower Endothelial Injury and Procedure Time Compared to Cryoballoon Ablation in Paroxysmal Atrial Fibrillation
Abstract
1. Introduction
2. Materials and Methods
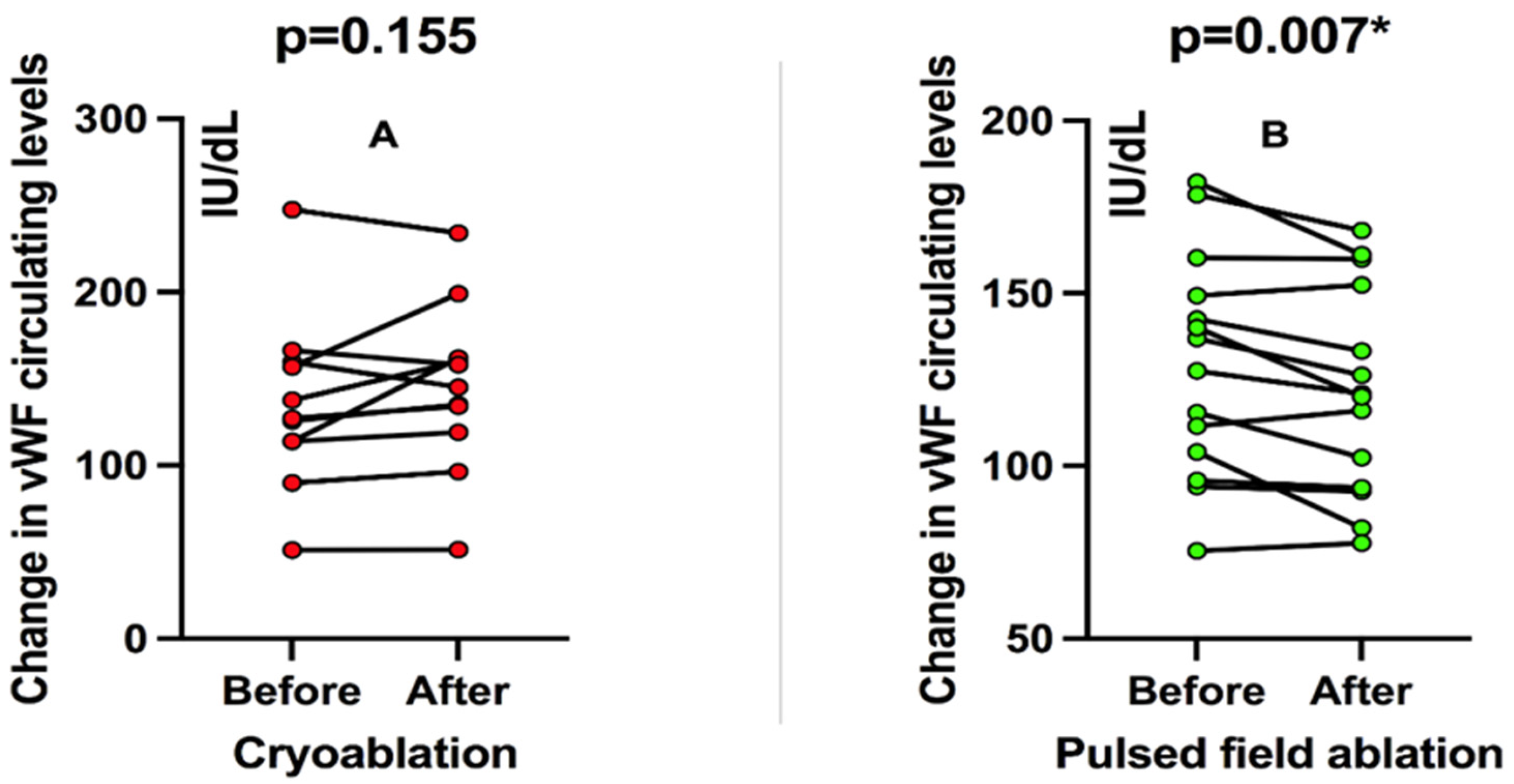
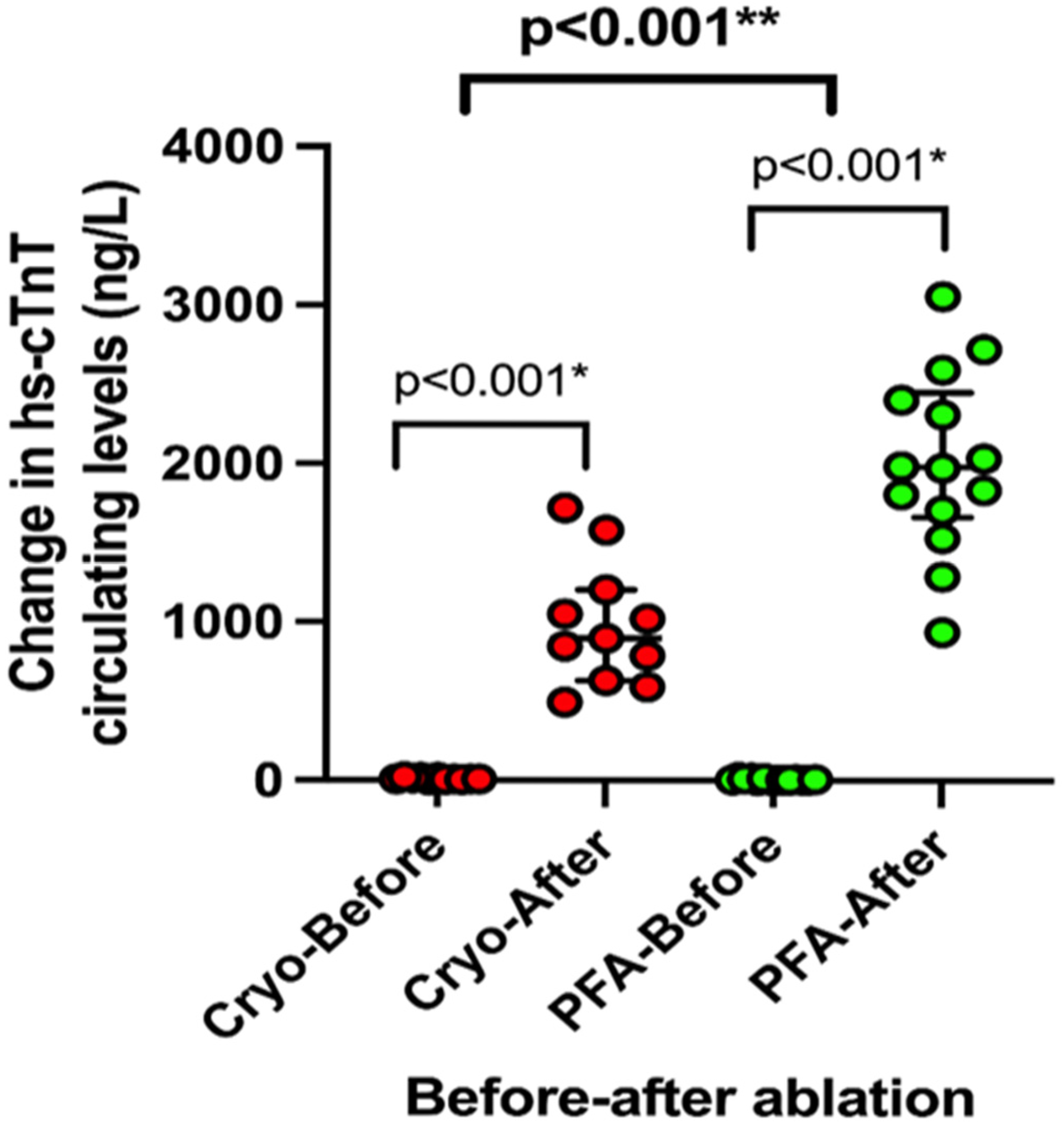
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, J.J.; Elafros, M.A.; Mullen, M.T.; Muser, D.; Hayashi, T.; Enriquez, A.; Pathak, R.K.; Zado, E.S.; Santangeli, P.; Arkles, J.S.; et al. Anticoagulation use and clinical outcomes after catheter ablation in patients with persistent and longstanding persistent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2018, 29, 823–832. [Google Scholar] [CrossRef]
- Tzeis, S.; Gerstenfeld, E.P.; Kalman, J.; Saad, E.B.; Sepehri Shamloo, A.; Andrade, J.G.; Barbhaiya, C.R.; Baykaner, T.; Boveda, S.; Calkins, H.; et al. 2024 European Heart Rhythm Association/Heart Rhythm Society/Asia Pacific Heart Rhythm Society/Latin American Heart Rhythm Society expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace 2024, 26, e31–e149. [Google Scholar] [CrossRef]
- Bulava, A.; Slavík, L.; Fiala, M.; Heinc, P.; Škvařilova, M.; Lukl, J.; Krčová, V.; Indrák, K. Endothelial Damage and Activation of the Hemostatic System During Radiofrequency Catheter Isolation of Pulmonary Veins. J. Interv. Card. Electrophysiol. 2004, 10, 271–279. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Koruth, J.; Jais, P.; Petru, J.; Timko, F.; Skalsky, I.; Hebeler, R.; Labrousse, L.; Barandon, L.; Kralovec, S.; et al. Ablation of Atrial Fibrillation with Pulsed Electric Fields. JACC Clin. Electrophysiol. 2018, 4, 987–995. [Google Scholar] [CrossRef]
- van Driel, V.J.H.M.; Neven, K.; van Wessel, H.; Vink, A.; Doevendans, P.A.F.M.; Wittkampf, F.H.M. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015, 12, 1838–1844. [Google Scholar]
- Reddy, V.Y.; Dukkipati, S.R.; Neuzil, P.; Anic, A.; Petru, J.; Funasako, M.; Cochet, H.; Minami, T.B.; Sikiric, I.; Sediva, L.; et al. Pulsed Field Ablation of Paroxysmal Atrial Fibrillation. JACC Clin. Electrophysiol. 2021, 7, 614–627. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.G.M.; De Potter, T.J.R.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2024, 45, 3314–3414. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, I.; Kotulska, M.; Stecka, A.; Saczko, J.; Drag-Zalesinska, M.; Wysocka, T.; Choromanska, A.; Skolucka, N.; Nowicki, R.; Marczak, J.; et al. Electroporation-induced changes in normal immature rat myoblasts (H9C2). Gen. Physiol. Biophys. 2012, 31, 19–25. [Google Scholar] [CrossRef]
- Maor, E.; Ivorra, A.; Rubinsky, B. Non Thermal Irreversible Electroporation: Novel Technology for Vascular Smooth Muscle Cells Ablation. PLoS ONE 2009, 4, e4757. [Google Scholar] [CrossRef]
- Osmancik, P.; Bacova, B.; Hozman, M.; Pistkova, J.; Kunstatova, V.; Sochorova, V.; Waldauf, P.; Hassouna, S.; Karch, J.; Vesela, J.; et al. Myocardial Damage, Inflammation, Coagulation, and Platelet Activity During Catheter Ablation Using Radiofrequency and Pulsed-Field Energy. JACC Clin. Electrophysiol. 2024, 10, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Pieragnoli, P.; Gori, A.M.; Ricciardi, G.; Carrassa, G.; Checchi, L.; Michelucci, A.; Priora, R.; Cellai, A.P.; Marcucci, R.; Padeletti, L.; et al. Effects of cryoablation and radiofrequency ablation on endothelial and blood clotting activation. Intern. Emerg. Med. 2014, 9, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Malmborg, H.; Christersson, C.; Lönnerholm, S.; Blomström-Lundqvist, C. Comparison of effects on coagulation and inflammatory markers using a duty-cycled bipolar and unipolar radiofrequency pulmonary vein ablation catheter vs. a cryoballoon catheter for pulmonary vein isolation. EP Eur. 2013, 15, 798–804. [Google Scholar] [CrossRef]
- Koruth, J.S.; Kuroki, K.; Iwasawa, J.; Viswanathan, R.; Brose, R.; Buck, E.D.; Donskoy, E.; Dukkipati, S.R.; Reddy, V.Y. Endocardial ventricular pulsed field ablation: A proof-of-concept preclinical evaluation. EP Eur. 2020, 22, 434–439. [Google Scholar] [CrossRef]
- Miklavčič, D.; Verma, A.; Krahn, P.R.P.; Štublar, J.; Kos, B.; Escartin, T.; Lombergar, P.; Coulombe, N.; Terricabras, M.; Jarm, T.; et al. Biophysics and electrophysiology of pulsed field ablation in normal and infarcted porcine cardiac ventricular tissue. Sci. Rep. 2024, 14, 32063. [Google Scholar] [CrossRef]
- Yavin, H.D.; Higuchi, K.; Sroubek, J.; Younis, A.; Zilberman, I.; Anter, E. Pulsed-Field Ablation in Ventricular Myocardium Using a Focal Catheter: The Impact of Application Repetition on Lesion Dimensions. Circ. Arrhythm. Electrophysiol. 2021, 14, e010375. [Google Scholar]
- Hochstadt, A.; Barbhaiya, C.R.; Jankelson, L.; Levine, J. Pulsed field ablation and periprocedural stroke risk—A step in the right direction. Heart Rhythm. 2025. [Google Scholar] [CrossRef]
- Sánchez-Gómez, J.M.; Ortiz-Fernández, Á.; Soler-Martínez, J.; Gabaldón-Pérez, A.; Iraola-Viana, D.; Martínez-Brotons, Á.; Bondanza-Saavedra, L.; Domínguez-Mafé, E.; Redondo-Nieto, L.; Picazo-Campos, P.; et al. Incidence of brain lesions following pulsed field ablation for atrial fibrillation. Heart Rhythm. 2025. [Google Scholar] [CrossRef]
- Mohanty, S.; Torlapati, P.G.; Casella, M.; La Fazia, V.M.; Valeri, Y.; Gianni, C.; Al-Ahmad, A.; Burkhardt, J.D.; Gallinghouse, G.; Horton, R.; et al. Management of oral anticoagulation after pulsed field ablation for atrial fibrillation: Insights from a multicenter study. Heart Rhythm. 2025. [Google Scholar] [CrossRef]
- Krisai, P.; Knecht, S.; Badertscher, P.; Mühl, A.; Osswald, S.; Roten, L.; Reichlin, T.; Sticherling, C.; Kühne, M. Troponin release after pulmonary vein isolation using pulsed field ablation compared to radiofrequency and cryoballoon ablation. Heart Rhythm. 2022, 19, 1471–1472. [Google Scholar] [PubMed]
- My, I.; Lemoine, M.D.; Butt, M.; Mencke, C.; Loeck, F.W.; Obergassel, J.; Rottner, L.; Wenzel, J.; Schleberger, R.; Moser, J.; et al. Acute lesion extension following pulmonary vein isolation with two novel single shot devices: Pulsed field ablation versus multielectrode radiofrequency balloon. J. Cardiovasc. Electrophysiol. 2023, 34, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, I.; Neuzil, P.; Shivamurthy, P.; Kuroki, K.; Lam, J.; Musikantow, D.; Chu, E.; Turagam, M.K.; Minami, K.; Funasako, M.; et al. How does the level of pulmonary venous isolation compare between pulsed field ablation and thermal energy ablation (radiofrequency, cryo, or laser)? Europace 2021, 23, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
| Variable | Pulsed-Field Ablation n = 14 | Cryoballoon Ablation n = 11 | p |
|---|---|---|---|
| Age | 61.79 ± 10 | 60.09 ± 8 | 0.677 |
| Female sex | 9 (64.3%) | 5 (45.5%) | 0.346 |
| Body mass index, (kg/m2) | 30 ± 5 | 27 ± 3 | 0.175 |
| Arterial hypertension | 7 (50%) | 6 (54.5%) | 0.821 |
| Smoking | 5 (36%) | 4 (36%) | 0.973 |
| Systolic blood pressure (mmHg) | 116 ± 13 | 120 ± 11 | 0.450 |
| Duration of AF since first documented episode (months) | 124.3 ± 95.6 | 81.2 ± 83.9 | 0.250 |
| RAAS inhibitors | 5 (36%) | 3 (27%) | 0.653 |
| Beta-blockers | 9 (64%) | 6 (54%) | 0.622 |
| Statins | 6 (42%) | 6 (54%) | 0.561 |
| Left atrium diameter (mm) | 41 ± 3.6 | 42 ± 7 | 0.415 |
| Left ventricular ejection fraction (%) | 62.8 ± 3.4 | 65.3 ± 6.7 | 0.240 |
| CHA2DS2-VASc score | 1.79 ± 1.31 | 1.45 ± 1.36 | 0.545 |
| NT-proBNP (pg/mL) | 300 ± 295 | 323 ± 303 | 0.848 |
| vWF levels baseline (%) | 129.5 ± 32.1 | 135.4 ± 49.8 | 0.726 |
| hs-troponin T baseline (ng/L) | 8.8 ± 6.0 | 10.3 ± 6.3 | 0.551 |
| D-dimer baseline (mg/L) | 0.34 ± 0.26 | 0.35 ± 0.45 | 0.937 |
| Platelets level baseline (×103) | 256.5 ± 54.24 | 246.4 ± 49.82 | 0.638 |
| eGFR baseline | 76.14 ± 13.52 | 74.7 ± 23.19 | 0.850 |
| White blood cells (103 × cells/lL) baseline | 7.5 ± 0.9 | 7.3 ± 1.8 | 0.854 |
| Hemoglobin (g/L) | 142.2 ± 15.5 | 144.3 ± 11.6 | 0.718 |
| Fasting glucose (g/dL) | 5.8 ± 1.3 | 5.8 ± 0.7 | 0.921 |
| Total cholesterol (mmol/L) | 5.5 ± 1.9 | 4.4 ± 1.3 | 0.125 |
| Procedural Characteristics | Pulsed-Field Ablation n = 14 | Cryoballoon Ablation n = 11 | p |
|---|---|---|---|
| Procedure time (min.) | 59.3 ± 25.4 | 94.1 ± 28.4 | 0.005 |
| Fluoroscopy time (min.) | 12.8 ± 7.4 | 12 ± 7 | 0.888 |
| LA dwell time (min.) | 44.3 ± 14.8 | 79.3 ± 21.9 | <0.001 |
| Total heparin dose (IU) | 18,000 IU (IQR 13,500–20,000) | 20,000 (IQR 13,000–23,000) | 0.181 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katic, J.; Anic, A.; Breskovic, T.; Borovac, J.A.; Kresic, B.; Supe-Domic, D.; Kumric, M.; Bozic, J.; Jurisic, Z. Pulsed-Field Ablation Is Associated with Lower Endothelial Injury and Procedure Time Compared to Cryoballoon Ablation in Paroxysmal Atrial Fibrillation. Pathophysiology 2025, 32, 60. https://doi.org/10.3390/pathophysiology32040060
Katic J, Anic A, Breskovic T, Borovac JA, Kresic B, Supe-Domic D, Kumric M, Bozic J, Jurisic Z. Pulsed-Field Ablation Is Associated with Lower Endothelial Injury and Procedure Time Compared to Cryoballoon Ablation in Paroxysmal Atrial Fibrillation. Pathophysiology. 2025; 32(4):60. https://doi.org/10.3390/pathophysiology32040060
Chicago/Turabian StyleKatic, Josip, Ante Anic, Toni Breskovic, Josip Andelo Borovac, Branka Kresic, Daniela Supe-Domic, Marko Kumric, Josko Bozic, and Zrinka Jurisic. 2025. "Pulsed-Field Ablation Is Associated with Lower Endothelial Injury and Procedure Time Compared to Cryoballoon Ablation in Paroxysmal Atrial Fibrillation" Pathophysiology 32, no. 4: 60. https://doi.org/10.3390/pathophysiology32040060
APA StyleKatic, J., Anic, A., Breskovic, T., Borovac, J. A., Kresic, B., Supe-Domic, D., Kumric, M., Bozic, J., & Jurisic, Z. (2025). Pulsed-Field Ablation Is Associated with Lower Endothelial Injury and Procedure Time Compared to Cryoballoon Ablation in Paroxysmal Atrial Fibrillation. Pathophysiology, 32(4), 60. https://doi.org/10.3390/pathophysiology32040060










