Exploration of the Topical Nanoemulgel Bearing with Ferulic Acid and Essential Oil for Diabetic Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation and Characterization of Nanoemulsion
2.2. In Vitro Cell Line Studies
2.2.1. MTT Assay
2.2.2. Cellular Uptake Study
2.2.3. 2,7-Dichlorodihydrofluorescein (DCFDA) Staining
2.2.4. Acridine Orange and Ethidium Bromide (AO/EB) Dual Staining
2.2.5. Morphology Study
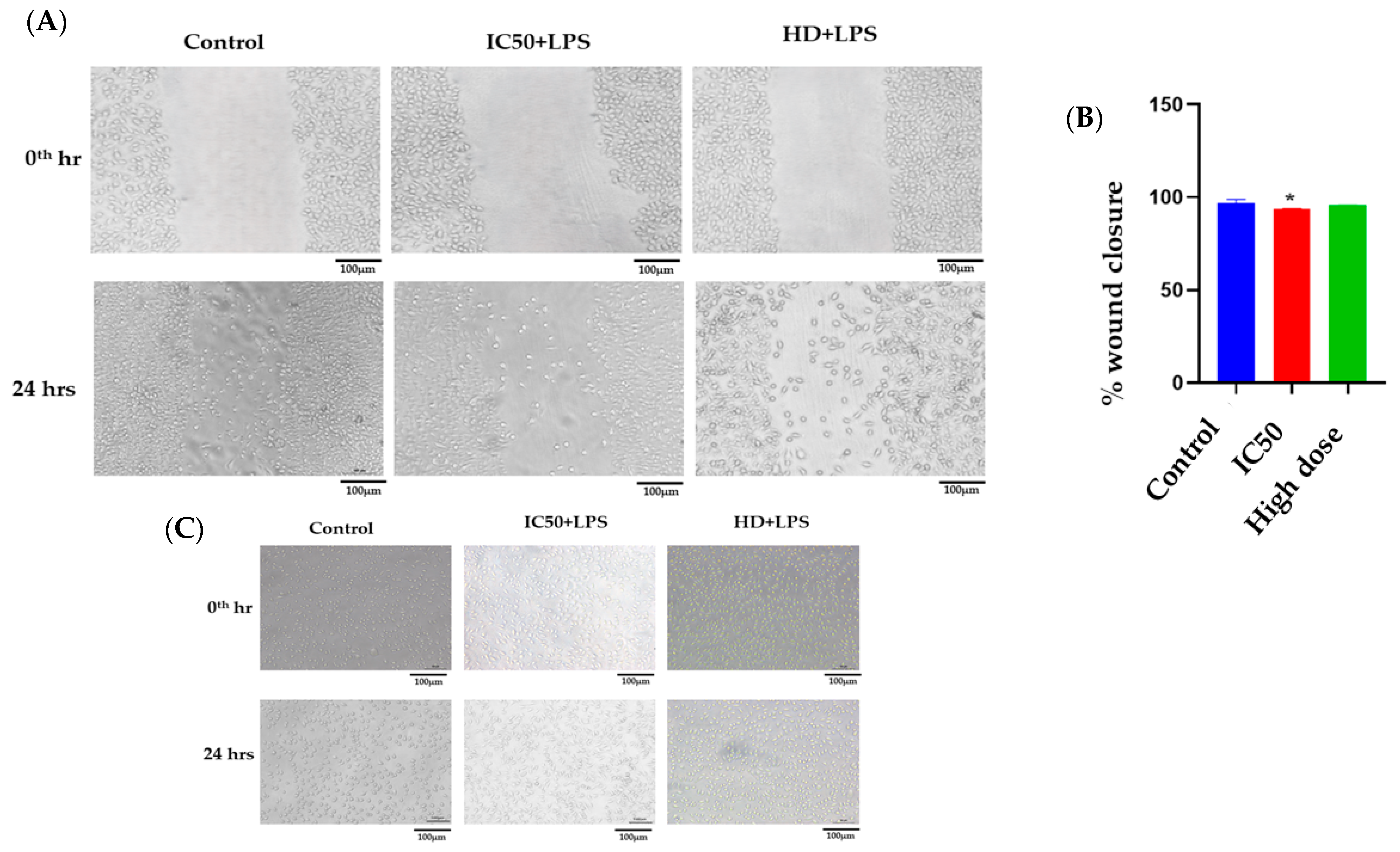
2.2.6. Scratch Assay
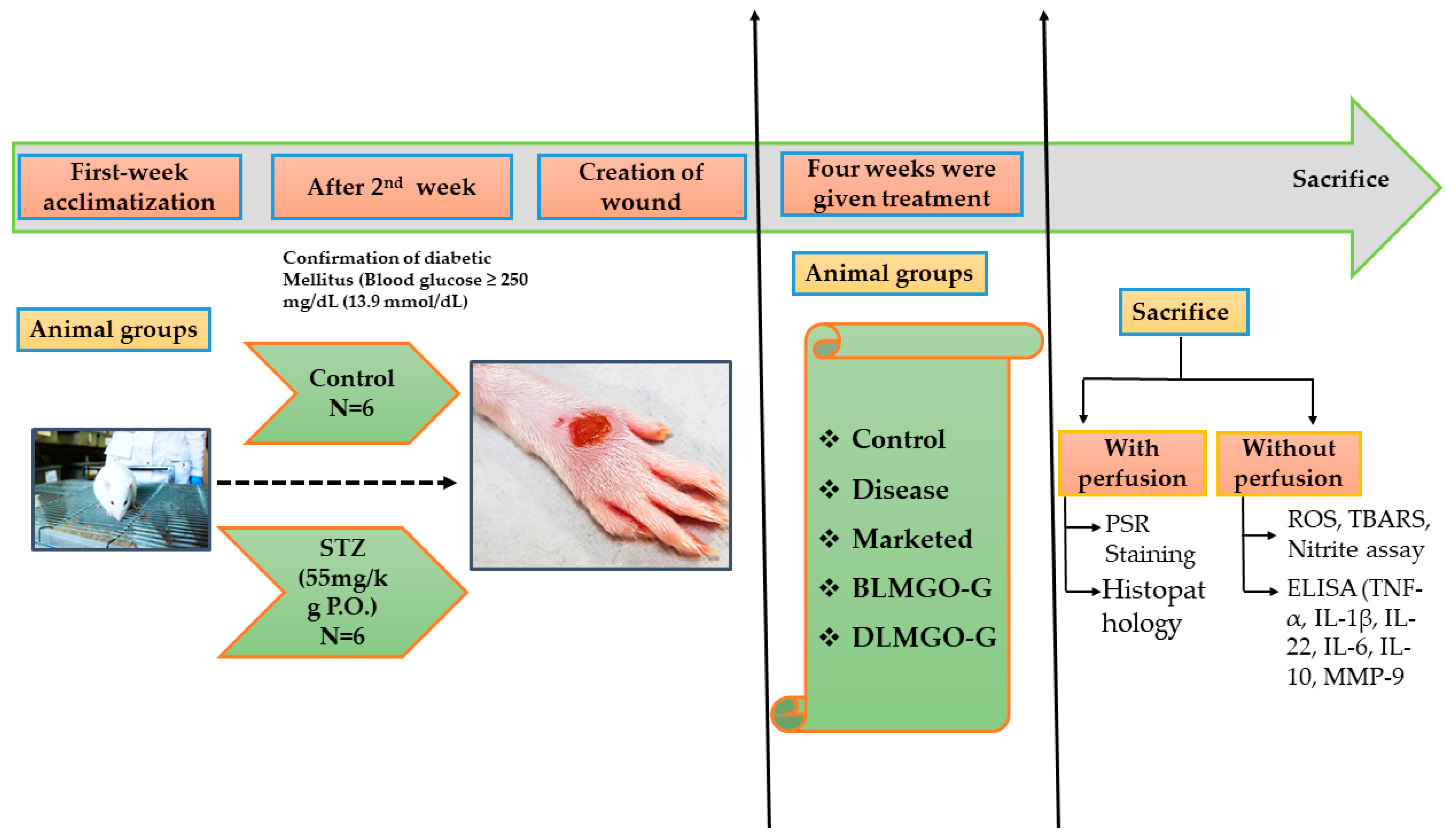
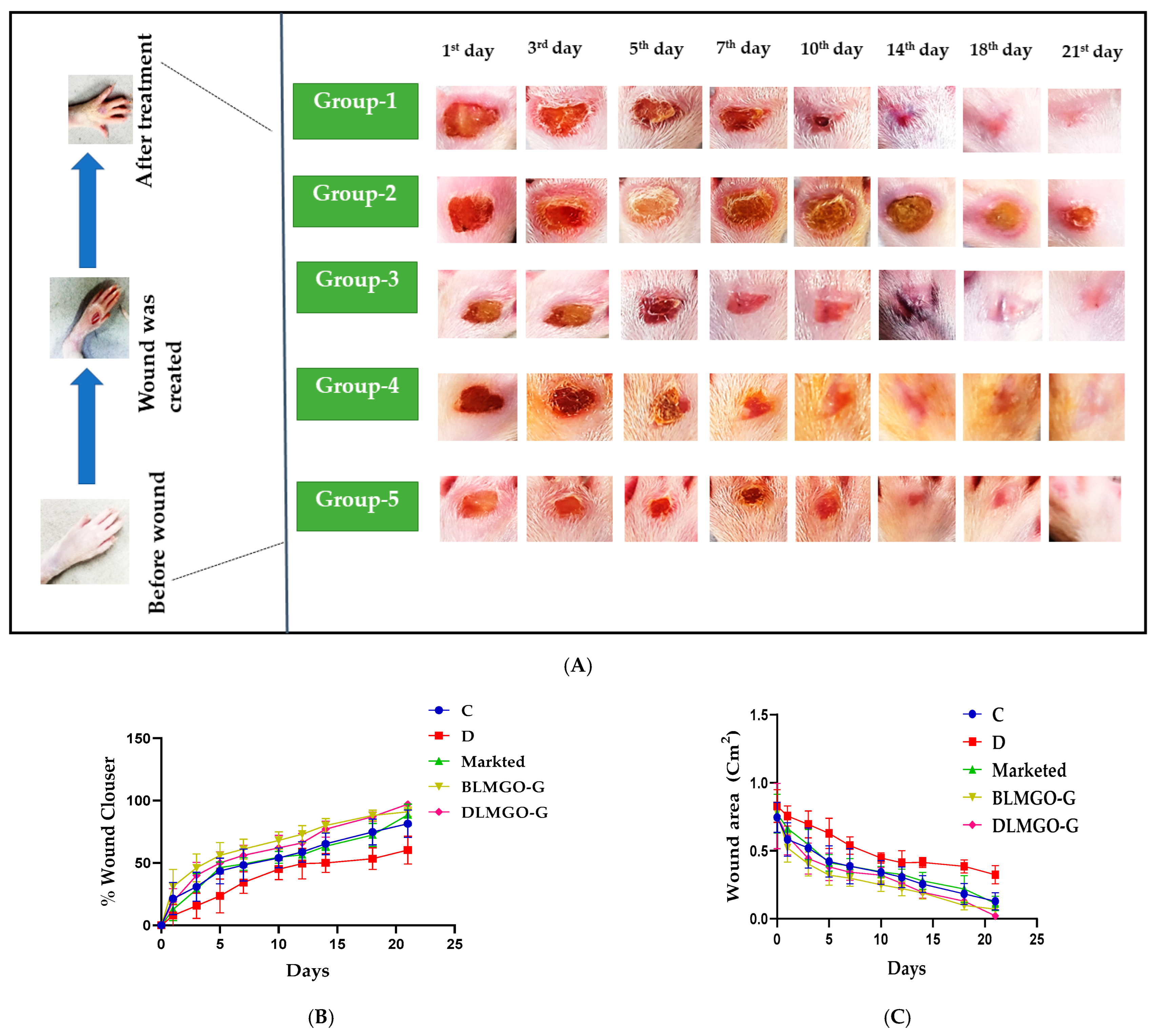
2.3. Evaluation of Wound Healing Processes Employing a Rat Excision Wound Model
2.3.1. Glucose Oxidase (GOD) Peroxidase (POD) Assay
2.3.2. Oxidative Stress Parameters
Nitrite Level
Thiobarbituric Acid Reactive Substances
2.3.3. Enzyme-Linked Immunosorbent (ELISA) Study of Pro- and Anti-Inflammatory Cytokines
2.3.4. Estimation of Metalloproteinase-9 (MMP-9) Levels
2.3.5. Histopathological Studies
2.3.6. Statistical Analysis
3. Results and Discussion
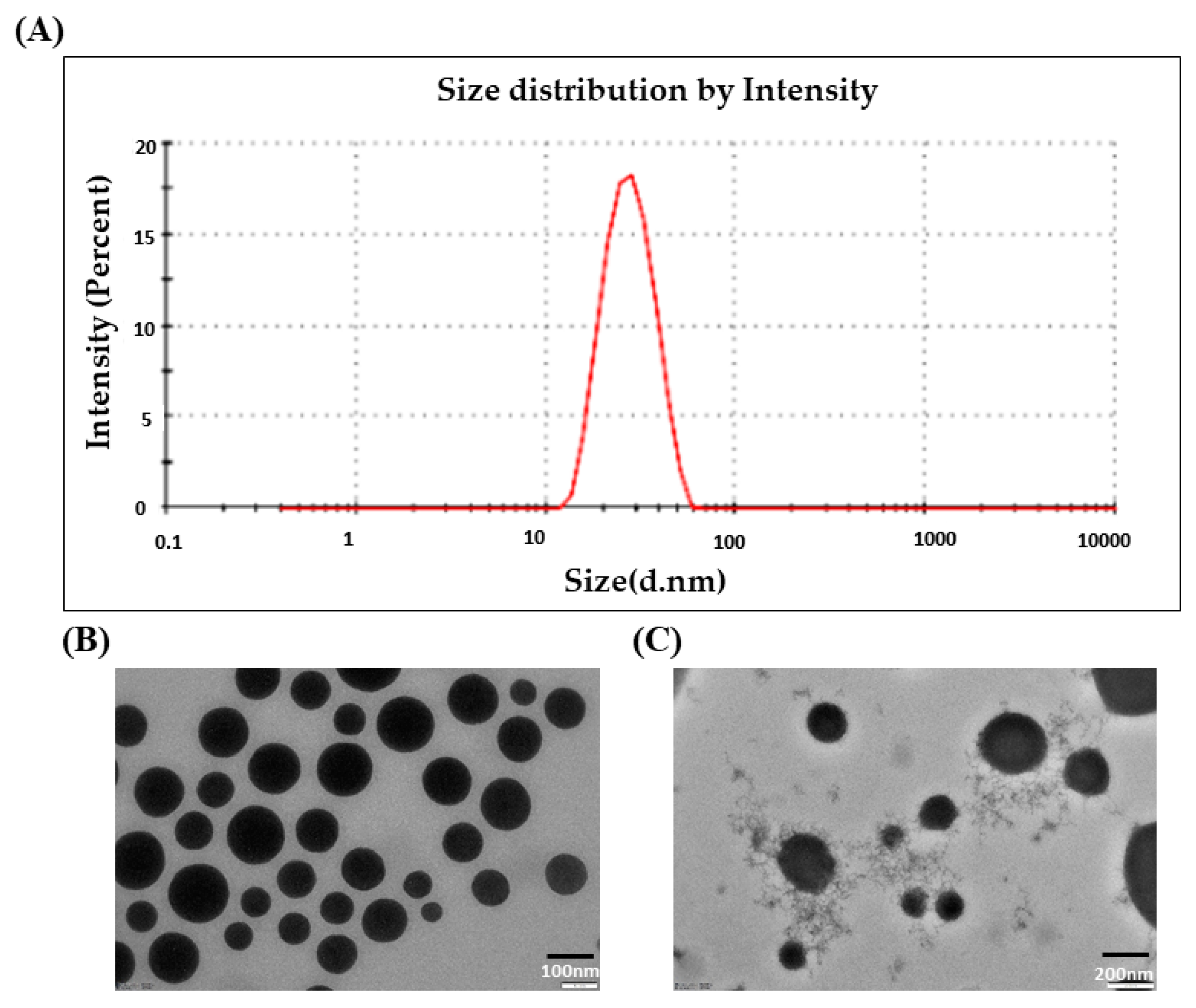
3.1. Droplet Size and PDI
3.2. Morphological Characterization of DLMGONE
3.3. Preparation, Characterization, and Morphology of FA-Loaded Emulgel of DLMGO-GEL
3.4. Morphological Characterization of DLMGO-G
3.5. In Vitro Antioxidant and Antimicrobial Assay
3.6. Cell Culture
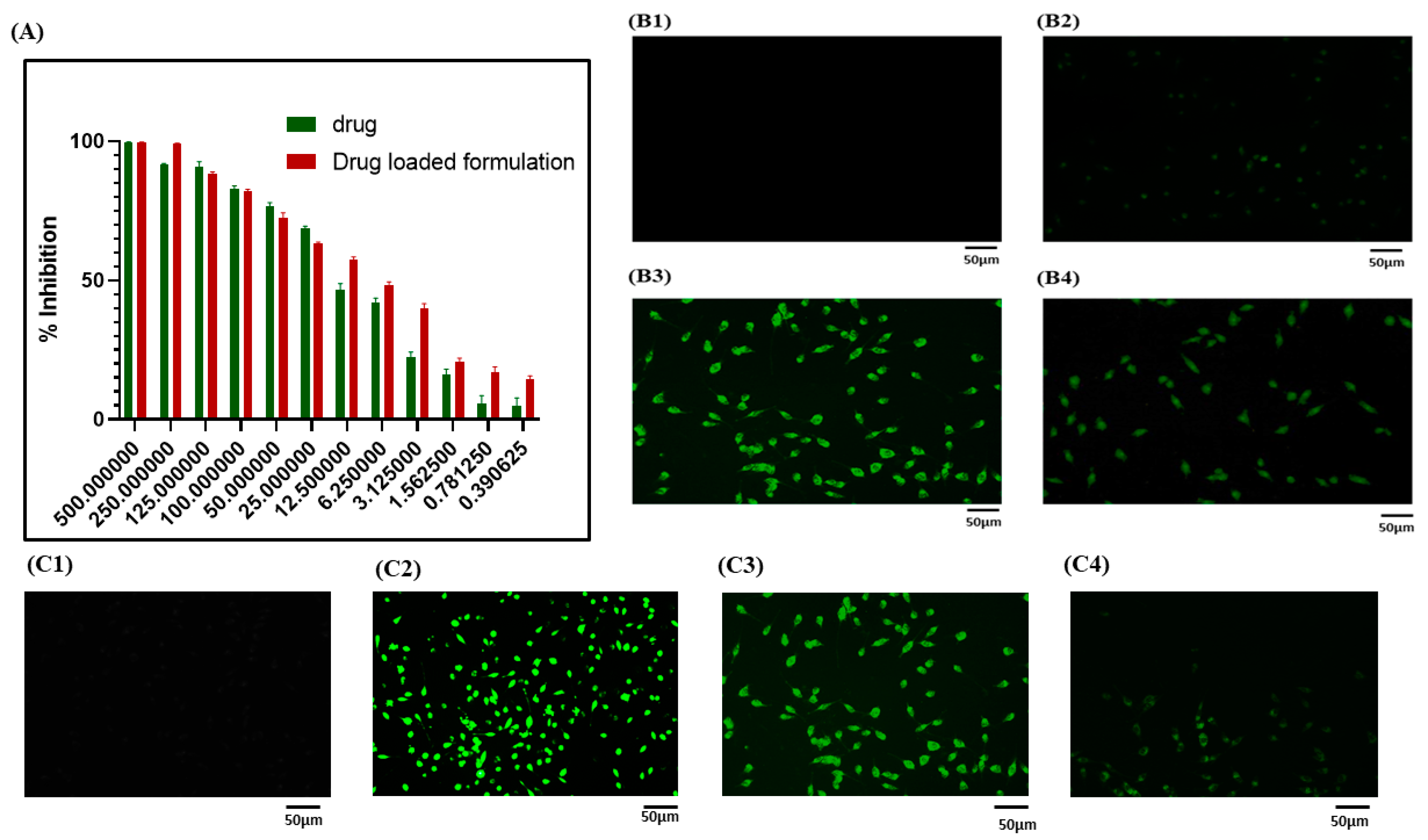
3.6.1. MTT Assay
3.6.2. Cellular Uptake Study
3.6.3. 2,7-Dichlorodihydrofluorescein (DCFDA) Staining
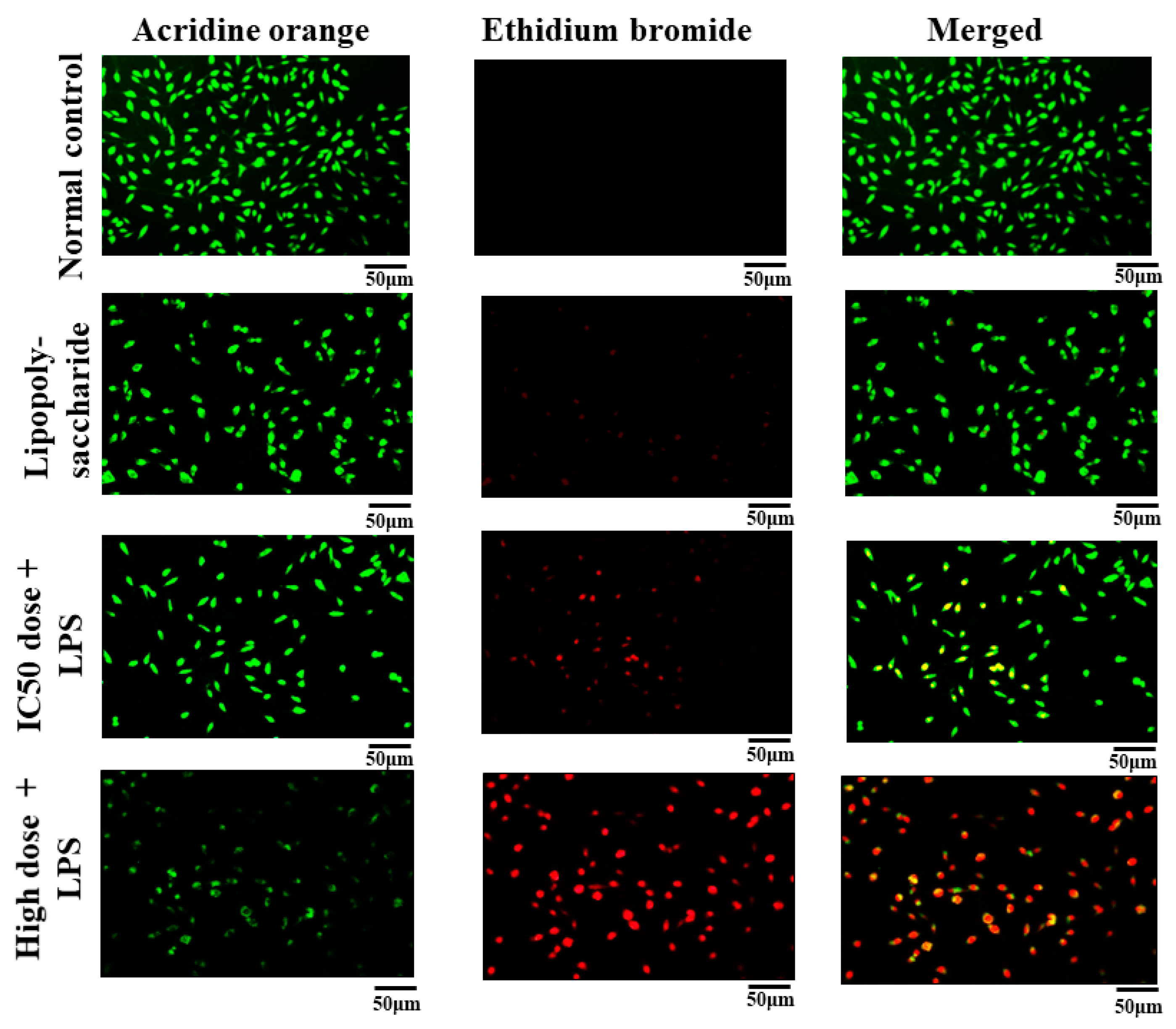
3.6.4. Acridine Orange and Ethidium Bromide (AO/EB) Staining
3.6.5. Morphology
3.6.6. Scratch Assay
3.7. Evaluation of Wound Healing Processes Employing a Rat Excision Wound Model
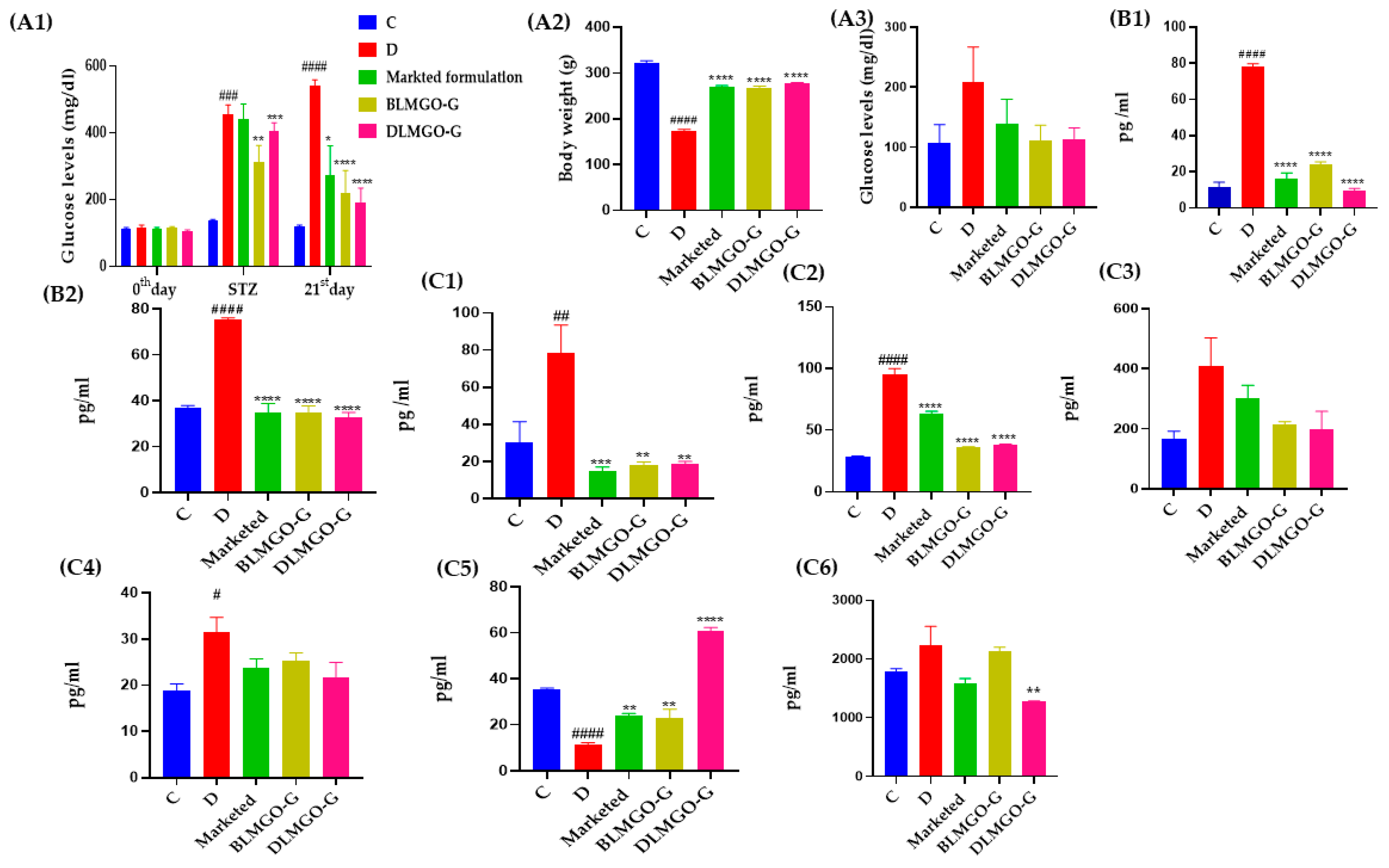
3.7.1. Effect of Body Weight and Blood Glucose Level
3.7.2. Oxidative Stress Parameters
3.7.3. Inflammatory and Anti-Inflammatory Markers
3.7.4. Estimation of MMP-9 Levels by ELISA Kit
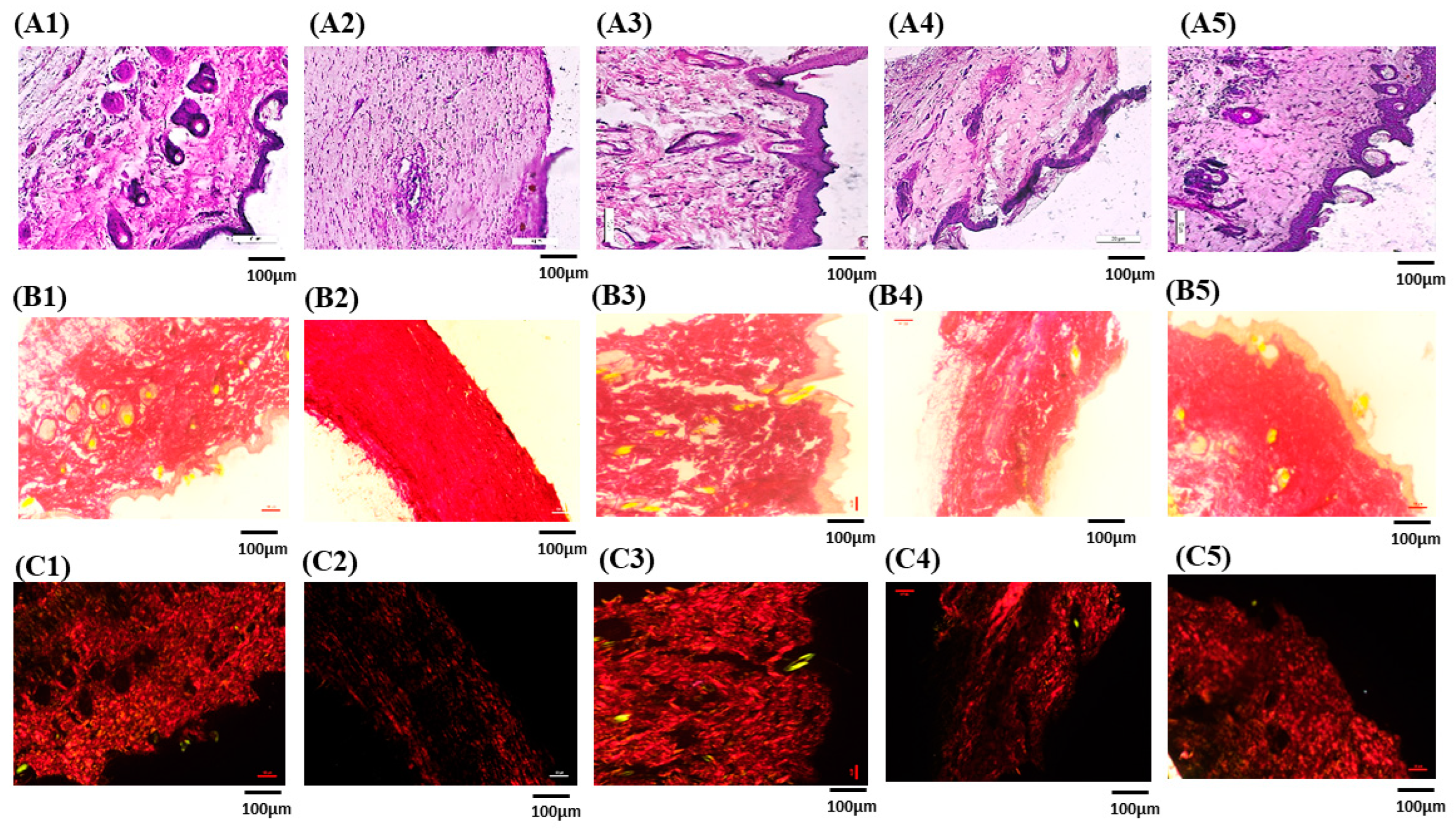
3.7.5. Histopathological Studies
3.7.6. Picro-Sirius Red Staining for Collagen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| DLMGNE | Drug-loaded nanoemulsion |
| DLMGO-G | Drug-loaded nanoemulgel |
| BLMGO-G | Blank nanoemulgel |
| FA | Ferulic acid |
| MMPs | Metalloproteinase |
| ROS | Reactive oxygen species |
| LGO | Lemon grass oil |
| DLS | Dynamic light scattering |
| TEM | Transmission electron microscopy |
| TNF-α | Tumor necrosis factor-alpha |
| IL-1β | Interleukin-beta |
| IL-6 | Interleukin-6 |
| IL-10 | Interleukin-10 |
| IL-22 | Interleukin-22 |
| MDA | Malondialdehyde |
| TBARS | Thiobarbituric acid-reactive substance |
| MMP-9 | Metalloproteinase-9 |
| ELISA | Enzyme-linked immunosorbent |
| MIC | Minimum inhibitory concentration |
| STZ | Streptozocin |
| BG | Blood glucose |
| GOD | Glucose oxidase |
| POD | Peroxidase |
References
- Higgins, G.C.; Coughlan, M.T. Mitochondrial dysfunction and mitophagy: The beginning and end to diabetic nephropathy? Br. J. Pharmacol. 2014, 171, 1917–1942. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.; Børsheim, E.; Carvalho, E. The Role of MicroRNAs in Diabetic Complications-Special Emphasis on Wound Healing. Genes 2014, 5, 926–956. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.S.; Ko, S.H.; Kim, J.H.; Moon, K.W.; Park, Y.M.; Yoo, K.D.; Ahn, Y.B. Diabetic retinopathy and endothelial dysfunction in patients with type 2 diabetes mellitus. Diabetes Metab. J. 2013, 37, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. J. Br. Diabet. Assoc. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Tiwari, P. Recent Trends in Therapeutic Approaches for Diabetes Management: A Comprehensive Update. J. Diabetes Res. 2015, 2015, 340838. [Google Scholar] [CrossRef]
- Tam, J.C.; Lau, K.M.; Liu, C.L.; To, M.H.; Kwok, H.F.; Lai, K.K.; Lau, C.P.; Ko, C.H.; Leung, P.C.; Fung, K.P.; et al. The in vivo and in vitro diabetic wound healing effects of a 2-herb formula and its mechanisms of action. J. Ethnopharmacol. 2011, 134, 831–838. [Google Scholar] [CrossRef]
- Kassab, A.; Piwowar, A. Cell oxidant stress delivery and cell dysfunction onset in type 2 diabetes. Biochimie 2012, 94, 1837–1848. [Google Scholar] [CrossRef]
- Dasari, N.; Jiang, A.; Skochdopole, A.; Chung, J.; Reece, E.M.; Vorstenbosch, J.; Winocour, S. Updates in Diabetic Wound Healing, Inflammation, and Scarring. Semin. Plast. Surg. 2021, 35, 153–158. [Google Scholar] [CrossRef]
- Cheel, J.; Theoduloz, C.; Rodríguez, J.; Schmeda-Hirschmann, G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.). J. Agric. Food Chem. 2005, 53, 2511–2517. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Lemongrass (Cymbopogon flexuosus) essential oil demonstrated anti-inflammatory effect in pre-inflamed human dermal fibroblasts. Biochim. Open 2017, 4, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, G.; Li, J.; Chen, J.; Li, L.; Li, Z.; Zhang, X.; Zhang, S.; Thorne, R.F.; Zhang, S. Antimicrobial Activity of Lemongrass Essential Oil (Cymbopogon flexuosus) and Its Active Component Citral Against Dual-Species Biofilms of Staphylococcus aureus and Candida Species. Front. Cell. Infect. Microbiol. 2020, 10, 603858. [Google Scholar] [CrossRef] [PubMed]
- Ghaisas, M.M.; Kshirsagar, S.B.; Sahane, R.S. Evaluation of wound healing activity of ferulic acid in diabetic rats. Int. Wound J. 2014, 11, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Kanimozhi, G.; Prasad, N.R.; Mahalakshmi, R. Radiosensitizing effect of ferulic acid on human cervical carcinoma cells in vitro. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2011, 25, 1366–1375. [Google Scholar] [CrossRef]
- Júnior, A.S.S.; Aidar, F.J.; Silva, L.A.S.; de B. Silva, T.; de Almeida, S.F.M.; Teles, D.C.S.; de L. Junior, W.; Schimieguel, D.M.; de Souza, D.A.; Nascimento, A.C.S.; et al. Influence of Lemongrass Essential Oil (Cymbopogon flexuosus) Supplementation on Diabetes in Rat Model. Life 2024, 14, 336. [Google Scholar] [CrossRef]
- Huang, C.; Huangfu, C.; Bai, Z.; Zhu, L.; Shen, P.; Wang, N.; Li, G.; Deng, H.; Ma, Z.; Zhou, W.; et al. Multifunctional carbomer based ferulic acid hydrogel promotes wound healing in radiation-induced skin injury by inactivating NLRP3 inflammasome. J. Nanobiotechnol. 2024, 22, 576. [Google Scholar] [CrossRef]
- Anand, S.; Pandey, P.; Begum, M.Y.; Chidambaram, K.; Arya, D.K.; Gupta, R.K.; Sankhwar, R.; Jaiswal, S.; Thakur, S.; Rajinikanth, P.S. Electrospun Biomimetic Multifunctional Nanofibers Loaded with Ferulic Acid for Enhanced Antimicrobial and Wound-Healing Activities in STZ-Induced Diabetic Rats. Pharmaceuticals 2022, 15, 302. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Li, W.; Yuan, H. Ferulic Acid Improves Functional Recovery after Acute Spinal Cord Injury in Rats by Inducing Hypoxia to Inhibit microRNA-590 and Elevate Vascular Endothelial Growth Factor Expressions. Front. Mol. Neurosci. 2017, 10, 183. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Ghosh, S.; Basak, P.; Dutta, S.; Chowdhury, S.; Sil, P.C. New insights into the ameliorative effects of ferulic acid in pathophysiological conditions. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017, 103, 41–55. [Google Scholar] [CrossRef]
- Bhavana, V.; Chary, P.S.; Rajana, N.; Devabattula, G.; Sau, S.; Godugu, C.; Kalia, N.P.; Singh, S.B.; Mehra, N.K. Multimodal lemongrass oil based topical nanoemulgel ingrained with ferulic acid for wound healing activity. J. Mol. Liq. 2023, 389, 122870. [Google Scholar] [CrossRef]
- Choudhury, H.; Gorain, B.; Pandey, M.; Chatterjee, L.A.; Sengupta, P.; Das, A.; Molugulu, N.; Kesharwani, P. Recent Update on Nanoemulgel as Topical Drug Delivery System. J. Pharm. Sci. 2017, 106, 1736–1751. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.; Dudhe, R.; Sharma, P.K. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef]
- Mahtab, A.; Anwar, M.; Mallick, N.; Naz, Z.; Jain, G.K.; Ahmad, F.J. Transungual Delivery of Ketoconazole Nanoemulgel for the Effective Management of Onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. [Google Scholar] [CrossRef]
- Gupta, A.; Bonde, S.R.; Gaikwad, S.; Ingle, A.; Gade, A.K.; Rai, M. Lawsonia inermis-mediated synthesis of silver nanoparticles: Activity against human pathogenic fungi and bacteria with special reference to formulation of an antimicrobial nanogel. IET Nanobiotechnol. 2014, 8, 172–178. [Google Scholar] [CrossRef]
- Lu, Y.; Ye, W.; Kang, W.; Wang, S.; Zhu, Z.; Chen, X.; Li, J. Wound-Healing Material with Antibacterial and Antioxidant Functions, Constructed Using Keratin, Hyperbranched Polymers, and MnO2. ACS Appl. Mater. Interfaces 2023, 15, 29841–29853. [Google Scholar] [CrossRef] [PubMed]
- Saka, R.; Jain, H.; Kommineni, N.; Chella, N.; Khan, W. Enhanced penetration and improved therapeutic efficacy of bexarotene via topical liposomal gel in imiquimod induced psoriatic plaque model in BALB/c mice. J. Drug Deliv. Sci. Technol. 2020, 58, 101691. [Google Scholar] [CrossRef]
- Chary, P.S.; Bansode, A.; Rajana, N.; Bhavana, V.; Singothu, S.; Sharma, A.; Guru, S.K.; Bhandari, V.; Mehra, N.K. Enhancing breast cancer treatment: Comprehensive study of gefitinib-loaded poloxamer 407/TPGS mixed micelles through design, development, in-silico modelling, In-Vitro testing, and Ex-Vivo characterization. Int. J. Pharm. 2024, 657, 124109. [Google Scholar] [CrossRef] [PubMed]
- Shanmugapriya, K.; Kim, H.; Lee, Y.W.; Kang, H.W. Multifunctional heteropolysaccharide hydrogel under photobiomodulation for accelerated wound regeneration. Ceram. Int. 2020, 46, 7268–7278. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Cereb. Blood Flow Metab. 2020, 40, 1769–1777. [Google Scholar] [CrossRef]
- Lin, C.-J.; Lan, Y.-M.; Ou, M.-Q.; Ji, L.-Q.; Lin, S.-D. Expression of miR-217 and HIF-1α/VEGF pathway in patients with diabetic foot ulcer and its effect on angiogenesis of diabetic foot ulcer rats. J. Endocrinol. Investig. 2019, 42, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Shaker, G.; Swift, C.J. Peroxidase-Coupled Glucose Method. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Urati, A.; Angati, A.; Singh Gautam, A.; Dey, M.; Pandey, S.K.; Singh, R.K. Neuroprotective responses of quercetin in regulation of biochemical, structural, and neurobehavioral effects in 28-day oral exposure of iron in rats. Toxicol. Mech. Methods 2024, 34, 57–71. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ghani, M.A.; Barril, C.; Bedgood, D.R.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Lai, H.-Y.; Chen, Y.-J.; Mersmann, H.J.; Lin, Y.-Y.; Ding, S.-T. Phyllanthus urinaria water extract ameliorates lipid accumulation, oxidative stress and inflammation in chickens with fatty liver syndrome. Ital. J. Anim. Sci. 2024, 23, 1233–1249. [Google Scholar] [CrossRef]
- Dayan, D.; Hiss, Y.; Hirshberg, A.; Bubis, J.J.; Wolman, M. Are the polarization colors of picrosirius red-stained collagen determined only by the diameter of the fibers? Histochemistry 1989, 93, 27–29. [Google Scholar] [CrossRef]
- Butenko, S.; Nagalla, R.R.; Guerrero-Juarez, C.F.; Palomba, F.; David, L.-M.; Nguyen, R.Q.; Gay, D.; Almet, A.A.; Digman, M.A.; Nie, Q.; et al. Hydrogel crosslinking modulates macrophages, fibroblasts, and their communication, during wound healing. Nat. Commun. 2024, 15, 6820. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Wang, Z.; Wang, R.; Chen, Q.; Yu, A.; Lu, A. Self-Healing, Injectable Hydrogel Dressing for Monitoring and Therapy of Diabetic Wound. Adv. Funct. Mater. 2024, 34, 2401209. [Google Scholar] [CrossRef]
- Polaka, S.; Katare, P.; Pawar, B.; Vasdev, N.; Gupta, T.; Rajpoot, K.; Sengupta, P.; Tekade, R.K. Emerging ROS-Modulating Technologies for Augmentation of the Wound Healing Process. ACS Omega 2022, 7, 30657–30672. [Google Scholar] [CrossRef]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Han, W.; Ding, H. Novel design and development of Centella Asiatica extract—Loaded poloxamer/ZnO nanocomposite wound closure material to improve anti-bacterial action and enhanced wound healing efficacy in diabetic foot ulcer. Regen. Ther. 2024, 27, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Narisepalli, S.; Salunkhe, S.A.; Chitkara, D.; Mittal, A. Asiaticoside polymeric nanoparticles for effective diabetic wound healing through increased collagen biosynthesis: In-vitro and in-vivo evaluation. Int. J. Pharm. 2023, 631, 122508. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, H.; Jangde, R.K. Development and characterization of ferulic acid-loaded chitosan nanoparticle embedded- hydrogel for diabetic wound delivery. Eur. J. Pharm. Biopharm. 2024, 201, 114371. [Google Scholar] [CrossRef]
- Selçuk, C.T.; Durgun, M.; Tekin, R.; Yolbas, L.; Bozkurt, M.; Akçay, C.; Alabalk, U.; Basarali, M.K. Evaluation of the effect of thymoquinone treatment on wound healing in a rat burn model. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2013, 34, e274–e281. [Google Scholar] [CrossRef]
- Doss, H.M.; Dey, C.; Sudandiradoss, C.; Rasool, M.K. Targeting inflammatory mediators with ferulic acid, a dietary polyphenol, for the suppression of monosodium urate crystal-induced inflammation in rats. Life Sci. 2016, 148, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Al Qaseer, S.M.; Salih, S.I.; Ali, R.M.; Khalaf, M.K. Comparative Study of Cold Physical Plasma Effect on Modulation of Basic-Fibroblast Growth Factor and Tumor Necrosis Factor Alpha in Full Thickness Skin Wound Healing Process in Normal and Diabetic Dogs. Indian J. Forensic Med. Toxicol. 2021, 15, 2505–2511. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Ren, J.; Yang, M.; Xu, F.; Chen, J.; Ma, S. Acceleration of wound healing activity with syringic acid in streptozotocin induced diabetic rats. Life Sci. 2019, 233, 116728. [Google Scholar] [CrossRef]
- Davis, F.M.; Kimball, A.; Boniakowski, A.; Gallagher, K. Dysfunctional Wound Healing in Diabetic Foot Ulcers: New Crossroads. Curr. Diabetes Rep. 2018, 18, 2. [Google Scholar] [CrossRef]
- Staniforth, V.; Huang, W.-C.; Aravindaram, K.; Yang, N.-S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Schultz, G.S. Molecular Wound Assessments: Matrix Metalloproteinases. Adv. Wound Care 2013, 2, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Cossermelli, W.; Brentani, R. Differential staining of collagens type I, II and III by Sirius Red and polarization microscopy. Arch. Histol. Jpn.=Nihon Soshikigaku Kiroku 1978, 41, 267–274. [Google Scholar] [CrossRef] [PubMed]
| Groups | Treatment | No. of Animals * |
|---|---|---|
| Group 1 | Control | 06 |
| Group 2 | Disease: Streptozocin (STZ: 55 mg/kg: i.p.) | 06 |
| Group 3 | Marketed formulation (Povidone iodine) + STZ | 12 |
| Group 4 | Blank nanoemulgel (BLMGO-GEL) + STZ | 12 |
| Group 5 | Drug (FA) loaded nanoemulgel (DLMGO-GEL) + STZ | 12 |
| Total | 58 * | |
| Considering 10% expected mortality Where * Is total number of animals, i.p. is intraperitoneal route of administration | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anuradha, U.; Bhavana, V.; Chary, P.S.; Kalia, N.P.; Mehra, N.K. Exploration of the Topical Nanoemulgel Bearing with Ferulic Acid and Essential Oil for Diabetic Wound Healing. Pathophysiology 2024, 31, 680-698. https://doi.org/10.3390/pathophysiology31040049
Anuradha U, Bhavana V, Chary PS, Kalia NP, Mehra NK. Exploration of the Topical Nanoemulgel Bearing with Ferulic Acid and Essential Oil for Diabetic Wound Healing. Pathophysiology. 2024; 31(4):680-698. https://doi.org/10.3390/pathophysiology31040049
Chicago/Turabian StyleAnuradha, Urati, Valamla Bhavana, Padakanti Sandeep Chary, Nitin Pal Kalia, and Neelesh Kumar Mehra. 2024. "Exploration of the Topical Nanoemulgel Bearing with Ferulic Acid and Essential Oil for Diabetic Wound Healing" Pathophysiology 31, no. 4: 680-698. https://doi.org/10.3390/pathophysiology31040049
APA StyleAnuradha, U., Bhavana, V., Chary, P. S., Kalia, N. P., & Mehra, N. K. (2024). Exploration of the Topical Nanoemulgel Bearing with Ferulic Acid and Essential Oil for Diabetic Wound Healing. Pathophysiology, 31(4), 680-698. https://doi.org/10.3390/pathophysiology31040049






