1. Introduction
Fibrillary glomerulonephritis (FGN) is an immune complex-mediated glomerulonephritis and a rare, poorly understood glomerular disease, accounting for less than 1% of glomerulopathies diagnosed by kidney biopsy (KB). Despite its low prevalence, FGN has a significant clinical impact due to its potential to cause irreversible kidney damage. This condition is characterized by the deposition of non-amyloid, Congo red-negative fibrils measuring 16 to 24 nm in diameter, as observed through electron microscopy (EM). Although first described in the 1980s, its pathophysiology and underlying immunological mechanisms remain an active area of research. While FGN is typically idiopathic, growing evidence suggests it may be an autoimmune disorder driven by complement activation, primarily via the alternative pathway, and by the deposition of immunoglobulins (Igs), predominantly of the IgG4 subtype [
1]. Moreover, up to 50% of patients with FGN have a history of underlying conditions, including malignancies, monoclonal gammopathy, autoimmune diseases, hepatitis C virus infection, diabetes mellitus, or chronic infections [
2,
3].
Clinically, FGN exhibits a broad spectrum of manifestations, ranging from asymptomatic proteinuria to nephrotic syndrome, nephritic syndrome, or even rapidly progressive glomerulonephritis (RPGN). The most common presentation is nephrotic syndrome, often accompanied by renal dysfunction and microscopic hematuria on urine sediment analysis. Notably, RPGN associated with FGN, although rare, represents a severe complication characterized by the rapid deterioration of renal function and an increased likelihood of requiring renal replacement therapy (RRT) [
4].
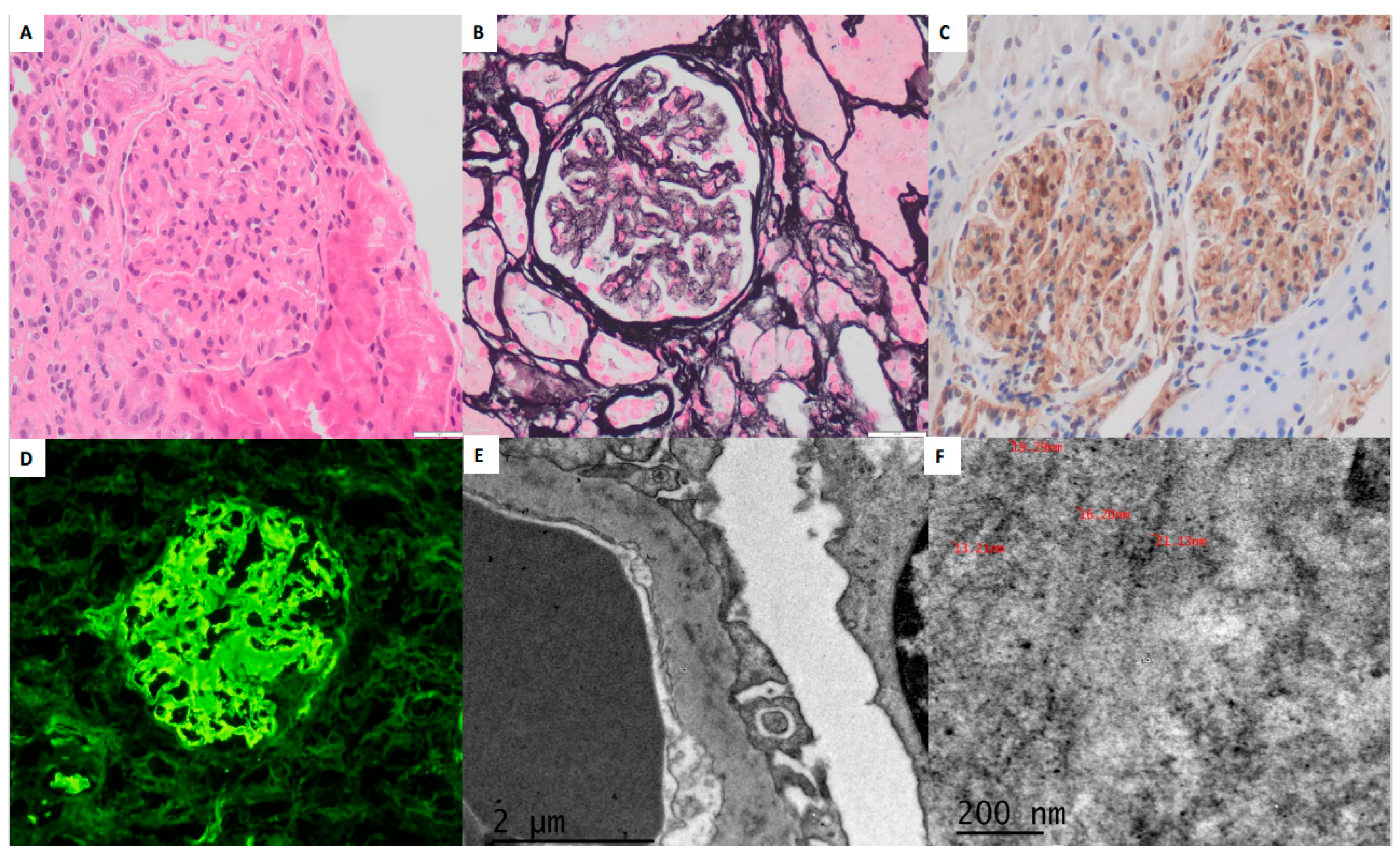
The diagnosis of FGN relies on KB, integrating electron microscopy (EM) findings, traditionally the gold standard, with immunofluorescence (IF) and light microscopy (LM) data. LM reveals mesangial expansion and capillary wall thickening due to the accumulation of deposits, while the identification of the characteristic fibrillar nature of the deposits requires confirmation by EM. The histopathological presentation is variable, ranging from minimal alterations to mesangial expansion with a non-methenamine silver- and Congo red-negative material that typically extends peripherally, thickening the capillary walls and allowing differentiation from amyloidosis. The presence of crescents has been reported in 27% to 50% of cases [
5], and approximately 4% may have a monoclonal origin.
IF typically demonstrates IgG deposition, predominantly of the IgG4 subclass, along with C3 deposits. A coarse and blurred staining pattern in the mesangium and capillary walls is characteristic; in some cases, deposits may be restricted to the capillary walls in a pseudolinear pattern, resembling that seen in anti-glomerular basement membrane (anti-GBM) antibody disease [
6]. EM remains essential for confirming the fibrillary nature and size of the deposits, distinguishing FGN from other fibrillary diseases such as immunotactoid glomerulonephritis (ITGN), where fibrillary deposits exceed 30 nm in diameter.
More recently, the overexpression of DNA J homolog subfamily B member 9 (DNAJB9) has been identified in both idiopathic FGN and FGN associated with other conditions. More recently, the overexpression of DNA J homolog subfamily B member 9 (DNAJB9) has been identified in both idiopathic FGN and FGN associated with other conditions, including systemic lupus erythematosus (SLE), rheumatoid arthritis, hepatitis C virus infection, monoclonal gammopathy, diabetes mellitus, and chronic infections [
7,
8,
9]. DNAJB9 positivity in fibrillary deposits, detectable via immunohistochemistry (IHC), has emerged as a valuable diagnostic tool. This marker can facilitate FGN diagnosis in the absence of EM or in cases where EM findings alone do not clearly differentiate amyloid, ITGN, or FGN based on fibril diameter or Congo red staining properties [
7,
8].
In patients with RPGN, rapid progression to acute kidney injury (AKI) may occur within weeks to months, underscoring the importance of early and accurate diagnosis. However, recognizing FGN remains challenging, as its clinical manifestations and laboratory findings can overlap with those of more common glomerulopathies, including IgA nephropathy (IgAN), membranoproliferative glomerulonephritis (MPGN), and even amyloidosis [
8].
From a therapeutic perspective, there is no clear consensus on the optimal management of FGN. This is largely due to the scarcity of controlled clinical studies and the heterogeneous nature of the disease. Although various immunosuppressive strategies have been employed, their effectiveness remains variable and is often associated with significant adverse effects. The identification of specific biomarkers and the development of targeted therapies are priority areas for future research [
10].
The prognosis of FGN varies depending on factors such as the severity of glomerular involvement, the presence of renal dysfunction at the time of diagnosis, and the response to treatment. In the absence of a standardized therapeutic approach, management is primarily supportive, focusing on symptom control and immunosuppressive therapies, including corticosteroids, cyclophosphamide, mycophenolate mofetil, and rituximab (RTX). However, the response to immunosuppressive therapy is often suboptimal, and a significant proportion of patients progress to end-stage renal disease (ESRD), ultimately requiring dialysis or kidney transplantation [
8].
We present a case series of three patients with FGN, each exhibiting a distinct clinical course that underscores the heterogeneous nature of the disease. One patient developed RPGN secondary to FGN, a rare but clinically significant complication that highlights the potential aggressiveness of this condition. Below, we detail the clinical, histological, and therapeutic aspects of each case, emphasizing the diagnostic and treatment challenges associated with FGN.
3. Discussion
We present a series of three cases of FGN, in which we review the clinical, histopathological, and therapeutic data, as well as the evolution of each case. FGN is an immune complex-mediated disease that is idiopathic in most cases and is a rare entity but when diagnosed quickly. Diagnosis primarily relies on histopathological findings, with the identification of non-amyloid fibrils that are randomly arranged, unbranched, and approximately 20 nm in diameter on EM, serving as its hallmark [
8]. It is a rare condition, accounting for less than 1% of kidney biopsies, yet it has a significant clinical impact due to its high progression rate to end-stage renal disease (ESRD) [
8].
FGN was first described by Rosenmann and Eliakim, who reported a case of nephrotic syndrome associated with the deposition of amyloid-like material [
11]. In 1987, Alpers et al. [
12] reported eight cases of patients with renal dysfunction, most of whom presented with nephrotic-range proteinuria. They coined the term “fibrillary nephritis” and characterized the fibrils as unbranched, randomly arranged structures in the mesangium and capillary walls, measuring approximately 20 nm in diameter.
The precise pathophysiology of FGN remains uncertain. However, there is evidence suggesting a genetic predisposition to the disease [
13]. FGN is an immune complex glomerulonephritis (GN), where immune complexes polymerize into fibrils due to their relative homogeneity, possibly related to the frequent IgG4 subclass restriction observed in FGN. In 2018, a better understanding of FGN was achieved when DNAJB9 was identified as being overexpressed in FGN glomeruli. DNAJB9 functions as a cochaperone to binding immunoglobulin protein, assisting in protein folding and the degradation of misfolded proteins, a process termed the “unfolded protein response.” DNAJB9 was shown to colocalize with IgG and components of the classical complement pathway in glomeruli, supporting the theory that it may act as an autoantigen in FGN, serving as a precursor that traps immunoglobulins and triggers an autoimmune response. An alternative theory suggests that DNAJB9 is not an autoantigen but rather a binding protein for misfolded IgG molecules, leading to their aggregation and deposition in the kidney as non-amyloid fibrils. Neither of these two theories has been confirmed to date [
13]. It is likely that the production of DNAJB9 occurs outside the kidney, as the disease tends to recur in allografts and evidence of fibril deposition has been found in extrarenal organs; however, the exact site of production remains unknown [
13]. While DNAJB9 staining assists diagnostic accuracy in FGN, staining levels do not so far correlate with clinical responses to rituximab or renal outcomes, suggesting the need for additional studies or that DNAJB9 use could be primarily as a diagnostic marker, at least until further validation as a prognostic or therapeutic indicator is accomplished.
In our series, all cases were presented with microscopic hematuria. Schober et al. [
14] reported a series of 42 patients with FGN, of whom 95% had hematuria. Additionally, the three patients of our series had proteinuria, with one meeting the criteria for nephrotic syndrome. Rosenstock et al. [
2] reported a series of 61 patients with FGN, all of whom presented with proteinuria, and 52% had nephrotic syndrome at the time of diagnosis. In our series, only one of the three patients had renal dysfunction, which differs from the findings in the literature. Andeen et al. [
3] reported a series of 66 patients with FGN, in which over 80% had renal dysfunction at presentation. All three patients in our series had a history of hypertension, consistent with findings by Gambella et al. [
7], who reported a cohort of 77 patients with FGN, in which hypertension was the most common clinical condition at diagnosis (34 cases).
Patient 1 of our series did not have any underlying diagnosis likely to be pathogenetically associated with the development of FGN; however, patient 2 and patient 3 had medical histories that could potentially be related to FGN. Patient 2 had a history of urothelial carcinoma. Malignant neoplasms, more frequently solid tumors than hematologic malignancies, have been reported in 4% to 23% of patients with FGN, with a wide variety of cancer types observed and no clear predilection for any specific type. The diagnosis of cancer may precede or follow the diagnosis of FGN [
5]. There is ongoing debate as to whether FGN represents a true paraneoplastic phenomenon or whether the increased prevalence of cancer is solely attributable to the advanced median age of FGN patients.
Patient 3 was diagnosed with T2DM, which is reported in 5.6% to 28% of patients with FGN. A possible association between T2DM and FGN has been proposed, based on the concept that accelerated protein glycosylation in diabetics and the accumulation of advanced glycation end products could cross-link with other structural and circulating proteins, thereby predisposing individuals to the development of FGN; however, this hypothesis has not yet been confirmed [
15].
The case of patient 3 underscores the importance of early diagnosis and aggressive treatment in rapidly progressive forms. The literature indicates that patients with RPGN have poorer outcomes due to the presence of crescents and fibrinoid necrosis in over 50% of glomeruli, as observed in this case [
16]. Studies by Hogan et al. emphasize that a combination of cyclophosphamide and corticosteroids remains the cornerstone of treatment in such severe cases, although the risk of progression to ESRD remains high [
17].
In contrast, patients 1 and 2 had more favorable outcomes. Both responded positively to rituximab treatment, consistent with findings by Attieh et al. [
8], who reported that patients with preserved renal function and a lower fibrillar burden achieved better clinical outcomes. This highlights the importance of considering rituximab as a therapeutic option in selected patients despite the lack of standardized guidelines [
18]. However, studies by Hogan et al. and Andeen et al. reported that up to 50% of patients with FGN progress to ESRD within an average of 2–4 years. While kidney transplantation is a viable option, graft recurrence poses a significant challenge, emphasizing the need for novel therapeutic strategies [
17,
18].
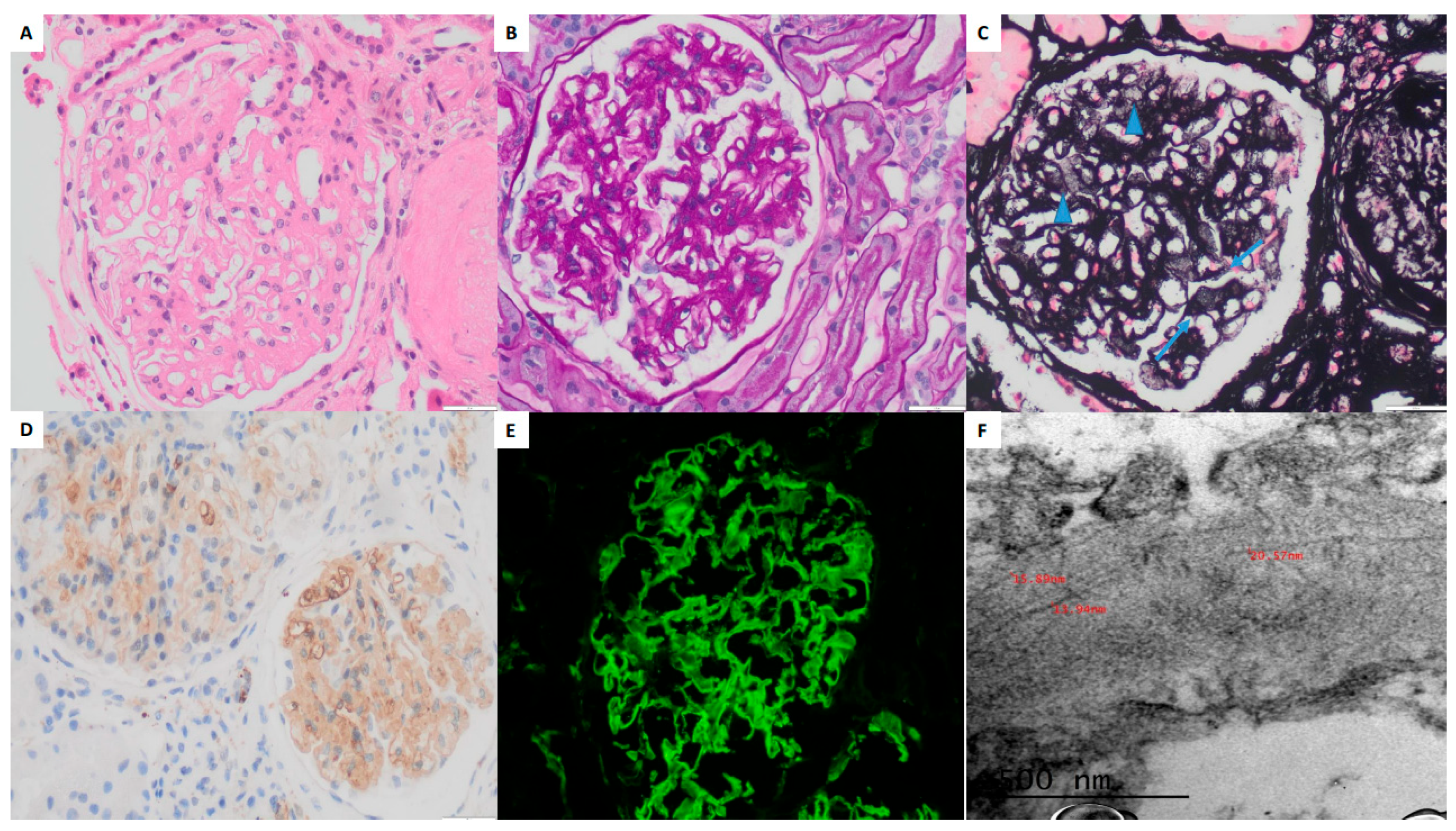
FGN can present with various histopathological patterns on light microscopy (LM), ranging from mesangial expansion without hypercellularity (MesGN) to a membranoproliferative glomerulonephritis (MPGN) pattern. Congo red staining is typically negative [
19]. In our three cases, electron microscopy (EM) revealed mesangial expansion and capillary wall thickening. Patient 3 exhibited an MPGN pattern of glomerular injury, eventually progressing to crescentic glomerulonephritis (GN). Additionally, this patient demonstrated Congo red positivity, a finding observed in approximately 5% of FGN cases. In general, the basis for congophilia in congophilic FGN remains unknown. However, considering the resemblance of the fibrillary quaternary structure between FGN and amyloid deposits observed by transmission EM, it is conceivable that FGN and amyloid fibrils may share certain similarities in their secondary structure [
20]. Alexander et al. described a case series of 18 patients with Congo red-positive FGN, reporting that these patients frequently had underlying conditions, particularly monoclonal gammopathy and hepatitis C virus (HCV) infection [
21]. In patient 3, the presence of congophilia prompted an extensive search for an underlying explanation, which remained inconclusive despite thorough investigation ruling out the following: (1) monoclonal gammopathy, (2) infection, and (3) malignancy. Thus, in our patient 3, the coexistence of congophilia and the clinical course of RPGN appears to be casual rather than causal.
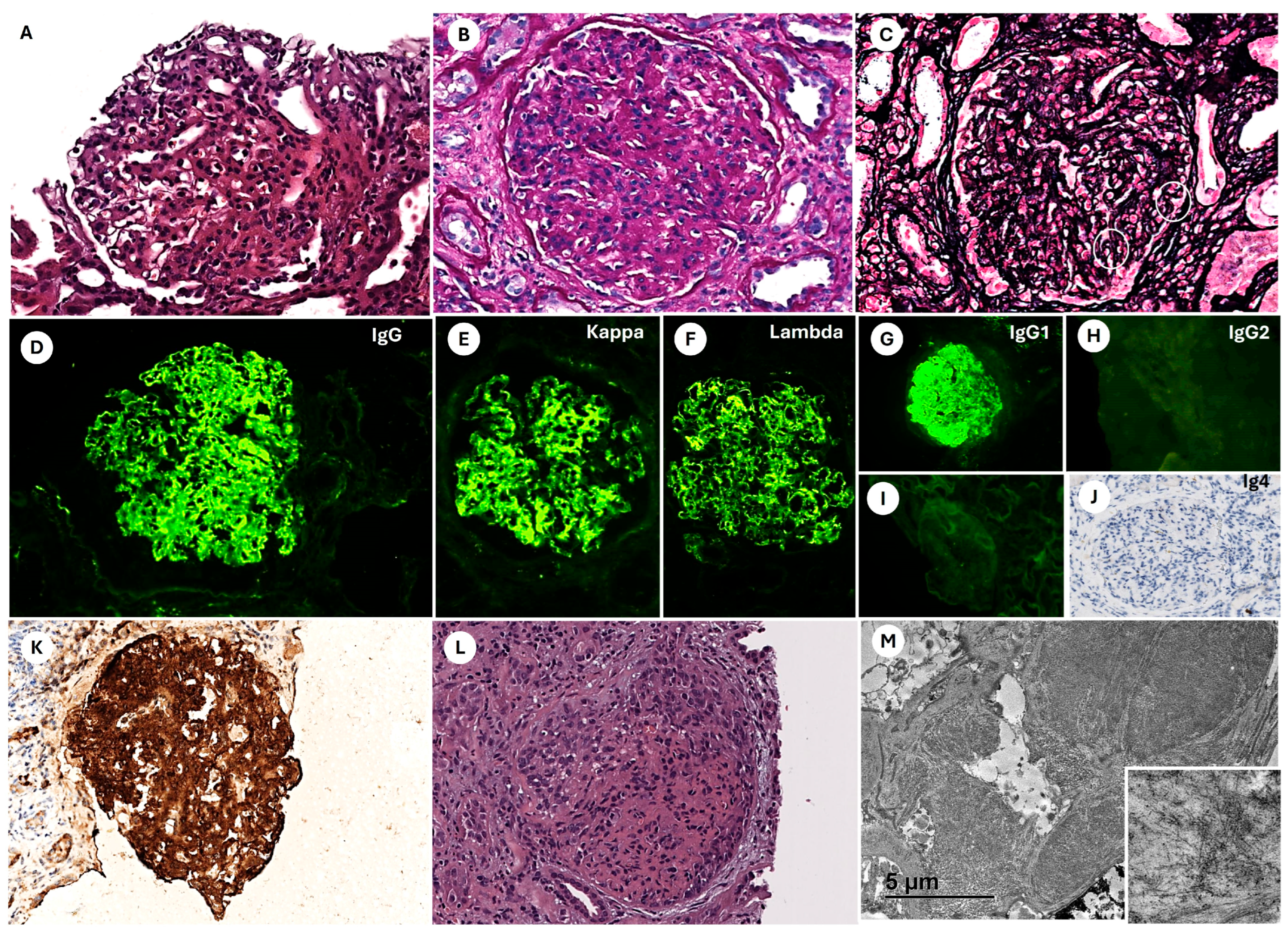
The most frequent IF findings in FGN patients are capillary wall and mesangial staining for polyclonal IgG and C3, with or without C1q. Most cases exhibit IgG4 dominance or IgG4 and IgG1 codominance within the IgG subclass [
22]. In our series, all three cases were positive for IgG, C3, C4, C1q, kappa, and lambda by IF. Nasr et al. reported a series of 66 FGN patients, in which 100%, 92%, 60%, 85%, and 90% of cases were positive for IgG, C3, C1q, kappa, and lambda, respectively [
5]. Gambella et al., in their series of 77 patients, had appropriate material for expression evaluation in 67 cases for IgG, 66 for C3, and 55 for light and heavy chains [
7]. Andeen et al. reported that only 6% of cases exhibited monoclonal staining (one light chain positive [κ or λ] with absent staining for the other) on IF [
13].
In our series, immunohistochemistry (IHC) demonstrated strong DNAJB9 positivity in all three cases, localized to the mesangium and capillary loops. DNAJB9-IHC staining has a reported sensitivity of 98% and specificity of 99% as a histological marker for FGN. DNAJB9 biomarker identification represents a major advancement in diagnosing FGN, revolutionizing its differentiation from other fibrillary glomerulopathies. DNAJB9 exhibits near-perfect sensitivity and specificity, facilitating early and accurate diagnosis. Furthermore, its potential role in the disease’s pathogenesis may provide opportunities for targeted therapies in the future [
19]. This staining is useful in distinguishing Congo red-positive FGN from amyloidosis and differentiating FGN from other conditions such as diabetic fibrillosis and collagen-fibrin glomerulopathy [
23]. In Gambella et al.’s series, DNAJB9 was detected in 73 of the 74 available cases of FGN, with predominantly homogeneous and strong positivity in 68 cases, while 5 cases exhibited moderate and scattered expression in mesangial areas and occasionally in the glomerular basement membrane (GBM) [
7].
EM findings in FGN typically include randomly oriented, straight, non-branching fibrils measuring approximately 20 nm in diameter (range: 12–24 nm), primarily deposited within the mesangium and occasionally extending along the GBM. Podocyte foot process effacement is frequently observed in regions with fibril accumulation [
24]. In our series, all three cases demonstrated non-branching fibrillar deposits, randomly arranged and measuring up to approximately 20 nm in diameter, within the mesangium and basement membranes. Additionally, extensive podocyte foot process effacement was observed. Javaugue et al. reported a series of 27 FGN patients, where EM revealed extracellular fibrils predominantly within the mesangium and subepithelial regions, with occasional extension into the dense lamina of the GBM. The mean fibril diameter was 15.4 nm (range: 7–26 nm) [
25]. Similarly, Alexander et al. described 18 FGN cases in which all patients exhibited glomerular deposits of non-branching, randomly oriented fibrils affecting the mesangium (100% of cases) and GBM (17 cases). The fibril size was measured in 15 cases, with a mean diameter of 14 nm (range: 11–18 nm) [
21].
FGN and immunotactoid glomerulonephritis (ITGN) share clinical and pathological features but are distinct entities. FGN is characterized by fibrillar deposits measuring 12–24 nm in diameter, whereas ITGN deposits are microtubular, larger (15–50 nm), and exhibit a different structural organization [
23]. Clinically, ITGN is more strongly associated with B-cell dyscrasias such as multiple myeloma or chronic lymphocytic leukemia, whereas FGN is predominantly idiopathic [
19,
23]. The differential diagnosis is further aided by DNAJB9 staining, which is positive in FGN but negative in ITGN, enabling precise differentiation even in the absence of electron microscopy [
23]. These differences highlight the importance of integrating advanced diagnostic tools into the clinical management of these conditions.
Regarding treatment, there are no prospective randomized controlled trials for FGN, and currently, no standardized therapeutic guidelines exist. Despite the lack of conclusive evidence supporting the benefit of immunosuppression in slowing renal progression, its use remains common. Various therapeutic strategies have been evaluated, ranging from corticosteroid monotherapy to combinations with cyclophosphamide, mycophenolate mofetil, rituximab (RTX), or calcineurin inhibitors (CNIs), with heterogeneous results. Few studies have described a beneficial impact on the disease course with steroids alone; as described by Dickenmann et al., three patients treated with prednisone showed an impressive reduction in proteinuria, which completely remitted in two cases, and no signs of declining renal function during the observation period [
26]. High-dose steroids and cyclophosphamide have shown relative success in some isolated case reports, mainly in cases of FGN with MPGN pattern, especially if there are crescents, as reported by Mahajan et al. [
27] and Blume et al. [
28], who described the cases of a patient with FGN MPGN and another with necrotizing FGN with good therapeutic response, respectively
Given that glomerular deposits in FGN consist of polyclonal IgG, some authors have proposed that FGN represents an autoimmune disease that may respond to RTX, a monoclonal anti-CD20 antibody [
29]. In a study by Hogan et al. involving 12 FGN patients with a median baseline creatinine of 2.1 mg/dL and proteinuria of 4497 mg/day, RTX treatment was associated with no disease progression in 33% of patients after 38 months of follow-up [
17]. Similarly, Javaugue et al. reported long-term follow-up of 27 FGN patients, of whom 7 received RTX as part of their treatment regimen. One patient achieved complete renal remission, four achieved partial remission, and two had no response to RTX therapy. The median estimated glomerular filtration rate (eGFR) before treatment among the five responders was 77 mL/min/1.73 m
2 (range: 35–96 mL/min/1.73 m
2) [
25]. On the other hand, the absence of predictive biomarkers to identify patients who may benefit from immunosuppression further complicates treatment decisions, leaving the therapeutic approach dependent on clinical severity and individual histologic features. In our series, all three patients received renin–angiotensin–aldosterone system (RAAS) blockade and sodium–glucose cotransporter-2 (SGLT2) inhibitors. Patients 1 and 2 were treated with rituximab (RTX) shortly after diagnosis and continued therapy according to the prescribed regimen for three years, maintaining adequate renal function and proteinuria control. These positive outcomes may be related to their preserved renal function and the short time interval between diagnosis and the initiation of RTX. This aligns with findings from Hogan et al., who reported that patients who did not progress to ESRD had lower serum creatinine levels, higher eGFR, and a shorter median duration from diagnosis to treatment compared to those who progressed [
17].
Conversely, patient 3, who presented with an MPGN-pattern FGN and did not receive RTX early, subsequently developed RPGN and remains dialysis-dependent despite treatment with cyclophosphamide, corticosteroids, and later RTX. While RTX has emerged as a promising therapy, its efficacy in patients with advanced renal dysfunction remains questionable. This supports Hogan’s hypothesis that RTX may only prevent or delay disease progression in patients with preserved renal function and may not be effective beyond a “point of no return” in renal dysfunction [
17]. Furthermore, the MPGN pattern itself represents an adverse prognostic factor, as it is associated with higher serum creatinine levels, greater proteinuria, and a shorter median time to ESRD, along with sclerosing histological patterns [
30]. Recently, Erikson et al. [
30] conducted a prospective pilot clinical trial in which 11 patients with idiopathic FGN received two cycles of RTX (1 g each) administered two weeks apart initially and then repeated at six months. The primary outcome was defined as preservation of renal function at 12 months, indicated by stable or increased creatinine clearance (CrCl). The secondary outcome included achieving complete remission (CR), defined as proteinuria <300 mg/24 h, or partial remission (PR), defined as proteinuria <3 g/24 h with at least a 50% reduction in proteinuria. Serum DNAJB9 levels were also measured at baseline and at 6 and 12 months. CrCl remained stable during follow-up, from 47.7 mL/min/1.73 m
2 at baseline to 43.7 mL/min/1.73 m
2 at 12 months (P = 0.15). Proteinuria decreased from 4.43 g/24 h (range: 1.6–5.53 g/24 h) at baseline to 1.9 g/24 h (range: 0.46–5.26 g/24 h) at 12 months, but the reduction did not reach statistical significance (P = 0.06). None of the patients achieved CR, while 3 of 11 achieved PR. Additionally, no significant changes in DNAJB9 levels were observed following RTX treatment. The study concluded that treatment with two cycles of RTX over six months was associated with stabilization of renal function but did not significantly improve renal function or proteinuria.
Currently, two clinical trials are underway to evaluate the efficacy of anti-CD20 therapy in FGN. The first study (NTC06680349), an Italian trial, is assessing the clinical and histological effects of two RTX-based regimens in DNAJB9-positive FGN. It compares RTX monotherapy (375 mg/m2 every four weeks) with intensive B-cell depletion therapy (IBCDT), which includes RTX (375 mg/m2 every four weeks followed by two additional doses at one and two months), cyclophosphamide (two 10 mg/kg pulses, adjusted for renal function, on days 4 and 17), and methylprednisolone (three bolus doses of 15 mg/kg) followed by oral prednisone (initial dose of 50 mg, gradually tapered over four months). The primary outcome will be the mean renal response at three and six months, defined as CR (proteinuria reduction to <0.5 g/day with stable renal function [<20% increase in serum creatinine]) or PR (≥50% reduction in proteinuria with stable renal function). Recruitment was completed in November 2024, with results pending. The second study, currently in the recruitment phase, aims to determine the efficacy and safety of obinutuzumab in the treatment of FGN. It is a single-center, phase 2, open-label trial evaluating obinutuzumab (NTC06295770). Patients with biopsy-proven FGN, proteinuria >1 g/24 h, and eGFR ≥20 mL/min/1.73 m2 will receive obinutuzumab at a dose of 1 g IV on day 1 and 1 g IV on day 15, followed by a second identical cycle at month 6. The primary outcome will be changes in proteinuria from baseline to 12 months post-treatment. Secondary outcomes include proteinuria reduction at six months, CR rate (proteinuria <0.5 g/24 h with no more than a 20% decrease in eGFR), PR rate (≥50% proteinuria reduction with proteinuria <3.5 g/24 h and no more than a 20% decrease in eGFR), improvement in serum albumin levels, stabilization of renal function (defined as no more than a 20% decrease in eGFR at six and 12 months), and the incidence of serious adverse events (SAEs), including severe infections such as urinary tract infections (UTIs), pyelonephritis, pneumonia, or other systemic infections requiring hospitalization.
The prognosis of FGN remains poor, with up to 50% of patients progressing to ESRD within two years of diagnosis [
31]. In cases where renal replacement therapy (RRT) becomes necessary, kidney transplantation may be a viable option; however, the risk of recurrence remains a significant concern [
32]. Although the pathophysiology of FGN is not entirely understood, a link between DNAJB9 and fibril formation was suggested [
33]. The development of targeted therapies aimed at inhibiting DNAJB9 formation or binding, tested in prospective controlled trials, could potentially alter the disease course.