Vitamin D Serum Levels and the Development of Intensive Care Unit-Acquired Weakness: Insights from a COVID-19 Intensive Care Cohort
Abstract
1. Introduction
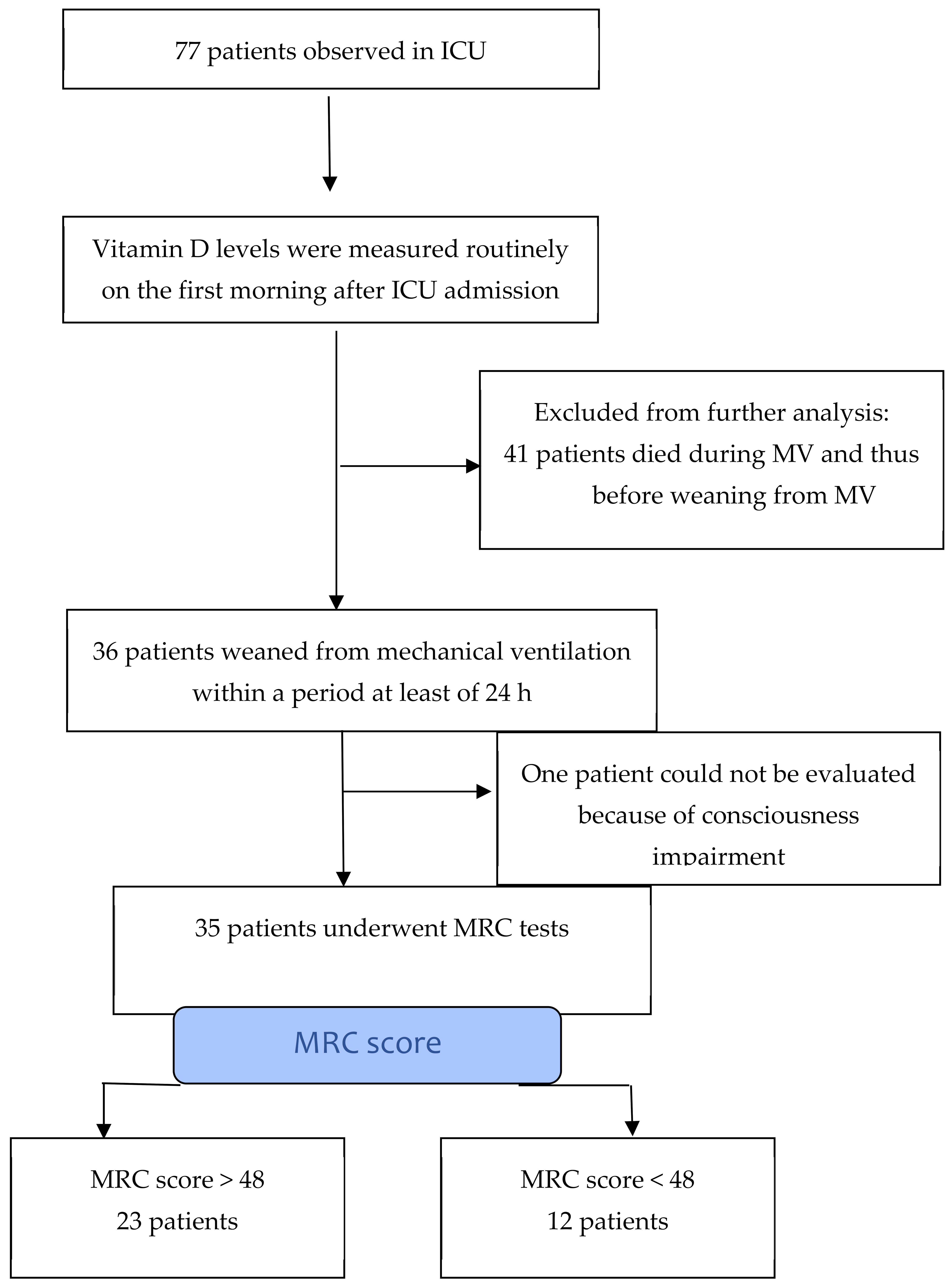
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yang, X.; Yang, L.; Zou, X.; Wang, Y.; Wu, Y.; Zhou, T.; Yuan, Y.; Qi, H.; Fu, S.; et al. Clinical course and predictors of 60-day mortality in 239 critically ill patients with COVID-19: A multicenter retrospective study from Wuhan, China. Crit. Care 2020, 24, 394. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health (US). Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/34003615/ (accessed on 28 July 2024).
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef] [PubMed]
- Van Aerde, N.; Van den Berghe, G.; Wilmer, A.; Gosselink, R.; Hermans, G. Intensive care unit acquired muscle weakness in COVID-19 patients. Intensive Care Med. 2020, 46, 2083–2085. [Google Scholar] [CrossRef]
- Chuang, Y.-C.; Shiu, S.-I.; Lee, Y.-C.; Tsai, Y.-L.; Cheng, Y.-Y. Prevalence and Risk Factors of Intensive Care Unit-acquired Weakness in Patients With COVID-19: A Systematic Review and Meta-analysis. J. Intensive Care Med. 2024. [Google Scholar] [CrossRef]
- Fan, E.; Cheek, F.; Chlan, L.; Gosselink, R.; Hart, N.; Herridge, M.S.; Hopkins, R.O.; Hough, C.L.; Kress, J.P.; Latronico, N.; et al. An Official American Thoracic Society Clinical Practice Guideline: The Diagnosis of Intensive Care Unit–acquired Weakness in Adults. Am. J. Respir. Crit. Care Med. 2014, 190, 1437–1446. [Google Scholar] [CrossRef]
- Kress, J.P.; Hall, J.B. ICU-acquired weakness and recovery from critical illness. N. Engl. J. Med. 2014, 370, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Lantronico, N.; Bolton, C.F. Critical illness polyneuropathy and myopathy; a major cause of muscle weakness and paralysis. Lancet Neurol. 2011, 10, 931–941. [Google Scholar] [CrossRef]
- Bednarík, J.; Vondracek, P.; Dusek, L.; Moravcova, E.; Cundrle, I. Risk factors for critical illness polyneuromyopathy. J. Neurol. 2005, 252, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Hermans, G.; Van den Berghe, G. Clinical review: Intensive care unit acquired weakness. Crit. Care 2015, 19, 274. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hermans, G.; Van Aerde, N.; Meersseman, P.; Van Mechelen, H.; Debaveye, Y.; Wilmer, A.; Gunst, J.; Casaer, M.P.; Dubois, J.; Wouters, P.; et al. Five-year mortality and morbidity impact of prolonged versus brief ICU stay: A propensity score matched cohort study. Thorax 2019, 74, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.K.; Pohlman, A.S.; Hall, J.B.; Kress, J.P. Impact of early mobilization on glyceic control and ICU-acquired weakness in critically ill patients who are mechanically ventilated. Chest 2014, 146, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Bercker, S.; Weber-Carstens, S.; Deja, M.; Grimm, C.; Wolf, S.; Behse, F.; Busch, T.; Falke, K.J.; Kaisers, U. Critical illness polyneuropathy and myopathy in patients with acute respiratory distress syndrome. Crit. Care Med. 2005, 33, 711–715. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe, B.; Sharshar, T.; Lefaucheur, J.P.; Authier, F.J.; Durand-Zaleski, I.; Boussarsar, M.; Cerf, C.; Renaud, E.; Mesrati, F.; Carlet, J.; et al. Paresis acquired in the intensive care unit: A prospective multicenter study. JAMA 2002, 288, 2859–2867. [Google Scholar] [CrossRef]
- Chlan, L.L.; Tracy, M.F.; Guttormson, J.; Savik, K. Peripheral muscle strength and correlates of muscle weakness in patients receiving mechanical ventilation. Am. J. Crit. Care 2015, 24, e91–e98. [Google Scholar] [CrossRef]
- Wolfe, K.S.; Patel, B.K.; MacKenzie, E.L.; Giovanni, S.P.; Pohlman, A.S.; Churpek, M.M.; Hall, J.B.; Kress, J.P. Impact of vasoactive medications on ICU-acquired weakness in mechanically ventilated patients. Chest 2018, 154, 781–787. [Google Scholar] [CrossRef]
- Jeong, B.H.; Nam, J.; Ko, M.G.; Chung, C.R.; Suh, G.Y.; Jeon, K. Impact of limb weakness on extubation failure after planned extubation in medical patients. Respirology 2018, 23, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Gil, Á.; Plaza-Diaz, J.; Mesa, M.D. Vitamin D: Classic and Novel Actions. Ann. Nutr. Metab. 2018, 72, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, K.R.; Cruzat, V.; Carlessi, R.; Newsholme, P. Mechanisms of vitamin D action in skeletal muscle. Nutr. Res. Rev. 2019, 32, 192–204. [Google Scholar] [CrossRef] [PubMed]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, J.; Huang, M. Intensive care unit-acquired weakness: Recent insights. J. Intensive Med. 2023, 4, 73–80. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- López-Muñoz, P.; Beltrán, B.; Sáez-González, E.; Alba, A.; Nos, P.; Iborra, M. Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in IBD Patients. Nutrients 2019, 11, 1059. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Azizieh, F.; Alyahya, K.O.; Raghupathy, R. Association between levels of vitamin D and inflammatory markers in healthy women. J. Inflamm. Res. 2016, 9, 51–57. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mansournia, M.A.; Ostadmohammadi, V.; Doosti-Irani, A.; Ghayour-Mobarhan, M.; Ferns, G.; Akbari, H.; Ghaderi, A.; Talari, H.R.; Asemi, Z. The Effects of Vitamin D Supplementation on Biomarkers of Inflammation and Oxidative Stress in Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Horm. Metab. Res. 2018, 50, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Dashti, F.; Mousavi, S.M.; Larijani, B.; Esmaillzadeh, A. The effects of vitamin D supplementation on inflammatory biomarkers in patients with abnormal glucose homeostasis: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2021, 170, 105727. [Google Scholar] [CrossRef] [PubMed]
- Bychinin, M.V.; Klypa, T.V.; Mandel, I.A.; Andreichenko, S.A.; Baklaushev, V.P.; Yusubalieva, G.M.; Kolyshkina, N.A.; Troitsky, A.V. Low Circulating Vitamin D in Intensive Care Unit-Admitted COVID-19 Patients as a Predictor of Negative Outcomes. J. Nutr. 2021, 151, 2199–2205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the Prevention of Disease: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Bleizgys, A. Dosing: Basic Principles and a Brief Algorithm (2021 Update). Nutrients 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424, Erratum in: Clin. Nutr. 2024, 43, 1024. https://doi.org/10.1016/j.clnu.2024.03.004. [Google Scholar] [CrossRef] [PubMed]
- van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. N. Am. 2017, 46, 845–870. [Google Scholar] [CrossRef] [PubMed]
- Ventura, J.; Kahlon, S.; Vota, S.; Sadeghian, H. Determining of the Prevalence of Vitamin D Deficiency in a Neuromuscular Clinic (P06.212). Am. Acad. Neurol. 2013, 80 (Suppl. S7), P06.212. [Google Scholar] [CrossRef]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Köstenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lucidarme, O.; Messai, E.; Mazzoni, T.; Arcade, M.; du Cheyron, D. Incidence and risk factors of vitamin D deficiency in critically ill patients: Results from a prospective observational study. Intensive Care Med. 2010, 36, 1609–1611. [Google Scholar] [CrossRef]
- Azim, A.; Ahmed, A.; Yadav, S.; Baronia, A.K.; Gurjar, M.; Godbole, M.M.; Poddar, B.; Singh, R.K. Prevalence of vitamin D deficiency in critically ill patients and its influence on outcome: Experience from a tertiary care centre in North India (an observational study). J. Intensive Care 2013, 1, 14. [Google Scholar] [CrossRef]
- Hassanein, M.M.; Huri, H.Z.; Baig, K.; Abduelkarem, A.R. Determinants and Effects of Vitamin D Supplementation in Postmenopausal Women: A Systematic Review. Nutrients 2023, 15, 685. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mei, Z.; Hu, H.; Zou, Y.; Li, D. The role of vitamin D in menopausal women’s health. Front. Physiol. 2023, 14, 1211896. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Holick, M.F.; Matsuoka, L.Y.; Wortsman, J. Age, vitamin D, and solar ultraviolet. Lancet 1989, 2, 1104–1105. [Google Scholar] [CrossRef]
- Plotnikoff, G.A.; Quigley, J.M. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin. Proc. 2003, 78, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Glerup, H.; Mikkelsen, K.; Poulsen, L.; Hass, E.; Overbeck, S.; Andersen, H.; Charles, P.; Eriksen, E.F. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif. Tissue Int. 2000, 66, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Deeg, D.J.; Lips, P.; Longitudinal Aging Study Amsterdam. Low vitamin D and high parathyroid hormone levels as determinants of loss of muscle strength and muscle mass (sarcopenia): The Longitudinal Aging Study Amsterdam. J. Clin. Endocrinol. Metab. 2003, 88, 5766–5772. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.Y.; Bruyère, O. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: A systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2011, 22, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Antoniak, A.E.; Greig, C.A. The effect of combined resistance exercise training and vitamin D3 supplementation on musculoskeletal health and function in older adults: A systematic review and meta-analysis. BMJ Open 2017, 7, e014619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cochet, C.; Belloni, G.; Buondonno, I.; Chiara, F.; D’Amelio, P. The Role of Nutrition in the Treatment of Sarcopenia in Old Patients: From Restoration of Mitochondrial Activity to Improvement of Muscle Performance, a Systematic Review. Nutrients 2023, 15, 3703. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG). Nutritional interventions to improve muscle mass, muscle strength, and physical performance in older people: An umbrella review of systematic reviews and meta-analyses. Nutr. Rev. 2021, 79, 121–147. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Triantafyllidis, K.K.; Kechagias, K.S.; Mesinovic, J.; Witard, O.C.; Scott, D. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 1642–1652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, S.H.; Chen, K.H.; Chen, C.; Chu, W.C.; Kang, Y.N. The Optimal Strategy of Vitamin D for Sarcopenia: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 3589. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández-Lepe, M.A.; Miranda-Gil, M.I.; Valbuena-Gregorio, E.; Olivas-Aguirre, F.J. Exercise Programs Combined with Diet Supplementation Improve Body Composition and Physical Function in Older Adults with Sarcopenia: A Systematic Review. Nutrients 2023, 15, 1998. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Gulišija, J.; Čapkun, V.; Stipić, S.S. Rani i konačni ishod bolesnika oboljelih od mišićne slabosti stečene u COVID jedinici intenzivnog liječenja—Koliko precizno možemo procijeniti rani i konačni ishod bolesnika po odvajanju bolesnika od mehaničke ventilacije? (Early and final outcome of patients suffered from muscle weakness acquired in COVID Intensive Care Unit—How precise we can be about early and final outcome of patients at the time of the end of mechanical ventilation?). Liječ. Vjesn. 2022, 144, 15–21. [Google Scholar] [CrossRef]
- Moonen, H.P.F.X.; Strookappe, B.; van Zanten, A.R.H. Physical recovery of COVID-19 pneumosepsis intensive care unit survivors compared with non-COVID pneumosepsis intensive care unit survivors during post–intensive care unit hospitalization: The RECOVID retrospective cohort study. J. Parenter. Enter. Nutr. 2021, 46, 798–804. [Google Scholar] [CrossRef]
- Dalakas, M.C. Inflammatory myopathies: Update on diagnosis, pathogenesis and therapies, and COVID-19-related implications. Acta Myol. 2020, 39, 289–301. [Google Scholar] [CrossRef]
- Côrtes, M.F.; de Almeida, B.L.; Espinoza, E.P.S.; Campos, A.F.; do Nascimento Moura, M.L.; Salomão, M.C.; Boszczowski, I.; Freire, M.P.; de Carvalho, L.B.; Paranhos-Baccalà, G.; et al. Procalcitonin as a biomarker for ventilator associated pneumonia in COVID-19 patients: Is it an useful stewardship tool? Diagn. Microbiol. Infect. Dis. 2021, 101, 115344. [Google Scholar] [CrossRef]
- Pink, I.; Raupach, D.; Fuge, J.; Vonberg, R.-P.; Hoeper, M.M.; Welte, T.; Rademacher, J. C-reactive protein and procalcitonin for antimicrobial stewardship in COVID-19. Infection 2021, 49, 935–943. [Google Scholar] [CrossRef]
- Luan, Y.-Y.; Yin, C.-H.; Yao, Y.-M. Update Advances on C-Reactive Protein in COVID-19 and Other Viral Infections. Front. Immunol. 2021, 12, 720363. [Google Scholar] [CrossRef]
- Giannini, M.; Ohana, M.; Nespola, B. Similarities between COVID-19 syndrome and MDA5 syndrome: What can we learn for better care? Eur. Respir. J. 2020, 56, 2001618. [Google Scholar] [CrossRef]
- Gokhale, Y.; Patankar, A.; Holla, U. Dermatomyositis during COVID-19 pandemic (a case series): Is there a cause effect relationship? J. Assoc. Physicians India 2020, 68, 20–24. [Google Scholar]
- Mehan, W.A.; Yoon, B.C.; Lang, M. Paraspinal myositis in patients with COVID-19 infection. AJNR Am. J. Neuroradiol. 2020, 41, 1949–1952. [Google Scholar] [CrossRef]
- Caress, J.B.; Castoro, R.J.; Simmons, Z.; Scelsa, S.N.; Lewis, R.A.; Ahlawat, A.; Narayanaswami, P. COVID-19–associated Guillain-Barré syndrome: The early pandemic experience. Muscle Nerve 2020, 62, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Fragiel, M.; Miró, Ò.; Llorens, P.; Jiménez, S.; Piñera, P.; Burillo, G.; Martín, A.; Martín-Sánchez, F.J.; García-Lamberechts, E.J.; Jacob, J.; et al. Incidence, clinical, risck factors and outcomes of Guillan-Barre in COVID-19. Ann. Neurol. 2021, 89, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Taga, A.; Lauria, G. COVID-19 and the peripheral nervous system. A 2-year from the pandemic to the vaccine era. J. Peripher. Nerv. Syst. 2022, 27, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Keddie, S.; Pakpoor, J.; Mousele, C.; Pipis, M.; Machado, P.M.; Foster, M.; Record, C.J.; Keh, R.Y.S.; Fehmi, J.; Paterson, R.W.; et al. Epidemiological and cohort study finds no associantion between COVID-19 and GBS syndrome. Brain 2021, 144, 682–693. [Google Scholar] [CrossRef]
- Girgis, C.M.; Clifton-Bligh, R.J.; Hamrick, M.W.; Holick, M.F.; Gunton, J.E. The roles of vitamin D in skeletal muscle: Form, function, and metabolism. Endocr. Rev. 2013, 34, 33–83. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Banerjee, M.; Bhadada, S.K.; Shetty, A.J.; Singh, B.; Vyas, A. Vitamin D supplementation and clinical outcomes in COVID-19: A systematic review and meta-analysis. J. Endocrinol. Investig. 2021, 45, 53–68. [Google Scholar] [CrossRef]
- Schmidt, D.; Piva, T.C.; Glaeser, S.S.; Piekala, D.M.; Berto, P.P.; Friedman, G.; Sbruzzi, G. Intensive Care Unit-Acquired Weakness in Patients With COVID-19: Occurrence and Associated Factors. Phys. Ther. 2022, 102, pzac028. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Appleton, R.; Kinsella, J. Intensive care unit-acquired weakness. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 62–66. [Google Scholar] [CrossRef]
- Sharshar, T.; Bastuji-Garin, S.; Stevens, R.D.; Durand, M.-C.; Malissin, I.; Rodriguez, P.; Cerf, C.; Outin, H.; De Jonghe, B. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit. Care Med. 2009, 37, 3047–3053. [Google Scholar] [CrossRef]
- Ali, N.A.; O’Brien, J.M., Jr.; Hoffmann, S.P.; Phillips, G.; Garland, A.; Finley, J.C.W.; Almoosa, K.; Hejal, R.; Wolf, K.M.; Lemeshow, S.; et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am. J. Respir. Crit. Care Med. 2008, 178, 261–268. [Google Scholar] [CrossRef]
- Feng, H.; Zhan, Q.; Huang, X.; Zhai, T.; Xia, J.; Yi, L.; Zhang, Y.; Wu, X.; Wang, Q.; Huang, L. Risk factors and diagnostic methods of intensive care unit-acquired weakness. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 460–465. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Drury, R.E.; Camara, S.; Chelysheva, I.; Bibi, S.; Sanders, K.; Felle, S.; Emary, K.; Phillips, D.; Voysey, M.; Ferreira, D.M.; et al. Multi-omics analysis reveals COVID-19 vaccine induced attenuation of inflammatory responses during breakthrough disease. Nat. Commun. 2024, 15, 3402. [Google Scholar] [CrossRef] [PubMed]
- Latronico, N.; Bertolini, G.; Guarneri, B.; Botteri, M.; Peli, E.; Andreoletti, S.; Bera, P.; Luciani, D.; Nardella, A.; Vittorielli, E.; et al. Simplified electrophysiological evaluation of peripheral nerves in critically ill patients: The Italian multi-centre CRIMYNE study. Crit. Care 2007, 11, R11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Novak, K.R.; Nardelli, P.; Cope, T.C.; Filatov, G.; Glass, J.D.; Khan, J.; Rich, M.M. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. J. Clin. Investig. 2009, 119, 1150–1158. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Characteristics | Categories | Total | Non-ICU-AW Group | ICU-AW Group | p-Value |
|---|---|---|---|---|---|
| Sex | Male | 23 | 18 (78%) | 5 (42%) | 0.059 * |
| Female | 12 | 5 (22%) | 7 (58%) | ||
| Age (years) | 68 (48–77) | 66 (47–75) | 71 (58–77) | 0.038 † | |
| Vaccinated | Yes | 5 | 4 (17%) | 1 (8%) | 0.640 * |
| No | 30 | 19 (83%) | 11 (92%) | ||
| Number of days from SARS-CoV-2 infection to respiratory failure | 8 (2–17) | 9 (2–15) | 7.5 (6–17) | 0.888 † | |
| Comorbidities | Hypertension | 21 | 12 | 9 | 0.282 * |
| Diabetes mellitus | 12 | 5 | 7 | 0.059 * | |
| Cardiovascular disease | 3 | 1 | 2 | 0.266 * | |
| Cerebrovascular disease | 2 | 0 | 2 | 0.111 * | |
| Chronic lung disease | 3 | 3 | 0 | 0.536 * | |
| Malignant disease | 3 | 3 | 0 | 0.536 * | |
| Autoimmune disease | 0 | 0 | 0 | 1.000 * | |
| Number of comorbidities | No comorbidities | 10 | 9 | 1 | 0.113 * |
| 1 | 14 | 5 | 5 | 0.258 * | |
| 2 | 12 | 8 | 4 | 1.000 * | |
| ≥3 | 3 | 1 | 2 | 0.266 * | |
| APACHE II score | 9 (3–19) | 9 (3–16) | 10 (5–19) | 0.151 † | |
| SOFA score | 2 (2–7) | 2 (2–3) | 2 (2–7) | 0.144 † | |
| Vitamin D level | 25.3 (7.5–121) | 25.2 (12.3–121) | 17 (7.5–73.3) | 0.567 † |
| Outcomes | Description | Total | Non-ICU-AW Group | ICU-AW Group | p-Value |
|---|---|---|---|---|---|
| Duration of mechanical ventilation (days) | Until first weaning from mechanical ventilation | 8 (3–84) | 6 (3–54) | 13.5 (8–84) | <0.001 † |
| Weaning from ventilator (number of tries) | 1 | 21 (60%) | 18 (78%) | 3 (25%) | 0.004 * |
| ≥2 | 14 (40%) | 5 (22%) | 9 (75%) | ||
| Duration of mechanical ventilation (days) | Total time | 11.5 (4–94) | 7 (4–54) | 32 (8–94) | 0.007 † |
| Duration of mechanical ventilation less than 7 days in non-ICU-AW group and ICU-AW group | 14 (40%) | 14 (61%) | 0 (0%) | NA | |
| Tracheotomy | Yes | 15 (43%) | 6 (26%) | 9 (75%) | 0.011 * |
| No | 20 (57%) | 17 (74%) | 3 (25%) | ||
| Number of days in ICU (n) | 32.5 (8–98) | 20 (8–80) | 54 (14–98) | 0.008 † | |
| Number of days in ward | 18 (6–103) | 12 (6–81) | 45 (10–103) | 0.001 † | |
| Final outcome | Discharged home | 21 (60%) | 17 (74%) | 4 (33%) | 0.012 * |
| Discharged home with oxygenator | 8 (23%) | 5 (22%) | 3 (25%) | ||
| Mortal outcome during hospitalization | 6 (17%) | 1 (4%) | 5 (42%) |
| ICUAW | Vitamin D Level | |||
|---|---|---|---|---|
| <30 nmol/L | 30–50 nmol/L | 50–75 nmol/L | >75 nmol/L | |
| Yes | 8 | 1 | 3 | 0 |
| No | 12 | 8 | 2 | 1 |
| Vitamin D Level | p-Value | ||
|---|---|---|---|
| <30 nmol/L | >30 nmol/L | 0.488 * | |
| ICU-AW group | 8 | 4 | |
| Non-ICU-AW group | 12 | 11 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulišija, J.; Čapkun, V.; Golic, S.; Stojanović Stipić, S. Vitamin D Serum Levels and the Development of Intensive Care Unit-Acquired Weakness: Insights from a COVID-19 Intensive Care Cohort. Pathophysiology 2025, 32, 21. https://doi.org/10.3390/pathophysiology32020021
Gulišija J, Čapkun V, Golic S, Stojanović Stipić S. Vitamin D Serum Levels and the Development of Intensive Care Unit-Acquired Weakness: Insights from a COVID-19 Intensive Care Cohort. Pathophysiology. 2025; 32(2):21. https://doi.org/10.3390/pathophysiology32020021
Chicago/Turabian StyleGulišija, Jelena, Vesna Čapkun, Stefan Golic, and Sanda Stojanović Stipić. 2025. "Vitamin D Serum Levels and the Development of Intensive Care Unit-Acquired Weakness: Insights from a COVID-19 Intensive Care Cohort" Pathophysiology 32, no. 2: 21. https://doi.org/10.3390/pathophysiology32020021
APA StyleGulišija, J., Čapkun, V., Golic, S., & Stojanović Stipić, S. (2025). Vitamin D Serum Levels and the Development of Intensive Care Unit-Acquired Weakness: Insights from a COVID-19 Intensive Care Cohort. Pathophysiology, 32(2), 21. https://doi.org/10.3390/pathophysiology32020021







