A Slow Hydrogen Sulfide Donor GYY-4137 Partially Improves Vascular Function in Spontaneously Hypertensive Rats Fed a High-Fat Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Animals
2.3. Experimental Design
2.4. Blood Pressure Measurement and Biometric Parameters
2.5. Body Weights and Diet Consumption
2.6. Blood Sample Analysis
2.7. ELISA
2.8. Vasoactive Responses of Isolated Arteries
2.9. Analysis of Protein Expression
2.10. Total NO Synthase Activity
2.11. Collagen Amount
2.12. Statistical Analysis
2.13. Drugs
3. Results
3.1. General Parameters of the Experimental Animals
3.2. Cardiac Parameters of the Experimental Animals
3.3. Blood Pressure of the Experimental Animals
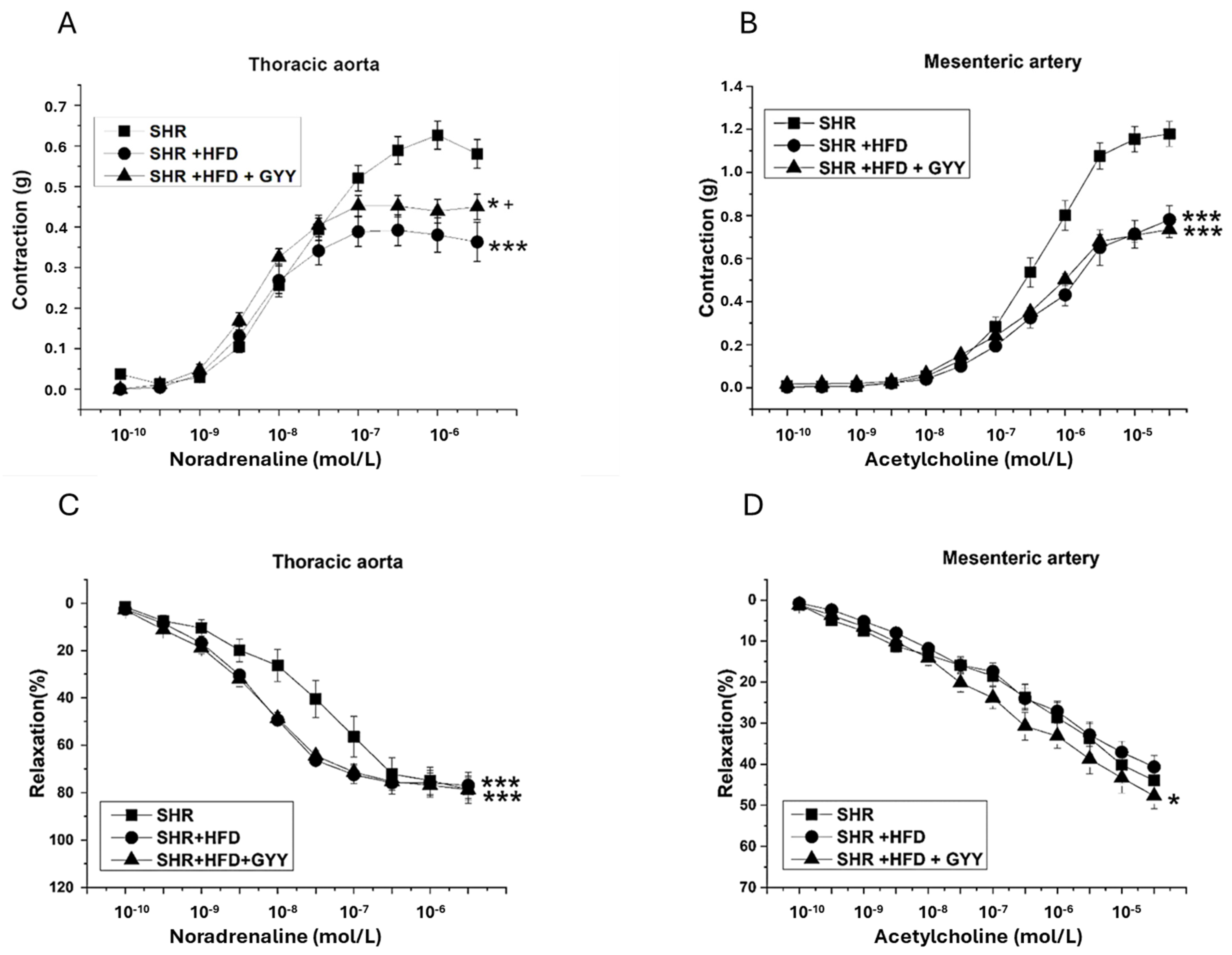
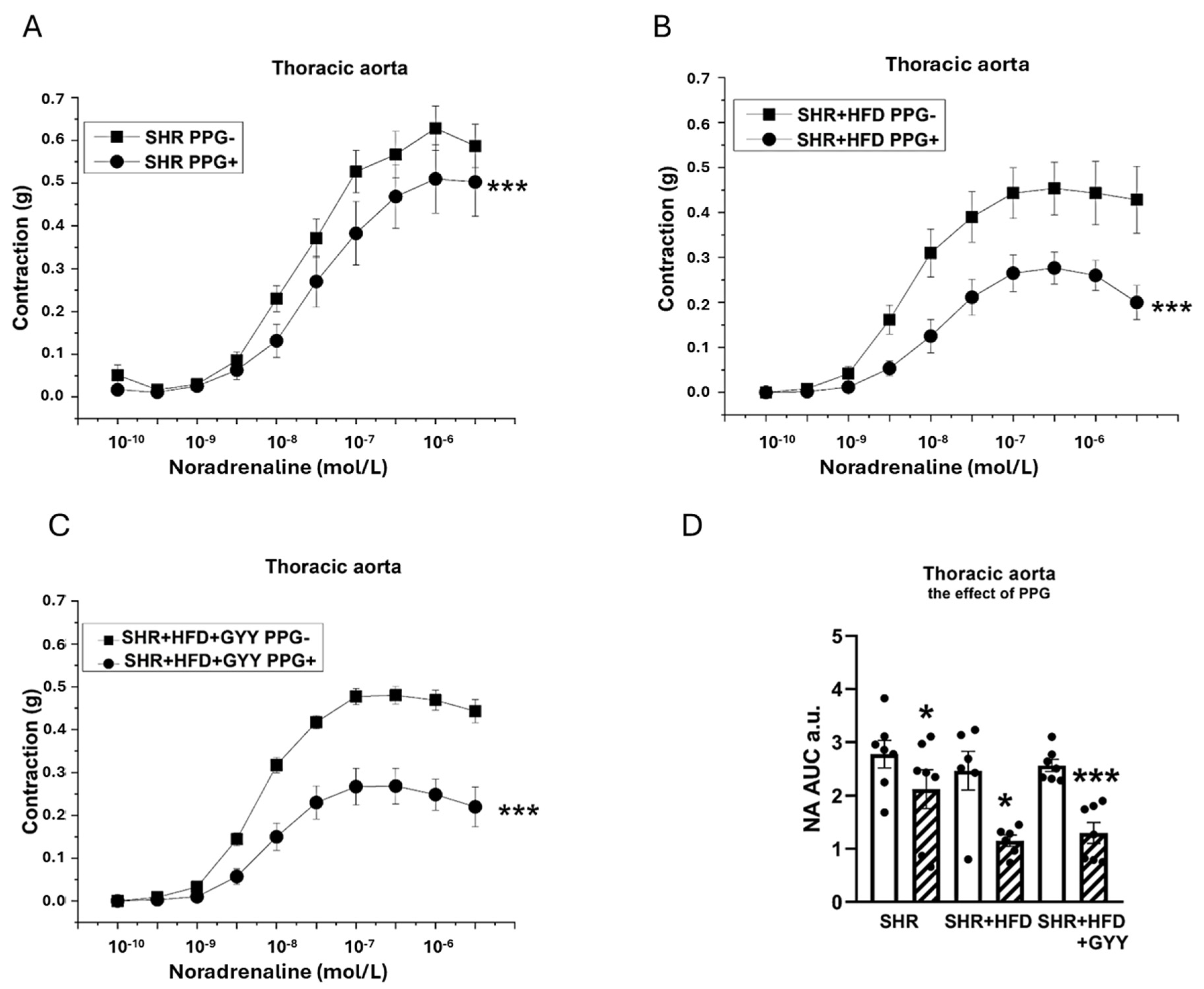
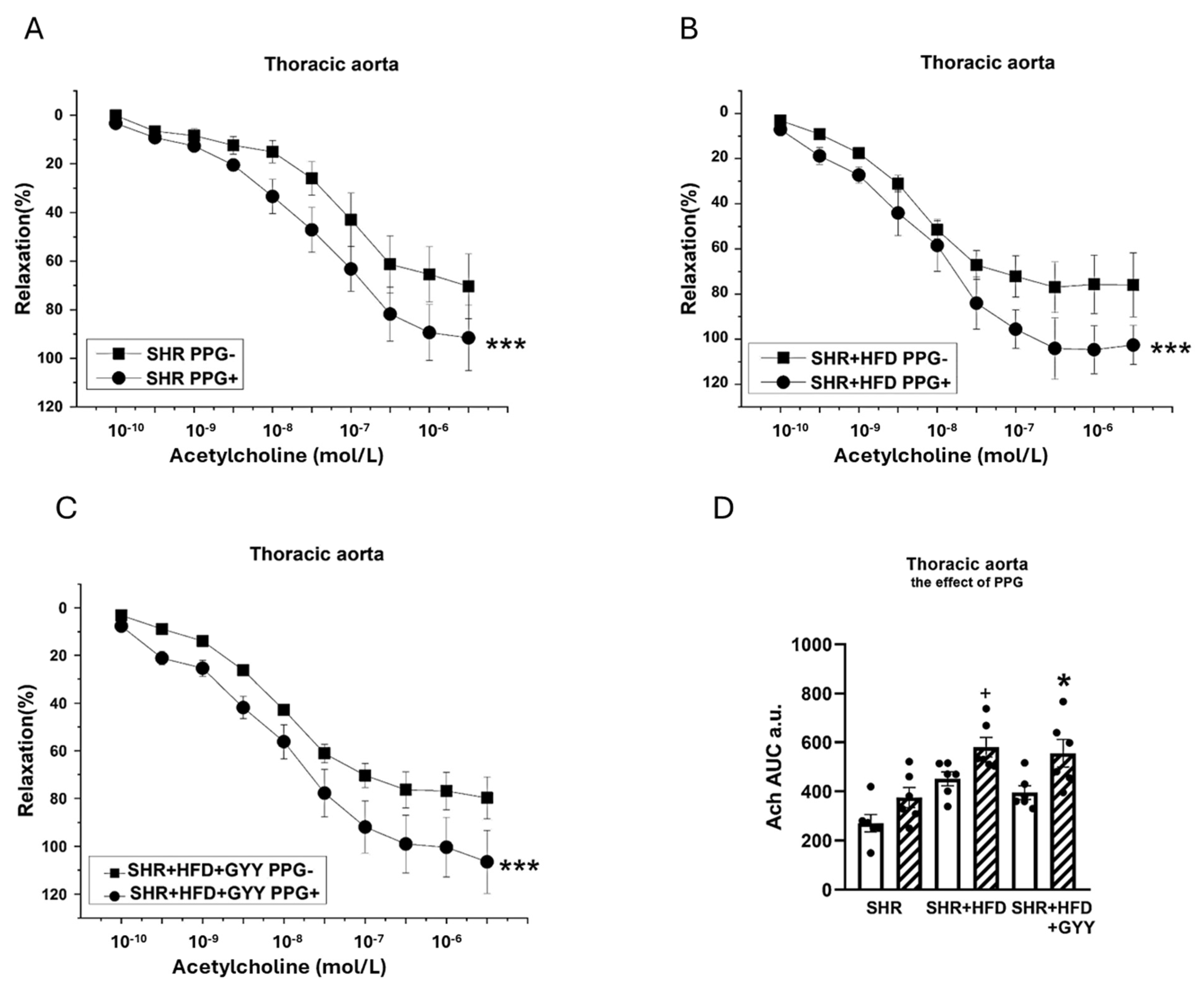
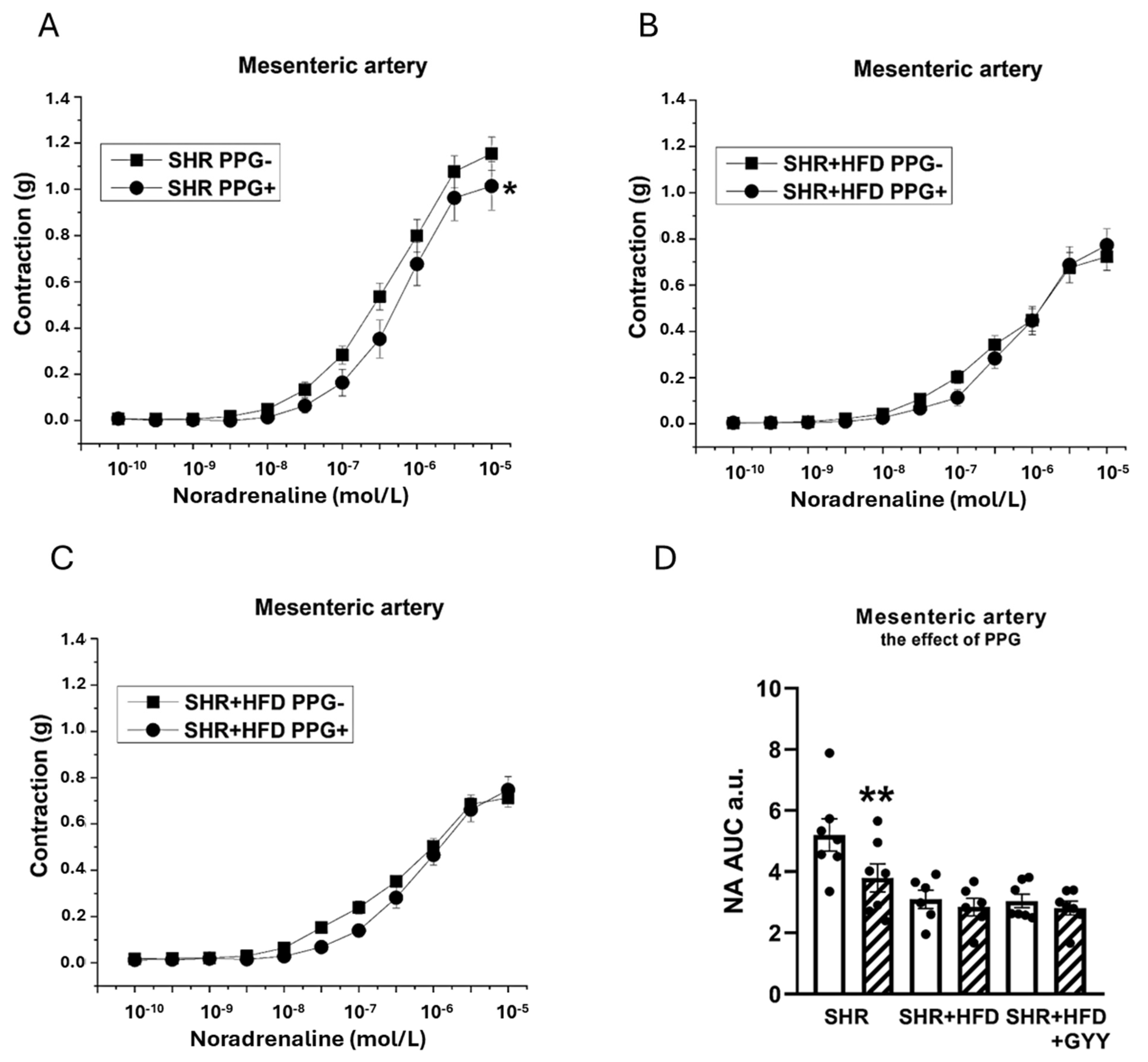
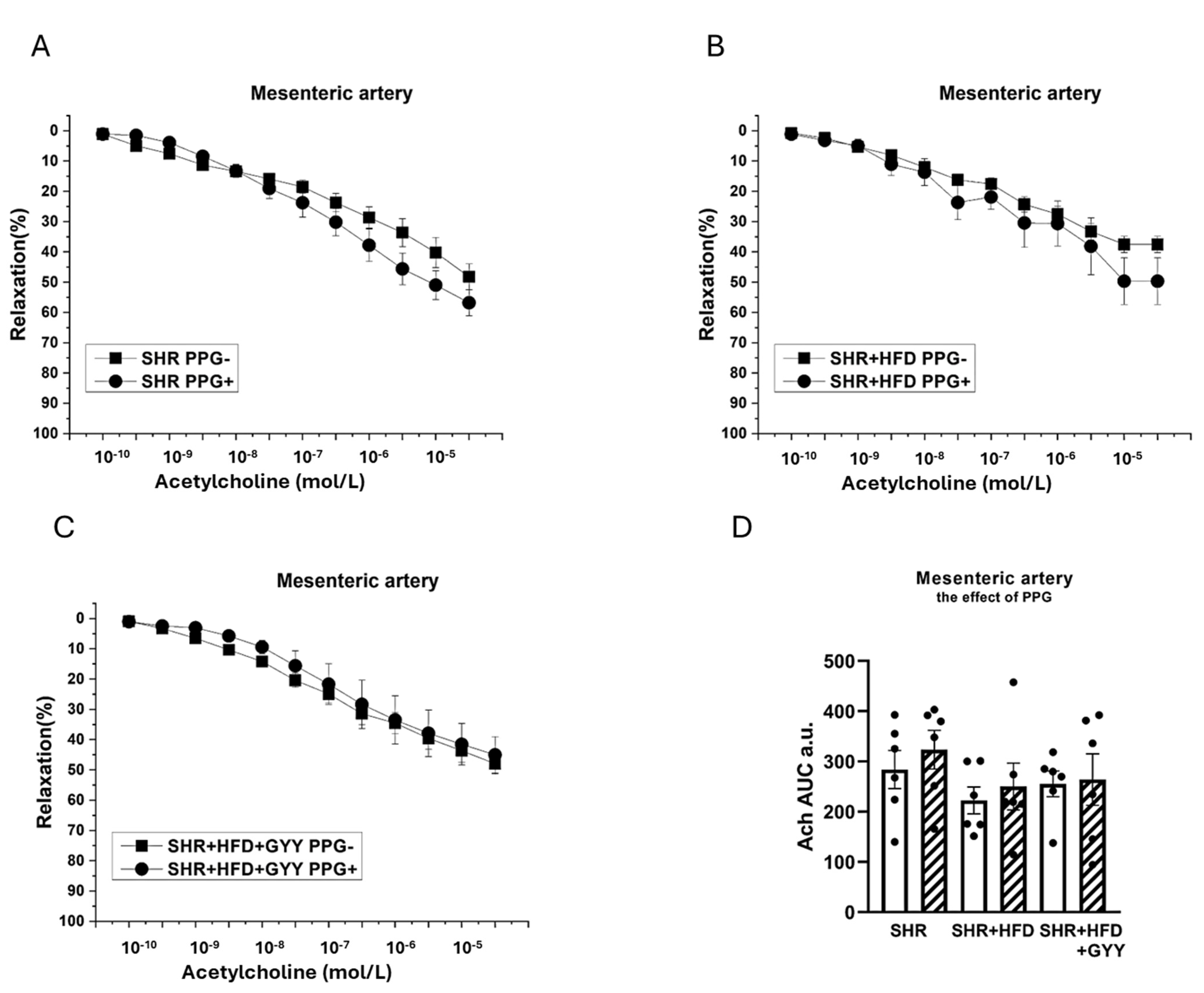
3.4. Endothelial Function and Contractility of the Thoracic Aorta and Mesenteric Artery
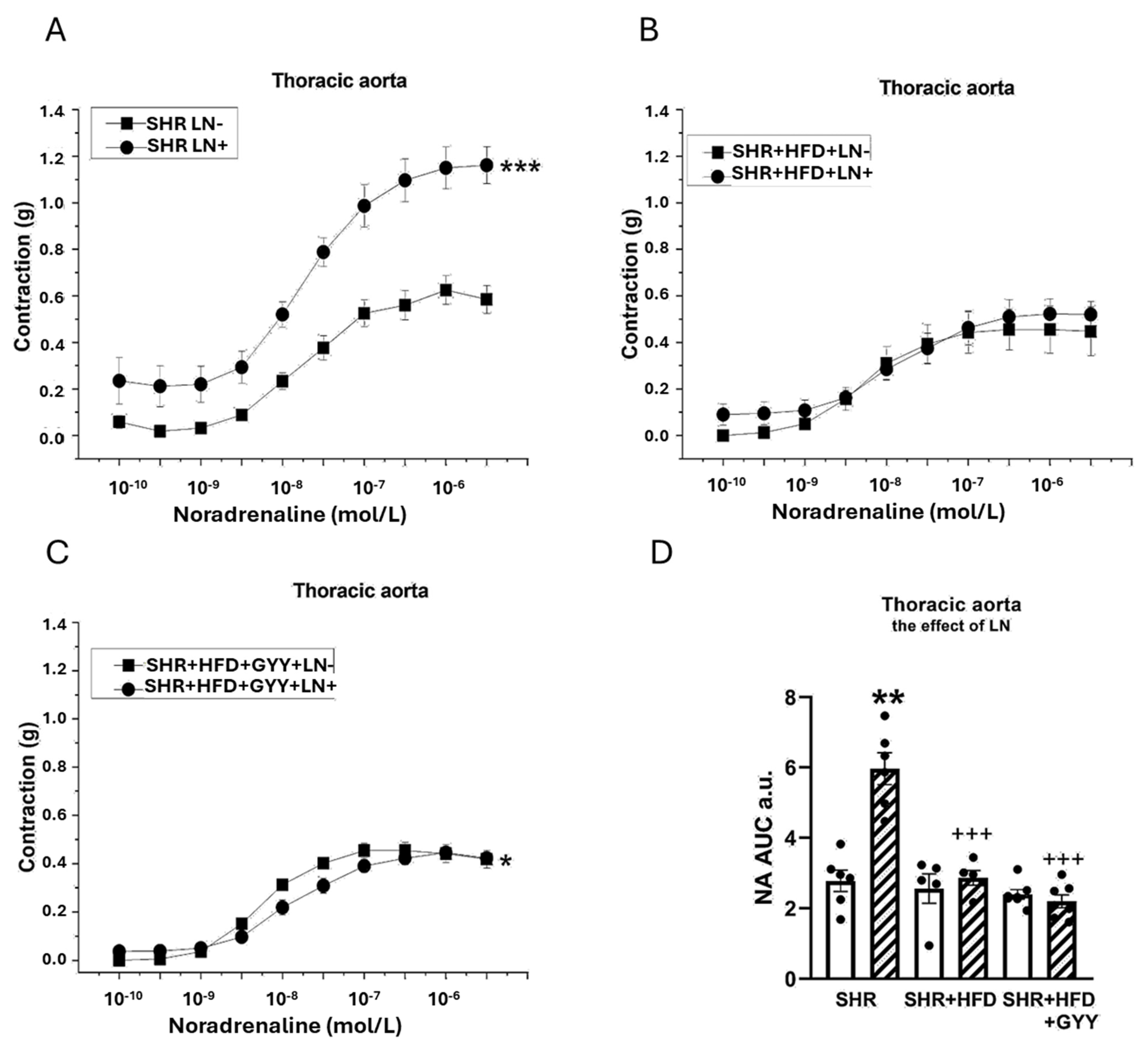
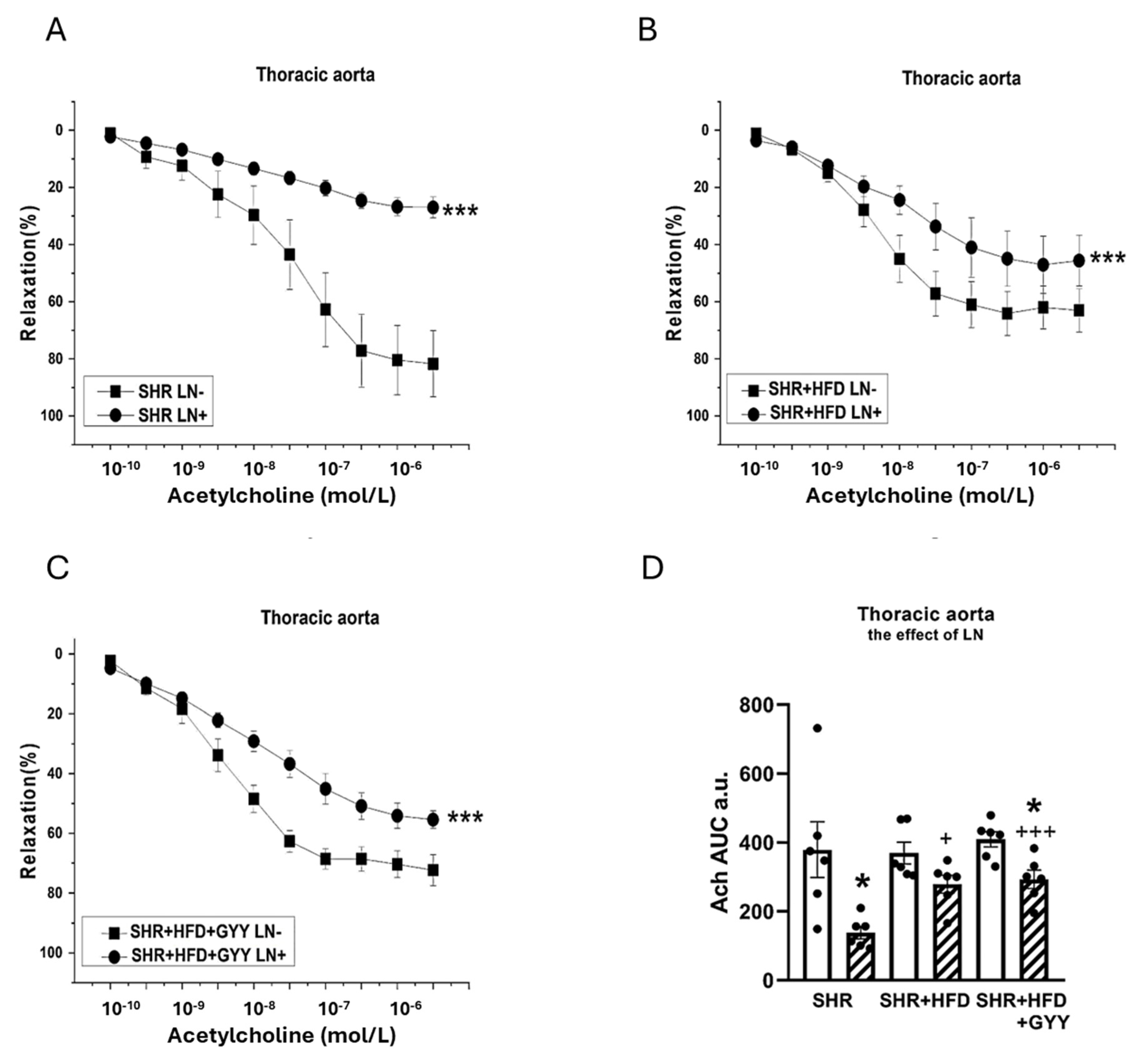
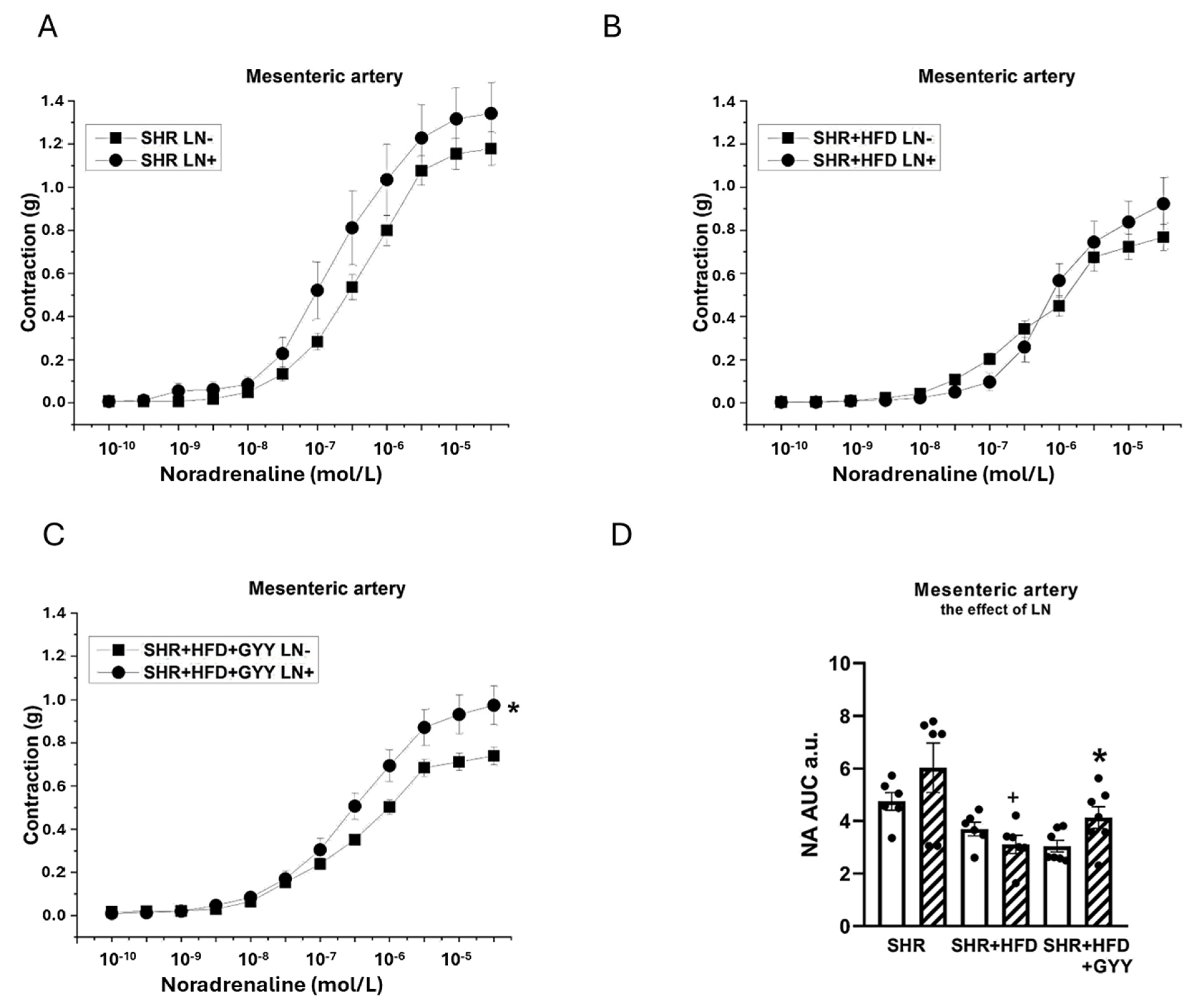
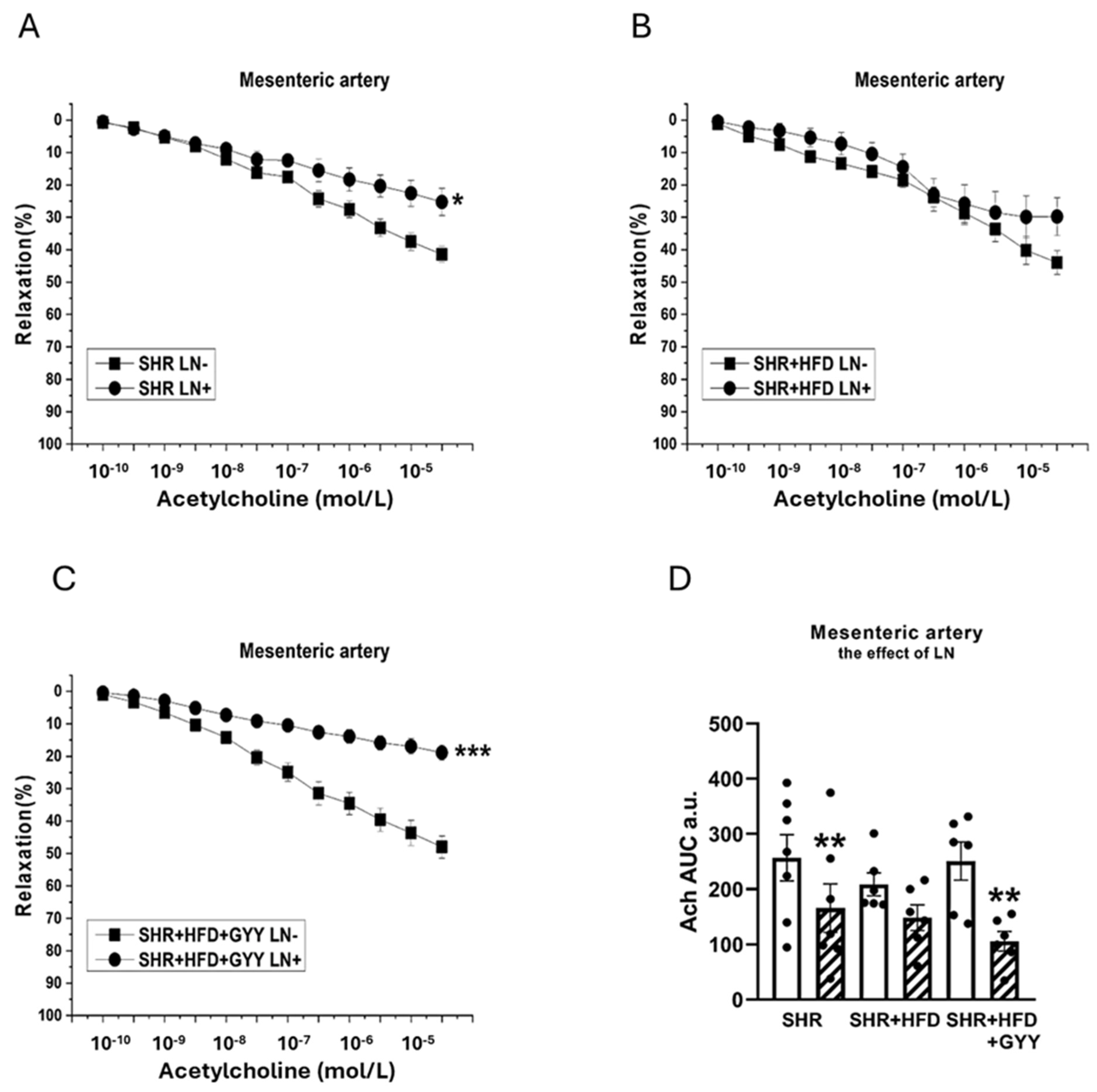
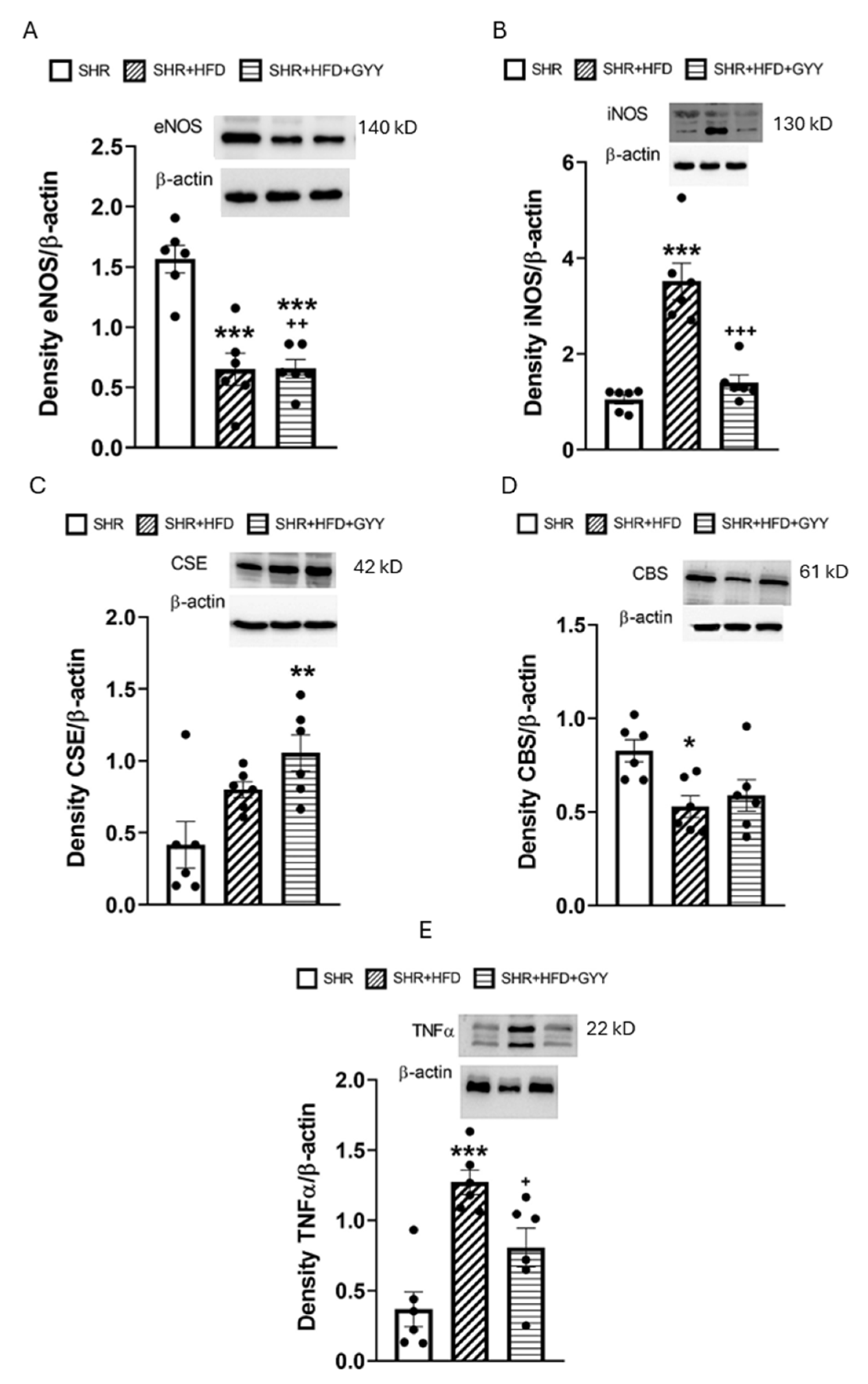
3.5. Evaluation of NO/NOS System Participation
3.6. Evaluation of H2S/CSE System Participation
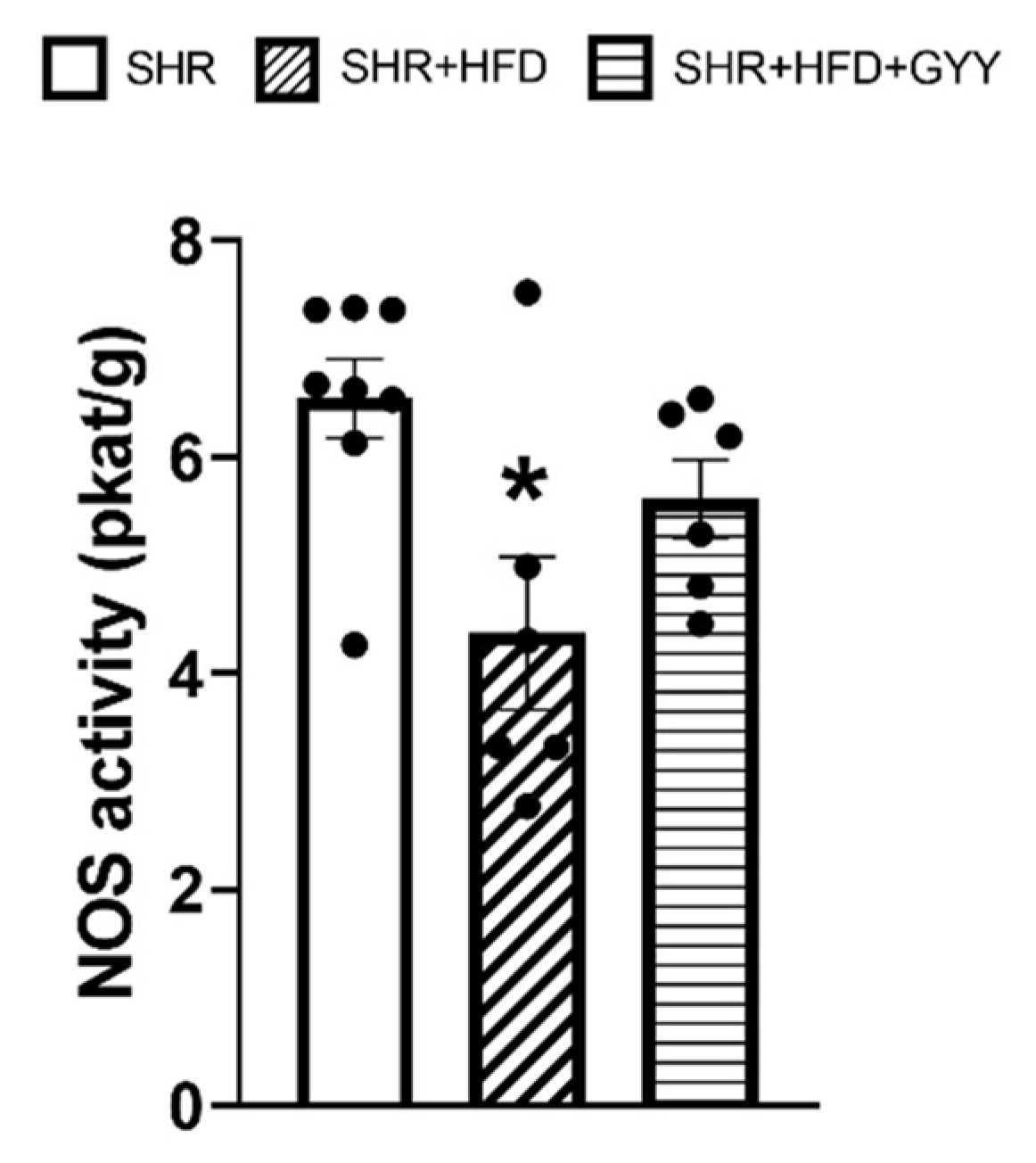
3.7. Total NOS Activity and Protein Expression Levels
4. Discussion
4.1. Effects of the High-Fat Diet
4.2. Effects of the GYY-4137 Treatment
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | acetylcholine |
| AUC | areas under the curve |
| BW | body weight |
| CaCl2 | calcium chloride |
| CaNa2EDTA | edetate calcium disodium |
| CBS | cystathionine β |
| synthase | |
| CHOL | cholesterol |
| CO2 | carbon dioxide |
| CRE | creatine |
| CSE | cystathionine γ |
| lyase | |
| DMSO | dimethyl sulfoxide |
| DMAB | dimethylaminobenzaldehyde |
| ECL | enhanced chemiluminescence |
| EDHF | endothelium |
| derived hyperpolarizing factor | |
| eNOS | endothelial nitric oxide synthase |
| GLU | glucose |
| H2S | hydrogen sulfide |
| HFD | high |
| fat diet | |
| HW | heart weight |
| HYP | hydroxyproline |
| IL | 6 |
| interleukin | 6 |
| iNOS | inducible nitric oxide synthase |
| KCl | potassium chloride |
| KH2PO4 | monopotassium phosphate |
| LN | N(G) |
| nitro | L |
| arginine methyl ester | |
| MA | mesenteric artery |
| MgSO4x7H2O | magnesium sulfate heptahydrate |
| NA | noradrenaline |
| Na2S | sodium sulfide |
| NaCl | sodium chloride |
| NaHCO3 | sodium hydrogen carbonate |
| NaHS | sodium hydrosulfide |
| NaOH | sodium hydroxide |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| O2 | oxygen |
| PPG | DL |
| propargylglycine | |
| PVAT | perivascular adipose tissue |
| RAAS | renin |
| angiotensin | aldosterone system |
| RTW | retroperitoneal adipose tissue weight |
| sBP | systolic blood pressure |
| sGC | soluble guanylate cyclase |
| SHR | spontaneously hypertensive rats |
| TA | thoraric aorta |
| TAG | triacylglycerol |
| TGF | β |
| transforming growth factor | beta |
| TL | the length of the tibia |
| TNFα | tumor necrosis factor alpha |
| WKY | Wistar |
| Kyoto |
References
- World Health Organization: Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 11 December 2024).
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory links between high fat diets and diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [PubMed]
- Gui, D.D.; Luo, W.; Yan, B.J.; Ren, Z.; Tang, Z.H.; Liu, L.S.; Zhang, J.F.; Jiang, Z.S. Effects of gut microbiota on atherosclerosis through hydrogen sulfide. Eur. J. Pharmacol. 2021, 896, 173916. [Google Scholar] [CrossRef]
- Berenyiova, A.; Cebova, M.; Aydemir, B.G.; Golas, S.; Majzunova, M.; Cacanyiova, S. Vasoactive Effects of Chronic Treatment with Fructose and Slow-Releasing H2S Donor GYY-4137 in Spontaneously Hypertensive Rats: The Role of Nitroso and Sulfide Signalization. Int. J. Mol. Sci. 2022, 23, 9215. [Google Scholar] [CrossRef] [PubMed]
- Bello, I.; Smimmo, M.; d’Emmanuele di Villa Bianca, R.; Bucci, M.; Cirino, G.; Panza, E.; Brancaleone, V. Erucin, an H2S-Releasing Isothiocyanate, Exerts Anticancer Effects in Human Triple-Negative Breast Cancer Cells Triggering Autophagy-Dependent Apoptotic Cell Death. Int. J. Mol. Sci. 2023, 24, 6764. [Google Scholar] [CrossRef]
- Corvino, A.; Scognamiglio, A.; Fiorino, F.; Perissutti, E.; Santagada, V.; Caliendo, G.; Severino, B. Pills of Multi-Target H2S Donating Molecules for Complex Diseases. Int. J. Mol. Sci. 2024, 25, 7014. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Whiteman, M.; Guan, Y.Y.; Neo, K.L.; Cheng, Y.; Lee, S.W.; Zhao, Y.; Baskar, R.; Tan, C.H.H.; Moore, P.K. Characterization of a novel, water-soluble hydrogen sulfide–releasing molecule (GYY4137): New insights into the biology of hydrogen sulfide. Circulation 2008, 117, 2351–2360. [Google Scholar] [CrossRef]
- Liu, Z.; Han, Y.; Li, L.; Lu, H.; Meng, G.; Li, X.; Shirhan, M.; Peh, M.T.; Xie, L.; Zhou, S.; et al. The hydrogen sulfide donor, GYY 4137, exhibits anti-atherosclerotic activity in high fat fed apolipoprotein E−/− mice. Br. J. Pharmacol. 2013, 169, 1795–1809. [Google Scholar] [CrossRef]
- Meng, G.; Zhu, J.; Xiao, Y.; Huang, Z.; Zhang, Y.; Tang, X.; Xie, L.; Chen, Y.; Shao, Y.; Ferro, A.; et al. Hydrogen sulfide donor GYY4137 protects against myocardial fibrosis. Oxid. Med. Cell Long. 2015, 2015, 691070. [Google Scholar] [CrossRef]
- Pechanova, O.; Bernatova, I.; Pelouch, V.; Simko, F. Protein remodelling of the heart in NO-deficient hypertension: The effect of captopril. J. Mol. Cell Cardiol. 1997, 29, 3365–3374. [Google Scholar] [CrossRef]
- Reddy, G.K.; Enwemeka, C.S. A simplified method for the analysis of hydroxyproline in biological tissues. Clin. Biochem. 1996, 29, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Gasparova, Z.; Ruskova, E.; Seckarova Michalikova, D.; Brnoliakova, Z.; Svik, K.; Slovak, L.; Bezek, S.; Knezl, V.; Sotnikova, R. High-fructose intake-induced dyslipidemia and oxidative stress accompanied by hippocampal dysfunctions in hypertensive but not hypertriacylglycerolemic rats. Gen. Physiol. Biophys. 2023, 42, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.D.; Yuan, Y.V.; Godin, D.V. Plasma and lipoprotein lipid composition and hepatic antioxidant status in spontaneously hypertensive (SHR) and normotensive (WKY) rats. Can. J. Physiol. Pharmacol. 1998, 76, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.V.; Kitts, D.D. Dietary fat source and cholesterol interactions alter plasma lipids and tissue susceptibility to oxidation in spontaneously hypertensive (SHR) and normotensive Wistar Kyoto (WKY) rats. Mol. Cell Biochem. 2002, 232, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Hojna, S.; Malínska, H.; Huttl, M.; Vanourkova, Z.; Markova, I.; Miklankova, D.; Hrdlicka, J.; Papousek, F.; Neckar, J.; Kujal, P.; et al. Hepatoprotective and cardioprotective effects of empagliflozin in spontaneously hypertensive rats fed a high-fat diet. Biomed. Pharmacoth 2024, 174, 116520. [Google Scholar] [CrossRef]
- Kurbel, S. Arterial Hypertension Due to Fructose Ingestion: Model Based on Intermittent Osmotic Fluid Trapping in the Small Bowel. Theor. Biol. Med. Modell. 2010, 7, 27. [Google Scholar] [CrossRef]
- Shiou, Y.L.; Huang, I.C.; Lin, H.T.; Lee, H.C. High fat diet aggravates atrial and ventricular remodeling of hypertensive heart disease in aging rats. J. Form. Med. Assoc. 2018, 117, 621–631. [Google Scholar] [CrossRef]
- Gabbiani, G. The myofibroblast in wound healing and fibrocontractive diseases. J. Pathol. 2003, 200, 500–503. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Transforming growth factor-β in myocardial disease. Nat. Rev. Cardiol. 2022, 19, 435–455. [Google Scholar] [CrossRef]
- Precek, J.; Hutyra, M.; Kovacik, F.; Orsag, J.; Taborsky, M. Biomarkers of renal function in prognostic stratification of patients with acute coronary syndrome. Cor et Vasa 2018, 60, e148–e154. [Google Scholar] [CrossRef]
- Caillard, P.; Bennis, Y.; Boudot, C.; Chatelain, D.; Rybarczyk, P.; Boullier, A.; Poirot, S.; Titeca-Beauport, D.; Bodeau, S.; Choukroun, G.; et al. Acute kidney disease in mice is associated with early cardiovascular dysfunction. Ren. Fail. 2024, 46, 2415510. [Google Scholar] [CrossRef]
- Bosse, J.D.; Lin, H.Y.; Sloan, C.; Zhang, Q.J.; Abel, E.D.; Pereira, T.J.; Dolinsky, V.W.; Symons, J.D.; Jalili, T. A low-carbohydrate/high-fat diet reduces blood pressure in spontaneously hypertensive rats without deleterious changes in insulin resistance. Am. J. Physiol. 2013, 304, H1733–H1742. [Google Scholar] [CrossRef] [PubMed]
- Ceriello, A.; Motz, E. Is Oxidative Stress the Pathogenic Mechanism Underlying Insulin Resistance, Diabetes, and Cardiovascular Disease? The Common Soil Hypothesis Revisited. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 816–823. [Google Scholar] [CrossRef]
- Haddad, Y.; Couture, R. Interplay between the kinin B1 receptor and inducible nitric oxide synthase in insulin resistance. Br. J. Pharmacol. 2016, 173, 1988–2000. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Ying, C.; Xu, M.; Zuo, X.; Ye, X.; Liu, L.; Nara, Y.; Sun, X. High-fat diet up-regulates caveolin-1 expression in aorta of diet-induced obese but not in diet-resistant rats. Cardiovasc. Res. 2007, 76, 167–174. [Google Scholar] [CrossRef]
- Kobayashi, T.; Taguchi, K.; Nemoto, S.; Nogami, T.; Matsumoto, T.; Kamata, K. Activation of the PDK-1/Akt/eNOS pathway involved in aortic endothelial function differs between hyperinsulinemic and insulin-deficient diabetic rats. Am. J. Physiol. 2009, 297, H1767–H1775. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Q.; Song, P.; Zhu, Y.; Zou, M.H. Redox Regulation of the AMP-Activated Protein Kinase. PLoS ONE 2010, 5, e15420. [Google Scholar] [CrossRef]
- Tomada, I.; Negrão, R.; Almeida, H.; Neves, D. Long-term high-fat consumption leads to downregulation of Akt phosphorylation of eNOS at Ser1177 and upregulation of Sirtuin-1 expression in rat cavernous tissue. Age 2014, 36, 597–611. [Google Scholar] [CrossRef]
- Feletou, M. Endothelium-dependent hyperpolarization and endothelial dysfunction. J. Cardiovasc. Pharmacol. 2016, 67, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Jebelovszki, E.; Kiraly, C.; Erdei, N.; Feher, A.; Pasztor, E.T.; Rutkai, I.; Forster, T.; Edes, I.; Koller, A.; Bagi, Z. High-fat diet-induced obesity leads to increased NO sensitivity of rat coronary arterioles: Role of soluble guanylate cyclase activation. Am. J. Physiol. 2008, 294, H2558–H2564. [Google Scholar] [CrossRef]
- Tang, G.; Yang, G.; Jiang, B.; Ju, Y.; Wu, L.; Wang, R. H2S is an endothelium-derived hyperpolarizing factor. Antiox Red. Signal 2013, 19, 1634–1646. [Google Scholar] [CrossRef]
- Cacanyiova, S.; Golas, S.; Zemancikova, A.; Majzunova, M.; Cebova, M.; Malinska, H.; Huttl, M.; Markova, I.; Berenyiova, A. The vasoactive role of perivascular adipose tissue and the sulfide signaling pathway in a nonobese model of metabolic syndrome. Biomolecules 2021, 11, 108. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Huang, W.; Skibba, M.; Fang, Q.; Xie, L.; Wei, T.; Feng, Z.; Liang, G. Inhibition of ROS and inflammation by an imidazopyridine derivative X22 attenuate high fat diet-induced arterial injuries. Vasc. Pharmacol. 2015, 72, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Berenyiova, A.; Drobna, M.; Cebova, M.; Kristek, F.; Cacanyiova, S. Changes in the vasoactive effects of nitric oxide, hydrogen sulfide and the structure of the rat thoracic aorta: The role of age and essential hypertension. J. Physiol. Pharmacol. 2018, 69, 4. [Google Scholar] [CrossRef]
- Neves, K.B.; Lobato, N.S.; Lopes, R.A.M.; Filgueira, F.P.; Zanotto, C.Z.; Oliveira, A.M.; Tostes, R.C. Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: A link to vascular dysfunction in obesity? Clin. Scie 2014, 127, 111–122. [Google Scholar] [CrossRef]
- Szpakowicz, A.; Szpakowicz, M.; Lapinska, M.; Paniczko, M.; Lawicki, S.; Raczkowski, A.; Kondraciuk, M.; Sawicka, E.; Chlabicz, M.; Kozuch, M.; et al. Serum Chemerin Concentration Is Associated with Proinflammatory Status in Chronic Coronary Syndrome. Biomolecules 2021, 11, 1149. [Google Scholar] [CrossRef]
- Eichelmann, F.; Schulze, M.B.; Wittenbecher, C.; Menzel, J.; Weikert, C.; di Giuseppe, R.; Biemann, R.; Isermann, B.; Fritsche, A.; Boeing, H.; et al. Chemerin as a Biomarker Linking Inflammation and Cardiovascular Diseases. J. Am. Coll. Cardiol. 2019, 73, 378–379. [Google Scholar] [CrossRef]
- Trovato, F.M.; Castrogiovanni, P.; Szychlinska, M.A.; Purrello, F.; Musumeci, G. Impact of Western and Mediterranean diets and vitamin D on muscle fibers of sedentary rats. Nutrients 2018, 10, 231. [Google Scholar] [CrossRef]
- Casili, G.; Randi, E.; Panagaki, T.; Zuhra, K.; Petrosino, M.; Szabo, C. Inhibition of the 3-mercaptopyruvate sulfurtransferase—Hydrogen sulfide system promotes cellular lipid accumulation. GeroScience 2022, 44, 2271–2289. [Google Scholar] [CrossRef]
- Zhao, S.; Song, T.; Gu, Y.; Zhang, Y.; Cao, S.; Miao, Q.; Zhang, X.; Chen, H.; Gao, Y.; Zhang, L.; et al. Hydrogen sulfide alleviates liver injury via S-sulfhydrated-Keap1/Nrf2/LRP1 pathway. Hepatology 2021, 73, 282–302. [Google Scholar] [CrossRef]
- Geng, B.; Cai, B.; Liao, F.; Zheng, Y.; Zeng, Q.; Fan, X.; Gong, Y.; Yang, J.; Cui, Q.; Tang, C.; et al. Increase or decrease hydrogen sulfide exert opposite lipolysis but reduce global insulin resistance in high fatty diet induced obese mice. PLoS ONE 2013, 8, e73892. [Google Scholar] [CrossRef] [PubMed]
- Qabazard, B.; Yousif, M.; Mousa, A.; Phillips, O.A. GYY4137 attenuates functional impairment of corpus cavernosum and reduces fibrosis in rats with STZ-induced diabetes by inhibiting the TGF-β1/Smad/CTGF pathway. Biomed. Pharmacother. 2021, 138, 111486. [Google Scholar] [CrossRef]
- Alshahwan, H.; Qabazard, B.; Mousa, A.; Chandrasekhar, B.; Santhosh, K.; Yousif, M.H.M. Hydrogen sulfide donor GYY4137 attenuates vascular complications in mesenteric bed of streptozotocin-induced diabetic rats. Eur. J. Pharmacol. 2022, 933, 175265. [Google Scholar] [CrossRef] [PubMed]
- Cebova, M.; Kristek, F. Age-dependent ultrastructural changes of coronary artery in spontaneously hypertensive rats. Gen. Physiol. Biophys. 2011, 30, 364–372. [Google Scholar] [CrossRef]
- Simko, F.; Baka, T.; Stanko, P.; Repova, K.; Krajcirovicova, K.; Aziriova, S.; Domenig, O.; Zorad, S.; Adamcova, M.; Paulis, L. Sacubitril/Valsartan and Ivabradine Attenuate Left Ventricular Remodelling and Dysfunction in Spontaneously Hypertensive Rats: Different Interactions with the Renin–Angiotensin–Aldosterone System. Biomedicines 2022, 10, 1844. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Patel, S.; Mani, S.; Hussain, T. Role of angiotensin type 2 receptor in improving lipid metabolism and preventing adiposity. Mol. Cell Biochem. 2019, 461, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Laggner, H.; Hermann, M.; Esterbauer, H.; Muellner, M.K.; Exner, M.; Gmeiner, B.M.; Kapiotis, S. The novel gaseous vasorelaxant hydrogen sulfide inhibits angiotensin-converting enzyme activity of endothelial cells. J. Hypertens. 2007, 25, 2100–2104. [Google Scholar] [CrossRef]
- Drobna, M.; Misak, A.; Holland, T.; Kristek, F.; Grman, M.; Tomasova, L.; Berenyiova, A.; Cacanyiova, S.; Ondrias, K. Captopril Partially Decreases the Effect of H2S on Rat Blood Pressure and Inhibits H2S-Induced Nitric Oxide Release From S-Nitrosoglutathione. Phys. Res. 2015, 64, 479–486. [Google Scholar] [CrossRef]
- Altaany, Z.; Ju, Y.; Yang, G.; Wang, R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal 2014, 7, ra87. [Google Scholar] [CrossRef]
- Grumbach, I.M.; Chen, W.; Mertens, S.A.; Harrison, D.G. A negative feedback mechanism involving nitric oxide and nuclear factor kappa-B modulates endothelial nitric oxide synthase transcription. J. Mol. Cell Cardiol. 2005, 39, 595–603. [Google Scholar] [CrossRef]
- Soares, A.G.; Catelli de Carvalho, M.H.; Akamine, E. Obesity Induces Artery-Specific Alterations: Evaluation of Vascular Function and Inflammatory and Smooth Muscle Phenotypic Markers. BioMed Res. Int. 2017, 2017, 5038602. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, Z.; Zhu, Y.Z. Regional Heterogeneity of Perivascular Adipose Tissue: Morphology, Origin, and Secretome. Front. Pharmacol. 2021, 12, 697720. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Gaddam, R.R. Hydrogen sulfide in inflammation: A novel mediator and therapeutic target. Antiox Red. Signal 2021, 34, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fox, B.; Keeble, J.; Salto-Tellez, M.; Winyard, P.G.; Wood, M.E.; Moore, P.K.; Whiteman, M. The complex effects of the slow-releasing hydrogen sulfide donor GYY 4137 in a model of acute joint inflammation and in human cartilage cells. J. Cell Mol. Med. 2013, 17, 365–376. [Google Scholar] [CrossRef]
- Cui, W.; Chen, J.; Yu, F.; Liu, W.; He, M. GYY4137 protected the integrity of the blood-brain barrier via activation of the Nrf2/ARE pathway in mice with sepsis. FASEB J. 2021, 35, e21902. [Google Scholar] [CrossRef]

| Parameters | SHR | n | SHR + HFD | n | SHR + HFD + GYY | n |
|---|---|---|---|---|---|---|
| BW (g) | 282.1 ± 13.3 | 8 | 332.4 ± 6.3 *** | 7 | 327.3 ± 7.3 ** | 8 |
| HW (g) | 1.047 ± 0.05 | 8 | 1.335 ± 0.03 *** | 7 | 1.372 ± 0.05 *** | 8 |
| RTW (g) | 2.07 ± 0.24 | 8 | 3.40 ± 0.20 ** | 7 | 3.44 ± 0.24 ** | 8 |
| TL (mm) | 34.42 ± 0.27 | 8 | 37.51 ± 0.9 ** | 7 | 37.05 ± 0.55 ** | 8 |
| RTW/BW (mg/g) | 7.21 ± 0.6 | 8 | 10.21 ± 0.46 ** | 7 | 10.48 ± 0.55 *** | 8 |
| RTW/TL (mg/mm) | 60.38 ± 7.14 | 8 | 90.59 ± 4.38 ** | 7 | 93.24 ± 6.71 ** | 8 |
| GLU (mmol/L) | 8.26 ± 0.4 | 8 | 8.68 ± 0.4 | 7 | 8.09 ± 0.3 | 8 |
| TAG (mmol/L) | 1.20 ± 0.07 | 8 | 2.42 ± 0.20 *** | 7 | 2.07 ± 0.24 ** | 8 |
| CHOL (mmol/L) | 2.67 ± 0.19 | 8 | 2.32 ± 0.07 * | 7 | 2.53 ± 0.10 | 8 |
| Urea (mmol/L) | 7.86 ± 0.11 | 8 | 7.09 ± 0.13 ** | 7 | 7.26 ± 0.26 | 8 |
| CRE (umol/L) | 23.57 ± 3.40 | 8 | 33.14 ± 4.38 | 7 | 34 ± 11.4 | 8 |
| Urea/CRE | 361.72 ± 32.76 | 8 | 237.27 ± 31.90 * | 7 | 305.05 ± 031.06 | 8 |
| Chemerin (ng/mL) | 158.25 ± 3.28 | 6 | 220.94 ± 7.66 * | 7 | 161.31 ± 5.91 + | 8 |
| Food intake (g/day) | 20.87 ± 0.45 | 8 | 15.56 ± 0.15 *** | 7 | 14.90 ± 0.16 ***+ | 8 |
| Parameters | SHR | n | SHR + HFD | n | SHR + HFD + GYY | n |
|---|---|---|---|---|---|---|
| HW (g) | 1.047 ± 0.05 | 8 | 1.335 ± 0.03 *** | 7 | 1.372 ± 0.05 *** | 8 |
| HW/BW (mg/g) | 3.72 ± 0.78 | 8 | 4.02 ± 0.07 * | 7 | 4.19 ± 0.13 * | 8 |
| HW/TL (mg/mm) | 30.43 ± 1.52 | 8 | 35.69 ± 0.85 * | 7 | 37.08 ± 1.44 ** | 8 |
| TGF-β (Density TGF-β/ β-actin) | 0.11 ± 0.01 | 6 | 2.45 ± 0.16 *** | 6 | 1.41 ± 0.17 +++ | 6 |
| Collagen (μg/mg) | 6.65 ± 0.77 | 6 | 9.14 ± 0.57 * | 6 | 6.79 ± 0.46 * | 6 |
| Thoracic Aorta | ||||||
|---|---|---|---|---|---|---|
| SHR | n | SHR + HFD | n | SHR + HFD + GYY | n | |
| NAmax (g) | 0.58 ± 0.03 | 7 | 0.37 ± 0.07 *** | 7 | 0.44 ± 0.03 *+ | 8 |
| NA EC50 (−log mol/L) | 8.39 ± 0.25 | 7 | 9.08 ± 0.26 | 7 | 9.53 ± 0.07 *** | 8 |
| Achmax (%) | 78.59 ± 4.75 | 7 | 76.95 ± 5.54 | 6 | 78.74 ± 5.79 | 7 |
| Ach EC50 (−log mol/L) | 8.70 ± 0.19 | 7 | 9.30 ± 0.17 * | 6 | 9.37 ± 0.09 ** | 7 |
| Mesenteric Artery | ||||||
|---|---|---|---|---|---|---|
| SHR | n | SHR + HFD | n | SHR + HFD + GYY | n | |
| NAmax (g) | 1.18 ± 0.06 | 7 | 0.78 ± 0.06 *** | 6 | 0.74 ± 0.04 *** | 7 |
| NA EC50 (−log mol/L) | 7.43 ± 0.07 | 7 | 7.41 ± 0.13 | 6 | 7.42 ± 0.10 | 7 |
| Achmax (%) | 43.97 ± 3.97 | 7 | 40.68 ± 2.80 | 6 | 47.72 ± 3.12 *+ | 6 |
| Ach EC50 (−log mol/L) | 7.56 ± 0.18 | 7 | 7.74 ± 0.29 | 6 | 8.18 ± 0.43 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aydemir, B.G.; Berenyiova, A.; Cebova, M.; Henderson, J.D.; Barta, A.; Cacanyiova, S. A Slow Hydrogen Sulfide Donor GYY-4137 Partially Improves Vascular Function in Spontaneously Hypertensive Rats Fed a High-Fat Diet. Pathophysiology 2025, 32, 27. https://doi.org/10.3390/pathophysiology32020027
Aydemir BG, Berenyiova A, Cebova M, Henderson JD, Barta A, Cacanyiova S. A Slow Hydrogen Sulfide Donor GYY-4137 Partially Improves Vascular Function in Spontaneously Hypertensive Rats Fed a High-Fat Diet. Pathophysiology. 2025; 32(2):27. https://doi.org/10.3390/pathophysiology32020027
Chicago/Turabian StyleAydemir, Basak G., Andrea Berenyiova, Martina Cebova, John D. Henderson, Andrej Barta, and Sona Cacanyiova. 2025. "A Slow Hydrogen Sulfide Donor GYY-4137 Partially Improves Vascular Function in Spontaneously Hypertensive Rats Fed a High-Fat Diet" Pathophysiology 32, no. 2: 27. https://doi.org/10.3390/pathophysiology32020027
APA StyleAydemir, B. G., Berenyiova, A., Cebova, M., Henderson, J. D., Barta, A., & Cacanyiova, S. (2025). A Slow Hydrogen Sulfide Donor GYY-4137 Partially Improves Vascular Function in Spontaneously Hypertensive Rats Fed a High-Fat Diet. Pathophysiology, 32(2), 27. https://doi.org/10.3390/pathophysiology32020027







