A Bird’s-Eye View of the Pathophysiologic Role of the Human Urobiota in Health and Disease: Can We Modulate It?
Abstract
1. Introduction
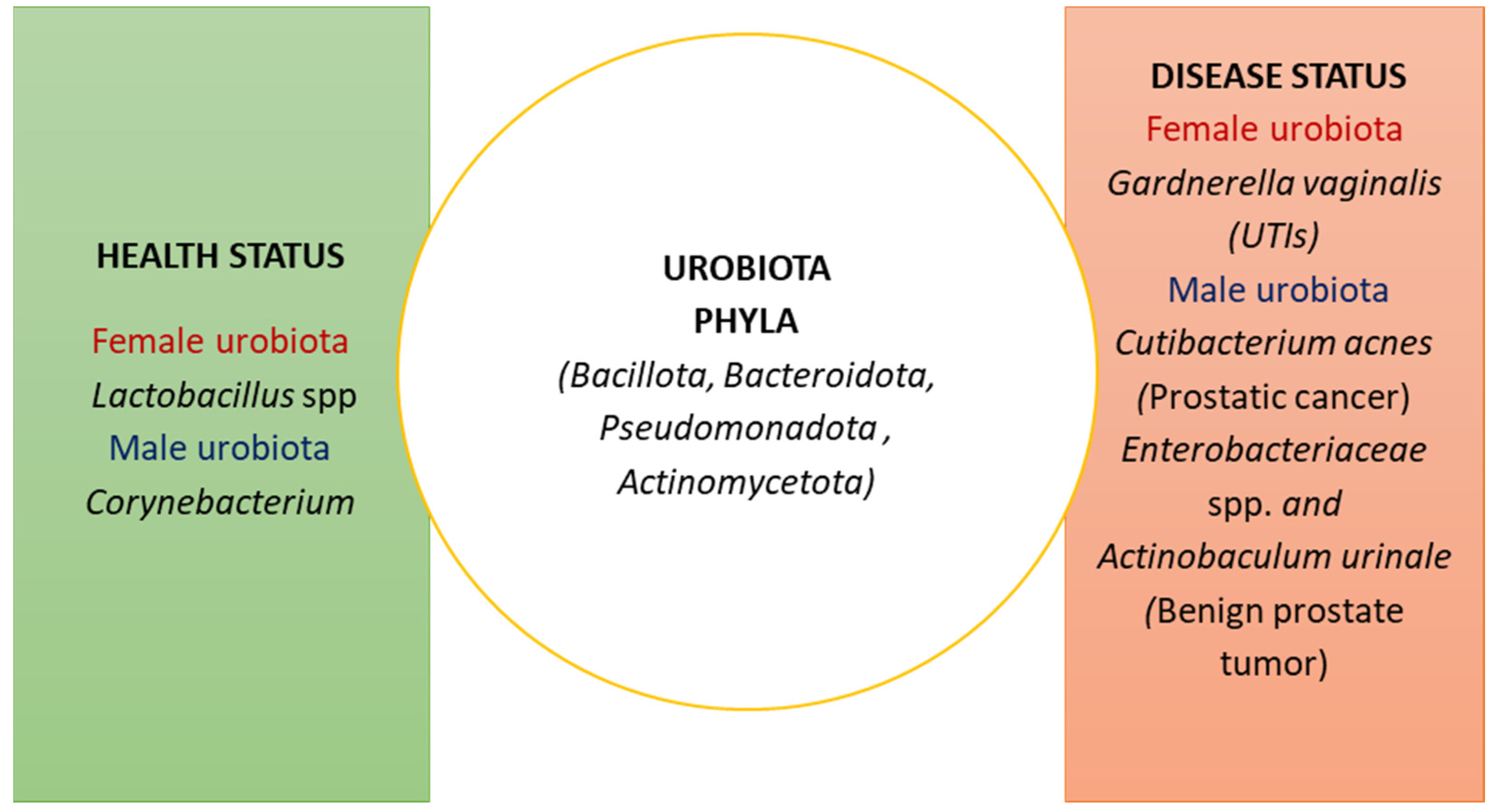
2. The Composition of the Urobiota
3. The Impact of the Gut Microbiota on UTI Occurrence
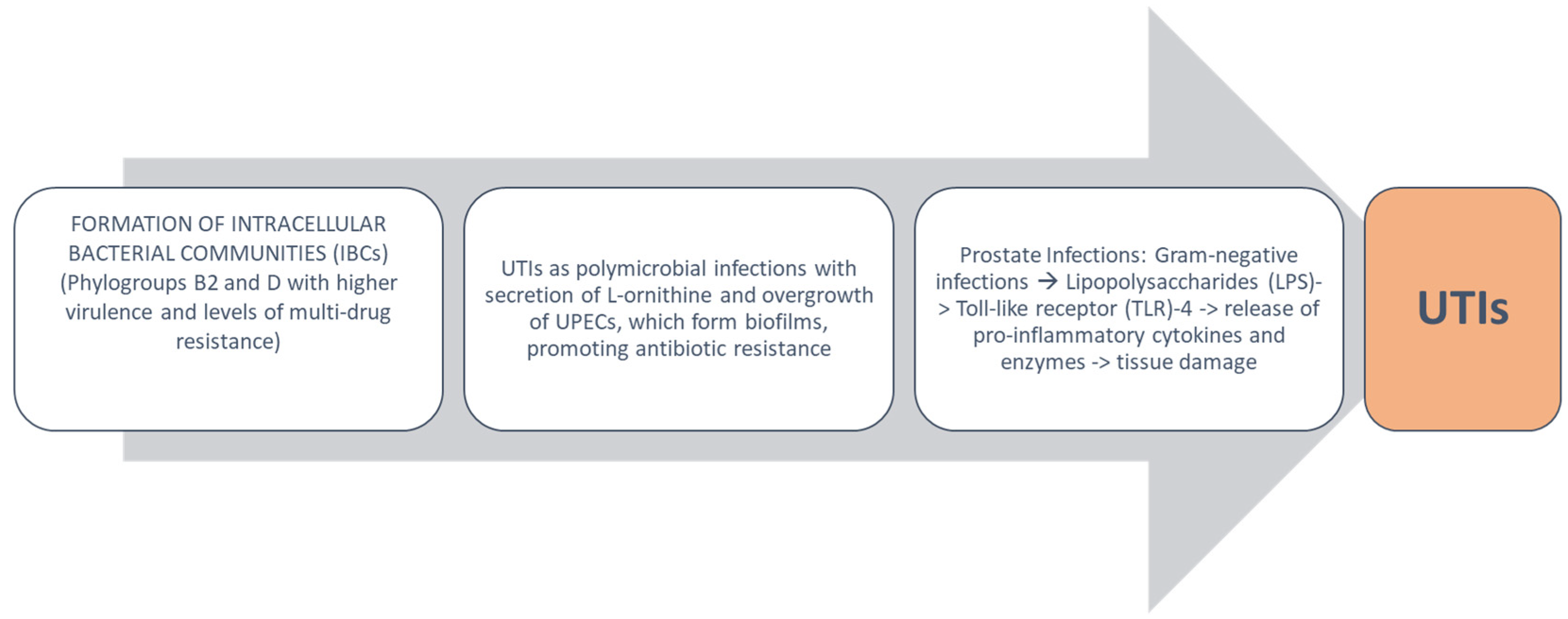
4. Urobiota Alteration in UTIs and Pathogenetic Mechanisms
5. New Therapeutic Approaches to Modulate the Urobiota and Prevent UTIs
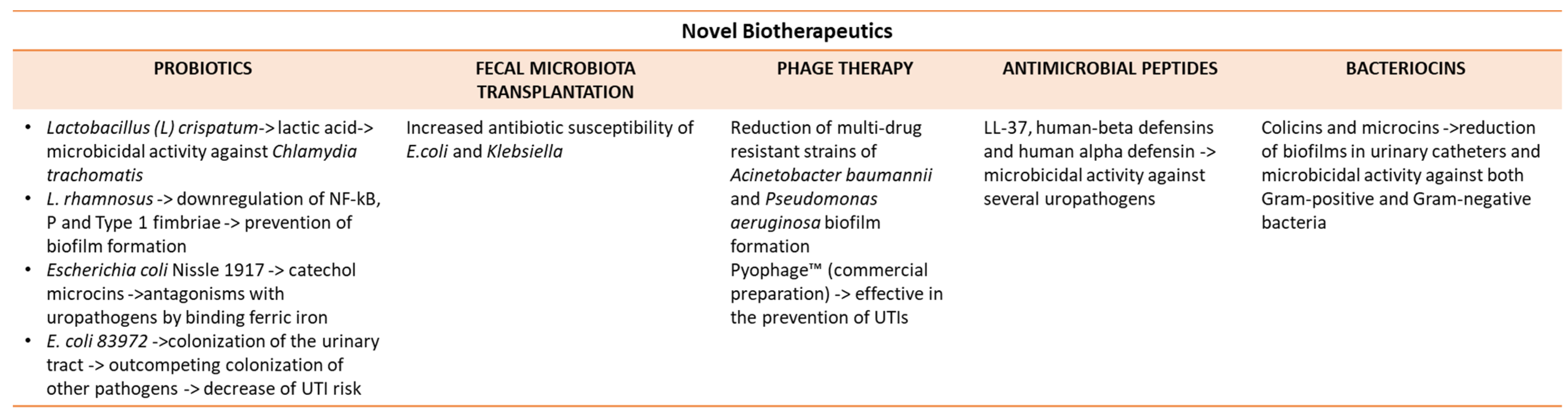
5.1. Probiotics
5.2. Fecal Microbiota Transplantation
5.3. Phage Therapy
5.4. Antimicrobial Peptides
5.5. Bacteriocins
5.6. Inhibitors of Bacterial Virulence
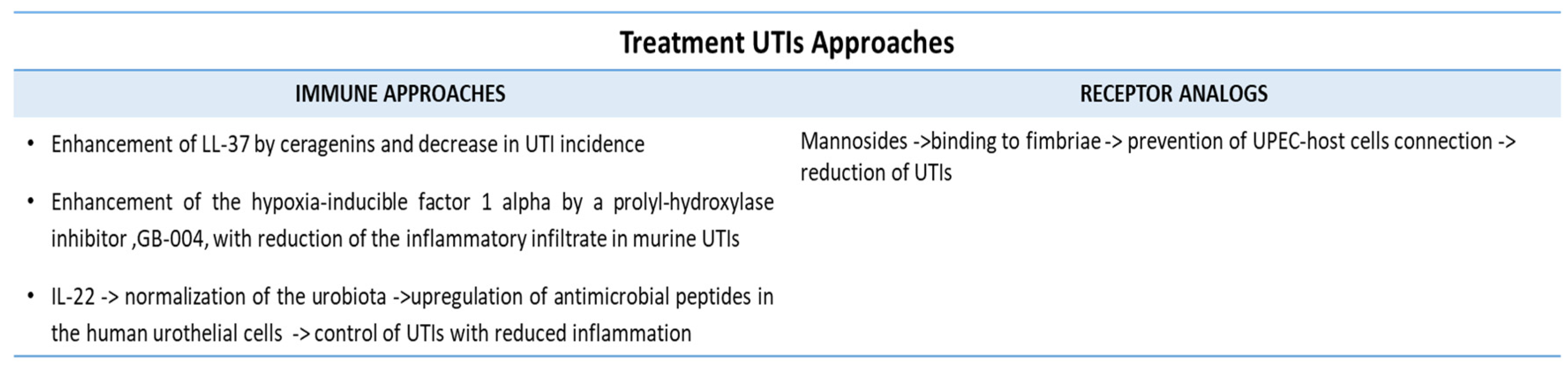
5.7. Immune-Mediated Therapies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| CAUTI | Catheter-associated UTI |
| CP | Chronic prostatitis |
| ExPEC | Extraintestinal pathogenic Escherichia coli |
| FMT | Fecal Microbiota Transplantation |
| HBD | Human-beta defensin |
| HD5 | Human-alpha defensin 5 |
| IBCs | Intracellular bacterial communities |
| IL | Interleukin |
| LPSs | Lipopolysaccharides |
| R | Recurrent |
| UTIs | Urinary tract infections |
| UPEC | Uropathogenic Escherichia coli |
| Th | T helper |
| TREG | T regulatory cells |
References
- Sleator, R.D. The human superorganism—Of microbes and men. Med. Hypotheses 2010, 74, 214–215. [Google Scholar] [CrossRef]
- Magrone, T.; Jirillo, E. The interplay between the gut immune system and microbiota in health and disease: Nutraceutical intervention for restoring intestinal homeostasis. Curr. Pharm. Des. 2013, 19, 1329–1342. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Visconti, A.; Le Roy, C.I.; Rosa, F.; Rossi, N.; Martin, T.C.; Mohney, R.P.; Li, W.; de Rinaldis, E.; Bell, J.T.; Venter, J.C.; et al. Interplay between the human gut microbiome and host metabolism. Nat. Commun. 2019, 10, 4505. [Google Scholar] [CrossRef]
- De Santis, S.; Cavalcanti, E.; Mastronardi, M.; Jirillo, E.; Chieppa, M. Nutritional Keys for Intestinal Barrier Modulation. Front. Immunol. 2015, 6, 612. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; FitzGerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of Uncultivated Bacteria in the Adult Female Bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Murphy, C.P.; Sleator, R.D.; Culligan, E.P. The urobiome, urinary tract infections, and the need for alternative therapeutics. Microb. Pathog. 2021, 161 Pt B, 105295. [Google Scholar] [CrossRef]
- Gasiorek, M.; Hsieh, M.H.; Forster, C.S. Utility of DNA Next-Generation Sequencing and Expanded Quantitative Urine Culture in Diagnosis and Management of Chronic or Persistent Lower Urinary Tract Symptoms. J. Clin. Microbiol. 2019, 58, e00204-19. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.; Kim, J. Urobiome: An outlook on the metagenome of urological diseases. Investig. Clin. Urol. 2021, 62, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, N.; Balachandran, A.; Krska, L.; Peppiatt-Wildman, C.; Wildman, S.; Duckett, J. Age, menopausal status and the bladder microbiome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Wolfe, A.J. The new world of the urinary microbiota in women. Am. J. Obstet. Gynecol. 2015, 213, 644–649. [Google Scholar] [CrossRef]
- Yıldırım, S.; Shoskes, D.; Kulkarni, S.; Laguna, P. Urinary microbiome in uncomplicated and interstitial cystitis: Is there any similarity? World J. Urol. 2020, 38, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Brubaker, L.; Wolfe, A.J. The female urinary microbiota, urinary health and common urinary disorders. Ann. Transl. Med. 2017, 5, 34. [Google Scholar] [CrossRef]
- Barber, A.E.; Norton, J.P.; Spivak, A.M.; Mulvey, M.A. Urinary tract infections: Current and emerging management strategies. Clin. Infect Dis. 2013, 57, 719–724. [Google Scholar] [CrossRef]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Bajic, P.; Van Kuiken, M.E.; Burge, B.K.; Kirshenbaum, E.J.; Joyce, C.J.; Wolfe, A.J.; Branch, J.D.; Bresler, L.; Farooq, A.V. Male Bladder Microbiome Relates to Lower Urinary Tract Symptoms. Eur. Urol. Focus 2020, 6, 376–382. [Google Scholar] [CrossRef]
- Pallares-Mendez, R.; Cervantes-Miranda, D.E.; Gonzalez-Colmenero, A.D.; Ochoa-Arvizo, M.A.; Gutierrez-Gonzalez, A. A Perspective of the Urinary Microbiome in Lower Urinary Tract Infections—A Review. Curr. Urol. Rep. 2022, 23, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Thomas-White, K.J.; Kliethermes, S.; Rickey, L.; Lukacz, E.S.; Richter, H.E.; Moalli, P.; Zimmern, P.; Norton, P.; Kusek, J.W.; Wolfe, A.J.; et al. Evaluation of the urinary microbiota of women with uncomplicated stress urinary incontinence. Am. J. Obstet. Gynecol. 2017, 216, 55.e1–55.e16. [Google Scholar] [CrossRef]
- Zandbergen, L.E.; Halverson, T.; Brons, J.K.; Wolfe, A.J.; de Vos, M.G.J. The Good and the Bad: Ecological Interaction Measurements between the Urinary Microbiota and Uropathogens. Front. Microbiol. 2021, 12, 659450. [Google Scholar] [CrossRef]
- Guglietta, A. Recurrent urinary tract infections in women: Risk factors, etiology, pathogenesis and prophylaxis. Futur. Microbiol. 2017, 12, 239–246. [Google Scholar] [CrossRef]
- Abelson, B.; Sun, D.; Que, L.; A Nebel, R.; Baker, D.; Popiel, P.; Amundsen, C.L.; Chai, T.; Close, C.; DiSanto, M.; et al. Sex differences in lower urinary tract biology and physiology. Biol. Sex. Differ. 2018, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Alperin, M.; Burnett, L.; Lukacz, E.M.; Brubaker, L. The mysteries of menopause and urogynecologic health: Clinical and scientific gaps. Menopause 2019, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kmiec, D.; Nchioua, R.; Sherrill-Mix, S.; Stürzel, C.M.; Heusinger, E.; Braun, E.; Gondim, M.V.P.; Hotter, D.; Sparrer, K.M.J.; Hahn, B.H.; et al. CpG Frequency in the 5′ Third of the env Gene Determines Sensitivity of Primary HIV-1 Strains to the Zinc-Finger Antiviral Protein. mBio 2020, 11, e02903-19. [Google Scholar] [CrossRef] [PubMed]
- Kostakioti, M.; Hultgren, S.J.; Hadjifrangiskou, M. Molecular blueprint of uropathogenic Escherichia coli virulence provides clues toward the development of anti-virulence therapeutics. Virulence 2012, 3, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Raz, R. Postmenopausal women with recurrent UTI. Int. J. Antimicrob. Agents 2001, 17, 269–271. [Google Scholar] [CrossRef]
- Magruder, M.; Sholi, A.N.; Gong, C.; Zhang, L.; Edusei, E.; Huang, J.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Crawford, C.; et al. Gut uropathogen abundance is a risk factor for development of bacteriuria and urinary tract infection. Nat. Commun. 2019, 10, 5521. [Google Scholar] [CrossRef]
- Thänert, R.; Reske, K.A.; Hink, T.; Wallace, M.A.; Wang, B.; Schwartz, D.J.; Seiler, S.; Cass, C.; Burnham, C.-A.D.; Dubberke, E.R.; et al. Comparative Genomics of Antibiotic-Resistant Uropathogens Implicates Three Routes for Recurrence of Urinary Tract Infections. mBio 2019, 10, e01977-19. [Google Scholar] [CrossRef]
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Van Kuiken, M.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 2018, 9, 1557. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Colella, M.; Charitos, I.A.; Di Domenico, M.; Palmirotta, R.; Jirillo, E. Microbial and Host Metabolites at the Backstage of Fever: Current Knowledge about the Co-Ordinate Action of Receptors and Molecules Underlying Pathophysiology and Clinical Implications. Metabolites 2023, 13, 461. [Google Scholar] [CrossRef]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Priadko, K.; Romano, L.; Olivieri, S.; Romeo, M.; Barone, B.; Sciorio, C.; Spirito, L.; Morelli, M.; Crocetto, F.; Arcaniolo, D.; et al. Intestinal microbiota, intestinal permeability and the urogenital tract: Is there a pathophysiological link? J. Physiol. Pharmacol. 2022, 73, 575–585. [Google Scholar] [CrossRef]
- Fourcade, C.; Canini, L.; Lavigne, J.P.; Sotto, A. A comparison of monomicrobial versus polymicrobial Enterococcus faecalis bacteriuria in a French University Hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Werneburg, G.T.; Nguyen, A.; Henderson, N.S.; Rackley, R.R.; Shoskes, D.A.; Le Sueur, A.L.; Corcoran, A.T.; Katz, A.E.; Kim, J.; Rohan, A.J.; et al. The Natural History and Composition of Urinary Catheter Biofilms: Early Uropathogen Colonization with Intraluminal and Distal Predominance. J. Urol. 2020, 203, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Amsel, D.; Kalsy, M.; Billion, A.; Dobrindt, U.; Vilcinskas, A. MicroRNAs regulate innate immunity against uropathogenic and commensal-like Escherichia coli infections in the surrogate insect model Galleria mellonella. Sci. Rep. 2020, 10, 2570. [Google Scholar] [CrossRef] [PubMed]
- Orazi, G.; O’Toole, G.A. “It Takes a Village”: Mechanisms Underlying Antimicrobial Recalcitrance of Polymicrobial Biofilms. J. Bacteriol. 2019, 202, e00530-19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Foxman, B.; Manning, S.D.; Tallman, P.; Marrs, C.F. Molecular epidemiologic approaches to urinary tract infection gene discovery in uropathogenic Escherichia coli. Infect. Immun. 2000, 8, 2009–2015. [Google Scholar] [CrossRef]
- Schwager, S.; Agnoli, K.; Köthe, M.; Feldmann, F.; Givskov, M.; Carlier, A.; Eberl, L. Identification of Burkholderia cenocepacia strain H111 virulence factors using nonmammalian infection hosts. Infect. Immun. 2013, 81, 143–153. [Google Scholar] [CrossRef]
- Arzola, J.M.; Hawley, J.S.; Oakman, C.; Mora, R.V. A case of prostatitis due to Burkholderia pseudomallei. Nat. Rev. Endocrinol. 2007, 4, 111–114. [Google Scholar] [CrossRef]
- Organ, M.; Grantmyre, J.; Hutchinson, J. Burkholderia cepacia infection of the prostate caused by inoculation ofcontaminated ultrasound gel during transrectal biopsy of the prostate. Can. Urol. Assoc. J. 2010, 4, 58–60. [Google Scholar] [CrossRef][Green Version]
- Shoskes, D.A.; Altemus, J.; Polackwich, A.S.; Tucky, B.; Wang, H.; Eng, C. The Urinary Microbiome Differs Significantly between Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Controls as Well as between Patients with Different Clinical Phenotypes. Urology 2016, 92, 26–32. [Google Scholar] [CrossRef]
- Santacroce, L.; Di Domenico, M.; Montagnani, M.; Jirillo, E. Antibiotic Resistance and Microbiota Response. Curr. Pharm. Des. 2023, 29, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lee, W.-C.; Chuang, Y.-C. Emerging Non-Antibiotic Options Targeting Uropathogenic Mechanisms for Recurrent Uncomplicated Urinary Tract Infection. Int. J. Mol. Sci. 2023, 24, 7055. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Palmirotta, R.; Bottalico, L.; Charitos, I.A.; Colella, M.; Topi, S.; Jirillo, E. Crosstalk between the Resident Microbiota and the Immune Cells Regulates Female Genital Tract Health. Life 2023, 13, 1531. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Lee, J.W. Urinary Tract Infection and Microbiome. Diagnostics 2023, 13, 1921. [Google Scholar] [CrossRef]
- Tariq, R.; Pardi, D.S.; Tosh, P.K.; Walker, R.C.; Razonable, R.R.; Khanna, S. Fecal Microbiota Transplantation for Recurrent Clostridium difficile Infection Reduces Recurrent Urinary Tract Infection Frequency. Clin. Infect. Dis. 2017, 65, 1745–1747. [Google Scholar] [CrossRef] [PubMed]
- Kalas, V.; Hibbing, M.E.; Maddirala, A.R.; Chugani, R.; Pinkner, J.S.; Mydock-McGrane, L.K.; Conover, M.S.; Janetka, J.W.; Hultgren, S.J. Structure-Based Discovery of Glycomimetic FmlH Ligands As Inhibitors of Bacterial Adhesion During Urinary Tract Infection. Proc. Natl. Acad. Sci. USA 2018, 115, E2819–E2828. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.; Silvis, M.R.; Talkington, K.C.; Budzik, J.M.; Dodd, C.E.; Paluba, J.M.; Oki, E.A.; Trotta, K.L.; Licht, D.J.; Jimenez-Morales, D.; et al. Ceragenins and Antimicrobial Peptides Kill Bacteria through Distinct Mechanisms. mBio 2022, 13, e0272621. [Google Scholar] [CrossRef] [PubMed]
- Chromek, M.; Slamová, Z.; Bergman, P.; Kovács, L.; Podracká, L.; Ehrén, I.; Hökfelt, T.; Gudmundsson, G.H.; Gallo, R.L.; Agerberth, B.; et al. The antimicrobial peptide cathelicidin protects the urinary tract against invasive bacterial infection. Nat. Med. 2006, 12, 636–641. [Google Scholar] [CrossRef]
- Colella, M.; Topi, S.; Palmirotta, R.; D’agostino, D.; Charitos, I.A.; Lovero, R.; Santacroce, L. An Overview of the Microbiota of the Human Urinary Tract in Health and Disease: Current Issues and Perspectives. Life 2023, 13, 1486. [Google Scholar] [CrossRef]
- Karstens, L.; Asquith, M.; Davin, S.; Stauffer, P.; Fair, D.; Gregory, W.T.; Rosenbaum, J.T.; McWeeney, S.K.; Nardos, R. Does the Urinary Microbiome Play a Role in Urgency Urinary Incontinence and Its Severity? Front. Cell. Infect. Microbiol. 2016, 6, 78. [Google Scholar] [CrossRef]
- E Fouts, D.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.-J.; Huang, S.-T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.N.; Sfanos, K.S.; Brüggemann, H. Draft Genome Sequences of Two Strains of Propionibacterium acnes Isolated from Radical Prostatectomy Specimens. Genome Announc. 2013, 1, e01071-13. [Google Scholar] [CrossRef]
- Shrestha, E.; White, J.R.; Yu, S.-H.; Kulac, I.; Ertunc, O.; De Marzo, A.M.; Yegnasubramanian, S.; Mangold, L.A.; Partin, A.W.; Sfanos, K.S. Profiling the Urinary Microbiome in Men with Positive versus Negative Biopsies for Prostate Cancer. J. Urol. 2018, 199, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Pearce, M.M.; Zilliox, M.J.; Rosenfeld, A.B.; Thomas-White, K.J.; Richter, H.E.; Nager, C.W.; Visco, A.G.; Nygaard, I.E.; Barber, M.D.; Schaffer, J.; et al. The female urinary microbiome in urgency urinary incontinence. Am. J. Obstet. Gynecol. 2015, 213, 347.e1–347.e11. [Google Scholar] [CrossRef] [PubMed]
- A Cadieux, P.; Burton, J.; Devillard, E.; Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol. 2009, 60 (Suppl. S6), 13–18. [Google Scholar] [PubMed]
- Charitos, I.A.; Topi, S.; Gagliano-Candela, R.; De Nitto, E.; Polimeno, L.; Montagnani, M.; Santacroce, L. The Toxic Effects of Endocrine Disrupting Chemicals (EDCs) on Gut Microbiota: Bisphenol A (BPA) A Review. Endocr. Metab. Immune Disord. Drug Targets 2022, 22, 716–727. [Google Scholar] [CrossRef]
- Gümüş, D.; Yüksek, F.K.; Sefer, Ö.; Yörük, E.; Uz, G.; Küçüker, M.A. The roles of hormones in the modulation of growth and virulence genes’ expressions in UPEC strains. Microb. Pathog. 2019, 132, 319–324. [Google Scholar] [CrossRef]
- Price, T.K.; Wolff, B.; Halverson, T.; Limeira, R.; Brubaker, L.; Dong, Q.; Mueller, E.R.; Wolfe, A.J. Temporal Dynamics of the Adult Female Lower Urinary Tract Microbiota. mBio 2020, 11, e00475-20. [Google Scholar] [CrossRef]
- Gholiof, M.; Adamson-De Luca, E.; Wessels, J.M. The female reproductive tract microbiotas, inflammation, and gynecological conditions. Front. Reprod. Health 2022, 9, 963752. [Google Scholar] [CrossRef]
- Morrill, S.; Gilbert, N.M.; Lewis, A.L. Gardnerella vaginalis as a Cause of Bacterial Vaginosis: Appraisal of the Evidence from in vivo Models. Front. Cell. Infect. Microbiol. 2020, 10, 168. [Google Scholar] [CrossRef]
- O’Brien, V.P.; Joens, M.S.; Lewis, A.L.; Gilbert, N.M. Recurrent Escherichia coli Urinary Tract Infection Triggered by Gardnerella vaginalis Bladder Exposure in Mice. J. Vis. Exp. 2020, 166, e61967. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, A.E.; Au-Yeung, M.; Hooton, T.M.; Fredricks, D.N.; Roberts, P.L.; Czaja, C.A.; Yarova-Yarovaya, Y.; Fiedler, T.; Cox, M.; Stamm, W.E. Randomized, Placebo-Controlled Phase 2 Trial of a Lactobacillus crispatus Probiotic Given Intravaginally for Prevention of Recurrent Urinary Tract Infection. Clin. Infect. Dis. 2011, 52, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Charitos, I.A.; Ballini, A.; Cafiero, C.; Topi, S.; Palmirotta, R.; Santacroce, L. Microbiota revolution: How gut microbes regulate our lives. World J. Gastroenterol. 2023, 29, 4368–4383. [Google Scholar] [CrossRef] [PubMed]
- Meštrović, T.; Matijašić, M.; Perić, M.; Paljetak, H.; Barešić, A.; Verbanac, D. The Role of Gut, Vaginal, and Urinary Microbiome in Urinary Tract Infections: From Bench to Bedside. Diagnostics 2020, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Forde, B.M.; Roberts, L.W.; Phan, M.-D.; Peters, K.M.; Fleming, B.A.; Russell, C.W.; Lenherr, S.M.; Myers, J.B.; Barker, A.P.; Fisher, M.A.; et al. Population dynamics of an Escherichia coli ST131 lineage during recurrent urinary tract infection. Nat. Commun. 2019, 10, 3643. [Google Scholar] [CrossRef]
- Khonsari, M.S.; Behzadi, P.; Foroohi, F. The prevalence of type 3 fimbriae in Uropathogenic Escherichia coli isolated from clinical urine samples. Meta Gene 2021, 28, 100881. [Google Scholar] [CrossRef]
- Behzadi, P. Classical chaperone-usher (CU) adhesive fimbriome: Uropathogenic Escherichia coli (UPEC) and urinary tract infections (UTIs). Folia Microbiol. 2020, 65, 45–65. [Google Scholar] [CrossRef]
- Hozzari, A.; Behzadi, P.; Khiabani, P.K.; Sholeh, M.; Sabokroo, N. Clinical cases, drug resistance, and virulence genes profiling in Uropathogenic Escherichia coli. J. Appl. Genet. 2020, 61, 265–273. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Stepanova, N. How Advanced Is Our Understanding of the Role of Intestinal Barrier Dysfunction in the Pathogenesis of Recurrent Urinary Tract Infections. Front. Pharmacol. 2022, 13, 780122. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13, 927. [Google Scholar] [CrossRef]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef]
- Whiteside, S.A.; Razvi, H.; Dave, S.; Reid, G.; Burton, J.P. The microbiome of the urinary tract—A role beyond infection. Nat. Rev. Urol. 2015, 12, 81–90. [Google Scholar] [CrossRef]
- Hsu, L.-N.; Hu, J.-C.; Chen, P.-Y.; Lee, W.-C.; Chuang, Y.-C. Metabolic Syndrome and Overactive Bladder Syndrome May Share Common Pathophysiologies. Biomedicines 2022, 10, 1957. [Google Scholar] [CrossRef] [PubMed]
- Nardos, R.; Leung, E.T.; Dahl, E.M.; Davin, S.; Asquith, M.; Gregory, W.T.; Karstens, L. Network-Based Differences in the Vaginal and Bladder Microbial Communities between Women with and without Urgency Urinary Incontinence. Front. Cell. Infect. Microbiol. 2022, 12, 759156. [Google Scholar] [CrossRef]
- Biggel, M.; Xavier, B.B.; Johnson, J.R.; Nielsen, K.L.; Frimodt-Møller, N.; Matheeussen, V.; Goossens, H.; Moons, P.; Van Puyvelde, S. Horizontally acquired papGII-containing pathogenicity islands underlie the emergence of invasive uropathogenic Escherichia coli lineages. Nat. Commun. 2020, 11, 5968. [Google Scholar] [CrossRef] [PubMed]
- Löffler, B.; Tuchscherr, L.; Niemann, S.; Peters, G. Staphylococcus aureus persistence in non-professional phagocytes. Int. J. Med. Microbiol. 2014, 304, 170–176. [Google Scholar] [CrossRef]
- Keogh, D.; Tay, W.H.; Ho, Y.Y.; Dale, J.L.; Chen, S.; Umashankar, S.; Williams, R.B.; Chen, S.L.; Dunny, G.M.; Kline, K.A. Enterococcal Metabolite Cues Facilitate Interspecies Niche Modulation and Polymicrobial Infection. Cell Host Microbe 2016, 20, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Topi, S.; Bottalico, L.; Charitos, I.A.; Colella, M.; Di Domenico, M.; Palmirotta, R.; Santacroce, L. Biomolecular Mechanisms of Autoimmune Diseases and Their Relationship with the Resident Microbiota: Friend or Foe? Pathophysiology 2022, 29, 507–536. [Google Scholar] [CrossRef]
- Croxall, G.; Weston, V.; Joseph, S.; Manning, G.; Cheetham, P.; McNally, A. Increased human pathogenic potential of Escherichia coli from polymicrobial urinary tract infections in comparison to isolates from monomicrobial culture samples. J. Med. Microbiol. 2011, 60 Pt 1, 102–109. [Google Scholar] [CrossRef]
- Magruder, M.; Edusei, E.; Zhang, L.; Albakry, S.; Satlin, M.J.; Westblade, L.F.; Malha, L.; Sze, C.; Lubetzky, M.; Dadhania, D.M.; et al. Gut commensal microbiota and decreased risk for Enterobacteriaceae bacteriuria and urinary tract infection. Gut Microbes 2020, 12, 1805281. [Google Scholar] [CrossRef] [PubMed]
- Bottery, M.J.; Matthews, J.L.; Wood, A.J.; Johansen, H.K.; Pitchford, J.W.; Friman, V.-P. Inter-species interactions alter antibiotic efficacy in bacterial communities. ISME J. 2022, 16, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Short, F.L.; Murdoch, S.L.; Ryan, R.P. Polybacterial human disease: The ills of social networking. Trends Microbiol. 2014, 22, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.U.; Ihsan, A.U.; Khan, H.U.; Jana, R.; Wazir, J.; Khongorzul, P.; Waqar, M.; Zhou, X. Comprehensive overview of prostatitis. Biomed. Pharmacother. 2017, 94, 1064–1076. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Ohnishi, K.; Hori, S.; Nakano, A.; Nakano, R.; Yano, H.; Ohnishi, S.; Owari, T.; Morizawa, Y.; Itami, Y.; et al. Mycoplasma genitalium Infection and Chronic Inflammation in Human Prostate Cancer: Detection Using Prostatectomy and Needle Biopsy Specimens. Cells 2019, 8, 212. [Google Scholar] [CrossRef]
- Salachan, P.V.; Rasmussen, M.; Fredsøe, J.; Ulhøi, B.; Borre, M.; Sørensen, K.D. Microbiota of the prostate tumor environment investigated by whole-transcriptome profiling. Genome Med. 2022, 14, 9. [Google Scholar] [CrossRef]
- Munteanu, R.; Feder, R.-I.; Onaciu, A.; Munteanu, V.C.; Iuga, C.-A.; Gulei, D. Insights into the Human Microbiome and Its Connections with Prostate Cancer. Cancers 2023, 15, 2539. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667, Erratum in Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 551. [Google Scholar] [CrossRef]
- Shukla, S.; MacLennan, G.T.; Fu, P.; Patel, J.; Marengo, S.R.; Resnick, M.L.; Gupta, S. Nuclear Factor-κB/p65 (Rel A) Is Constitutively Activated in Human Prostate Adenocarcinoma and Correlates with Disease Progression. Neoplasia 2004, 6, 390–400. [Google Scholar] [CrossRef]
- Jain, S.; Suklabaidya, S.; Das, B.; Raghav, S.K.; Batra, S.K.; Senapati, S. TLR4 activation by lipopolysaccharide confers survival advantage to growth factor deprived prostate cancer cells. Prostate 2015, 75, 1020–1033. [Google Scholar] [CrossRef] [PubMed]
- Simons, B.W.; Durham, N.M.; Bruno, T.C.; Grosso, J.F.; Schaeffer, A.J.; E Ross, A.; Hurley, P.J.; Berman, D.M.; Drake, C.G.; Thumbikat, P.; et al. A human prostatic bacterial isolate alters the prostatic microenvironment and accelerates prostate cancer progression. J. Pathol. 2015, 235, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ramnarine, V.R.; Bell, R.; Volik, S.; Davicioni, E.; Hayes, V.M.; Ren, S.; Collins, C.C. Metagenomic and metatranscriptomic analysis of human prostate microbiota from patients with prostate cancer. BMC Genom. 2019, 20, 146. [Google Scholar] [CrossRef] [PubMed]
- Kopa, Z.; Wenzel, J.; Papp, G.K.; Haidl, G. Role of granulocyte elastase and interleukin-6 in the diagnosis of male genital tract inflammation. Andrologia 2005, 37, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.E.; Memon, A.; Ahmed, S. Bladder Cancer and the Urinary Microbiome—New Insights and Future Directions: A Review. Clin. Genitourin. Cancer, 2024; Epub ahead of print. [Google Scholar] [CrossRef]
- Inchingolo, F.; Santacroce, L.; Cantore, S.; Ballini, A.; Del Prete, R.; Topi, S.; Saini, R.; Dipalma, G.; Arrigoni, R. Probiotics and EpiCor® in human health. J. Biol. Regul. Homeost. Agents 2019, 33, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Lev-Sagie, A.; Goldman-Wohl, D.; Cohen, Y.; Dori-Bachash, M.; Leshem, A.; Mor, U.; Strahilevitz, J.; Moses, A.E.; Shapiro, H.; Yagel, S.; et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat. Med. 2019, 25, 1500–1504. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Tkhruni, F.N.; Aghajanyan, A.E.; Balabekyan, T.R.; Khachatryan, T.V.; Karapetyan, K.J. Characteristic of Bacteriocins of Lactobacillus rhamnosus BTK 20-12 Potential Probiotic Strain. Probiotics Antimicrob. Proteins 2020, 12, 716–724. [Google Scholar] [CrossRef]
- van Hemert, S.; Meijerink, M.; Molenaar, D.; A Bron, P.; de Vos, P.; Kleerebezem, M.; Wells, J.M.; Marco, M.L. Identification of Lactobacillus plantarum genes modulating the cytokine response of human peripheral blood mononuclear cells. BMC Microbiol. 2010, 10, 293. [Google Scholar] [CrossRef]
- Santacroce, L.; Man, A.; Charitos, I.A.; Haxhirexha, K.; Topi, S. Current knowledge about the connection between health status and gut microbiota from birth to elderly. A narrative review. Front. Biosci. 2021, 26, 135–148. [Google Scholar] [CrossRef]
- Edwards, V.L.; Smith, S.B.; McComb, E.J.; Tamarelle, J.; Ma, B.; Humphrys, M.S.; Gajer, P.; Gwilliam, K.; Schaefer, A.M.; Lai, S.K.; et al. The Cervicovaginal Microbiota-Host Interaction Modulates Chlamydia trachomatis Infection. mBio 2019, 10, e01548-19. [Google Scholar] [CrossRef]
- Niu, X.-X.; Li, T.; Zhang, X.; Wang, S.-X.; Liu, Z.-H. Lactobacillus crispatus Modulates Vaginal Epithelial Cell Innate Response to Candida albicans. Chin. Med. J. 2017, 130, 273–279. [Google Scholar] [CrossRef]
- Man, A.; Ciurea, C.N.; Pasaroiu, D.; Savin, A.-I.; Toma, F.; Sular, F.; Santacroce, L.; Mare, A. New perspectives on the nutritional factors influencing growth rate of Candida albicans in diabetics. An in vitro study. Mem. Inst. Oswaldo. Cruz. 2017, 112, 587–592. [Google Scholar] [CrossRef]
- Petrova, M.I.; Imholz, N.C.E.; Verhoeven, T.L.A.; Balzarini, J.; Van Damme, E.J.M.; Schols, D.; Vanderleyden, J.; Lebeer, S. Lectin-Like Molecules of Lactobacillus rhamnosus GG Inhibit Pathogenic Escherichia coli and Salmonella Biofilm Formation. PLoS ONE 2016, 11, e0161337. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Scherbak, N.; Khalaf, H.; Olsson, P.-E.; Jass, J. Substances released from probiotic Lactobacillus rhamnosus GR-1 potentiate NF-κB activity in Escherichia coli-stimulated urinary bladder cells. FEMS Immunol. Med. Microbiol. 2012, 66, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Beerepoot, M.A.J.; ter Riet, G.; Nys, S.; van der Wal, W.M.; de Borgie, C.A.J.M.; de Reijke, T.M.; Prins, J.M.; Koeijers, J.; Verbon, A.; Stobberingh, E.; et al. Lactobacilli vs. antibiotics to prevent urinary tract infections: A randomized, double-blind, noninferiority trial in postmenopausal women. JAMA Intern. Med. 2012, 172, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Groah, S.L.; Rounds, A.K.; Ljungberg, I.H.; Sprague, B.M.; Frost, J.K.; Tractenberg, R.E. Intravesical Lactobacillus rhamnosus GG is safe and well tolerated in adults and children with neurogenic lower urinary tract dysfunction: First-in-human trial. Ther. Adv. Urol. 2019, 11, 1756287219875594. [Google Scholar] [CrossRef] [PubMed]
- Kenneally, C.; Murphy, C.P.; Sleator, R.D.; Culligan, E.P. The urinary microbiome and biological therapeutics: Novel therapies for urinary tract infections. Microbiol. Res. 2022, 259, 127010. [Google Scholar] [CrossRef] [PubMed]
- Thomas, X.; Destoumieux-Garzón, D.; Peduzzi, J.; Afonso, C.; Blond, A.; Birlirakis, N.; Goulard, C.; Dubost, L.; Thai, R.; Tabet, J.-C.; et al. Siderophore Peptide, a New Type of Post-translationally Modified Antibacterial Peptide with Potent Activity. J. Biol. Chem. 2004, 279, 28233–28242. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadis, G.; Destoumieux-Garzón, D.; Lombard, C.; Rebuffat, S.; Peduzzi, J. Isolation and characterization of two members of the siderophore-microcin family, microcins M and H47. Antimicrob. Agents Chemother. 2010, 54, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Zdziarski, J.; Brzuszkiewicz, E.; Wullt, B.; Liesegang, H.; Biran, D.; Voigt, B.; Grönberg-Hernandez, J.; Ragnarsdottir, B.; Hecker, M.; Ron, E.Z.; et al. Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients. PLoS Pathog. 2010, 6, e1001078. [Google Scholar] [CrossRef]
- Roos, V.; Ulett, G.C.; Schembri, M.A.; Klemm, P. The Asymptomatic Bacteriuria Escherichia coli Strain 83972 Outcompetes Uropathogenic E. coli Strains in Human Urine. Infect. Immun. 2006, 74, 615–624. [Google Scholar] [CrossRef]
- Stork, C.; Kovács, B.; Rózsai, B.; Putze, J.; Kiel, M.; Dorn, Á.; Kovács, J.; Melegh, S.; Leimbach, A.; Kovács, T.; et al. Characterization of Asymptomatic Bacteriuria Escherichia coli Isolates in Search of Alternative Strains for Efficient Bacterial Interference against Uropathogens. Front. Microbiol. 2018, 9, 214. [Google Scholar] [CrossRef]
- Trautner, B.W.; Hull, R.A.; Thornby, J.I.; Darouiche, R.O. Coating Urinary Catheters with an Avirulent Strain of Escherichia coli as a Means to Establish Asymptomatic Colonization. Infect. Control. Hosp. Epidemiol. 2007, 28, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Veziant, J.; Bonnet, M.; Occean, B.V.; Dziri, C.; Pereira, B.; Slim, K. Probiotics/Synbiotics to Reduce Infectious Complications after Colorectal Surgery: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2022, 14, 3066. [Google Scholar] [CrossRef]
- Bustamante, M.; Oomah, B.D.; Oliveira, W.P.; Burgos-Díaz, C.; Rubilar, M.; Shene, C. Probiotics and prebiotics potential for the care of skin, female urogenital tract, and respiratory tract. Folia Microbiol. 2020, 65, 245–264. [Google Scholar] [CrossRef] [PubMed]
- Baek, O.D.; Hjermitslev, C.K.; Dyreborg, L.; Baunwall, S.M.D.; Høyer, K.L.; Rågård, N.; Hammeken, L.H.; Povlsen, J.V.; Ehlers, L.H.; Hvas, C.L. Early Economic Assessment of Faecal Microbiota Transplantation for Patients with Urinary Tract Infections Caused by Multidrug-Resistant Organisms. Infect. Dis. Ther. 2023, 12, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Hocquart, M.; Pham, T.; Kuete, E.; Tomei, E.; Lagier, J.C.; Raoult, D. Successful Fecal Microbiota Transplantation in a Patient Suffering From Irritable Bowel Syndrome and Recurrent Urinary Tract Infections. Open Forum Infect. Dis. 2019, 6, ofz398. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Sidhu, P.K.; Rana, J.; Nehra, K. Managing urinary tract infections through phage therapy: A novel approach. Folia Microbiol. 2020, 65, 217–231. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Wojciuk, B.; Roszak, M.; Łubowska, N.; Błażejczak, P.; Jursa-Kulesza, J.; Rakoczy, R.; Masiuk, H.; Dołęgowska, B. Environmental Phage-Based Cocktail and Antibiotic Combination Effects on Acinetobacter baumannii Biofilm in a Human Urine Model. Microb. Drug Resist. 2021, 27, 25–35. [Google Scholar] [CrossRef]
- Chegini, Z.; Khoshbayan, A.; Vesal, S.; Moradabadi, A.; Hashemi, A.; Shariati, A. Bacteriophage therapy for inhibition of multi drug-resistant uropathogenic bacteria: A narrative review. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 30. [Google Scholar] [CrossRef]
- Liao, K.; Lehman, S.; Tweardy, D.; Donlan, R.; Trautner, B. Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. J. Appl. Microbiol. 2012, 113, 1530–1539. [Google Scholar] [CrossRef]
- Catte, A.; Ramaswamy, V.K.; Vargiu, A.V.; Malloci, G.; Bosin, A.; Ruggerone, P. Common recognition topology of mex transporters of Pseudomonas aeruginosa revealed by molecular modelling. Front. Pharmacol. 2022, 13, 1021916. [Google Scholar] [CrossRef]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2021, 21, 427–436. [Google Scholar] [CrossRef]
- Magrone, T.; Russo, M.A.; Jirillo, E. Dietary Approaches to Attain Fish Health with Special Reference to their Immune System. Curr. Pharm. Des. 2018, 24, 4921–4931. [Google Scholar] [CrossRef]
- Nagy-Bota, M.C.; Man, A.; Santacroce, L.; Brinzaniuc, K.; Pap, Z.; Pacurar, M.; Pribac, M.; Ciurea, C.N.; Pintea-Simon, I.A.; Kovacs, M. Essential Oils as Alternatives for Root-Canal Treatment and Infection Control against Enterococcus faecalis—A Preliminary Study. Appl. Sci. 2021, 11, 1422. [Google Scholar] [CrossRef]
- Spencer, J.D.; Schwaderer, A.L.; Becknell, B.; Watson, J.; Hains, D.S. The innate immune response during urinary tract infection and pyelonephritis. Pediatr. Nephrol. 2014, 29, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, T.; Nakazato, M.; Ihi, T.; Minematsu, T.; Chino, N.; Nakanishi, T.; Shimizu, A.; Kangawa, K.; Matsukura, S. Structural Analysis of Human β-Defensin-1 and Its Significance in Urinary Tract Infection. Nephron 2000, 85, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chromek, M. The role of the antimicrobial peptide cathelicidin in renal diseases. Pediatr. Nephrol. 2015, 30, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Zasloff, M. Antimicrobial Peptides, Innate Immunity, and the Normally Sterile Urinary Tract. J. Am. Soc. Nephrol. 2007, 18, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Babikir, I.H.; Abugroun, E.A.; Bilal, N.E.; Alghasham, A.A.; Abdalla, E.E.; Adam, I. The impact of cathelicidin, the human antimicrobial peptide LL-37 in urinary tract infections. BMC Infect. Dis. 2018, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Jackson, A.; DaJusta, D.; McLeod, D.; Alpert, S.; Jayanthi, V.; McHugh, K.; Schwaderer, A.; Becknell, B.; Ching, C. Urinary antimicrobial peptides: Potential novel biomarkers of obstructive uropathy. J. Pediatr. Urol. 2018, 14, 238.e1–238.e6. [Google Scholar] [CrossRef]
- Mao, X.; Yao, R.; Guo, H.; Bao, L.; Bao, Y.; Xu, Y.; Sun, J.; Guo, S.; Shi, Y.; Liu, S.; et al. Polysaccharides extract from Vaccaria segetalis seeds inhibits kidney infection by regulating cathelicidin expression. J. Ethnopharmacol. 2021, 267, 113505. [Google Scholar] [CrossRef]
- Wang, Y. The Function of OmpA in Escherichia coli. Biochem. Biophys. Res. Commun. 2002, 292, 396–401. [Google Scholar] [CrossRef]
- Kandaswamy, K.; Liew, T.H.; Wang, C.Y.; Huston-Warren, E.; Meyer-Hoffert, U.; Hultenby, K.; Schröder, J.M.; Caparon, M.G.; Normark, S.; Henriques-Normark, B.; et al. Focal targeting by human β-defensin 2 disrupts localized virulence factor assembly sites in Enterococcus faecalis. Proc. Natl. Acad. Sci. USA 2013, 110, 20230–20235. [Google Scholar] [CrossRef]
- Fenner, A. Antimicrobial peptide derived from moths can eradicate UPEC biofilms and could offer a novel therapeutic option. Nat. Rev. Urol. 2020, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Genet. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Hirakawa, H.; Suzue, K.; Tomita, H. Roles of the Tol/Pal System in Bacterial Pathogenesis and Its Application to Antibacterial Therapy. Vaccines 2022, 10, 422. [Google Scholar] [CrossRef]
- Trautner, B.W. Management of catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 2010, 23, 76–82. [Google Scholar] [CrossRef]
- Roy, S.M.; Riley, M.A. Evaluation of the potential of colicins to prevent extraluminal contamination of urinary catheters by Escherichia coli. Int. J. Antimicrob. Agents 2019, 54, 619–625. [Google Scholar] [CrossRef]
- Trivedi, D.; Jena, P.K.; Seshadri, S. Colicin E2 Expression in Lactobacillus brevis DT24, A Vaginal Probiotic Isolate, against Uropathogenic Escherichia coli. ISRN Urol. 2014, 2014, 869610. [Google Scholar] [CrossRef]
- Field, D.; Hill, C.; Cotter, P.D.; Ross, R.P. The dawning of a ‘Golden era’ in lantibiotic bioengineering. Mol. Microbiol. 2010, 78, 1077–1087. [Google Scholar] [CrossRef]
- Healy, B.; Field, D.; O’connor, P.M.; Hill, C.; Cotter, P.D.; Ross, R.P. Intensive mutagenesis of the nisin hinge leads to the rational design of enhanced derivatives. PLoS ONE 2013, 8, e79563. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Z.-Z.; Chen, X.-Z.; Yang, W.; Huan, L.-D. Site-directed mutagenesis of the hinge region of nisinZ and properties of nisinZ mutants. Appl. Microbiol. Biotechnol. 2004, 64, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Bottalico, L. A successful history: Probiotics and their potential as antimicrobials. Expert. Rev. Anti-Infect. Ther. 2019, 17, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, M.; Kobayashi, M.; Sano, Y.; Masaki, H. Construction and characterization of pyocin-colicin chimeric proteins. J. Bacteriol. 1996, 178, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bram, E.E.; Weiss, R. Genetically Programmable Pathogen Sense and Destroy. ACS Synth. Biol. 2013, 2, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Rebuffat, S. Ribosomally synthesized peptides, foreground players in microbial interactions: Recent developments and unanswered questions. Nat. Prod. Rep. 2022, 39, 273–310. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Imbimbo, C.; Ballini, A.; Crocetto, F.; Scacco, S.; Cantore, S.; Di Zazzo, E.; Colella, M.; Jirillo, E. Testicular Immunity and Its Connection with the Microbiota. Physiological and Clinical Implications in the Light of Personalized Medicine. J. Pers. Med. 2022, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, E.; Laviña, M. The proton channel is the minimal structure of ATP synthase necessary and sufficient for microcin h47 antibiotic action. Antimicrob. Agents Chemother. 2003, 47, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Patzer, S.I.; Baquero, M.R.; Bravo, D.; Moreno, F.; Hantke, K. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology 2003, 149, 2557–2570. [Google Scholar] [CrossRef]
- Parker, J.K.; Davies, B.W. Microcins reveal natural mechanisms of bacterial manipulation to inform therapeutic development. Microbiology 2022, 168, 001175. [Google Scholar] [CrossRef]
- Piscotta, F.J.; Tharp, J.M.; Liu, W.R.; Link, A.J. Expanding the chemical diversity of lasso peptide MccJ25 with genetically encoded noncanonical amino acids. Chem. Commun. 2015, 51, 409–412. [Google Scholar] [CrossRef]
- Yu, H.; Ma, Z.; Meng, S.; Qiao, S.; Zeng, X.; Tong, Z.; Jeong, K.C. A novel nanohybrid antimicrobial based on chitosan nanoparticles and antimicrobial peptide microcin J25 with low toxicity. Carbohydr. Polym. 2021, 253, 117309. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.; Cusumano, Z.; Han, Z.; Binkley, J.; Kostakioti, M.; Hannan, T.; Pinkner, J.S.; Klein, R.; Kalas, V.; Crowley, J.; et al. Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections. J. Med. Chem. 2016, 59, 9390–9408. [Google Scholar] [CrossRef]
- Ballini, A.; Charitos, I.A.; Cantore, S.; Topi, S.; Bottalico, L.; Santacroce, L. About Functional Foods: The Probiotics and Prebiotics State of Art. Antibiotics 2023, 12, 635. [Google Scholar] [CrossRef]
- Guiton, P.S.; Cusumano, C.K.; Kline, K.A.; Dodson, K.W.; Han, Z.; Janetka, J.W.; Henderson, J.P.; Caparon, M.G.; Hultgren, S.J. Combinatorial small-molecule therapy prevents uropathogenic Escherichia coli catheter-associated urinary tract infections in mice. Antimicrob. Agents Chemother. 2012, 56, 4738–4745. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L.; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Guo, M.; Zheng, Y.; Zhang, Y.; De, Y.; Xu, C.; Zhang, L.; Sun, R.; Lv, Y.; Liang, Y.; et al. Current Evidence of Interleukin-6 Signaling Inhibitors in Patients with COVID-19: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 615972. [Google Scholar] [CrossRef] [PubMed]
- Wnorowska, U.; Piktel, E.; Durnaś, B.; Fiedoruk, K.; Savage, P.B.; Bucki, R. Use of ceragenins as a potential treatment for urinary tract infections. BMC Infect. Dis. 2019, 19, 369. [Google Scholar] [CrossRef] [PubMed]
- Krakowska, A.; Cedzyński, M.; Wosiak, A.; Swiechowski, R.; Krygier, A.; Tkaczyk, M.; Zeman, K. Toll-like receptor (TLR2, TLR4) polymorphisms and their influence on the incidence of urinary tract infections in children with and without urinary tract malformation. Central Eur. J. Immunol. 2022, 47, 260–266. [Google Scholar] [CrossRef]
- Lin, A.E.; Beasley, F.C.; Olson, J.; Keller, N.; Shalwitz, R.A.; Hannan, T.J.; Hultgren, S.J.; Nizet, V. Role of Hypoxia Inducible Factor-1α (HIF-1α) in Innate Defense against Uropathogenic Escherichia coli Infection. PLoS Pathog. 2015, 11, e1004818. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Yun, C.; Sun, L.; Xia, J.; Wu, Q.; Wang, Y.; Wang, L.; Zhang, Y.; Liang, X.; Wang, L.; et al. Gut microbiota–bile acid–interleukin-22 axis orchestrates polycystic ovary syndrome. Nat. Med. 2019, 25, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, G.F.; Monticelli, L.A.; Alenghat, T.; Fung, T.C.; Hutnick, N.A.; Kunisawa, J.; Shibata, N.; Grunberg, S.; Sinha, R.; Zahm, A.M.; et al. Innate lymphoid cells promote anatomical containment of lymphoid-resident commensal bacteria. Science 2012, 336, 1321–1325. [Google Scholar] [CrossRef] [PubMed]
- Sabih, A.; Leslie, S.W. Complicated Urinary Tract Infections. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Huttner, A.; Hatz, C.; Dobbelsteen, G.v.D.; Abbanat, D.; Hornacek, A.; Frölich, R.; Dreyer, A.M.; Martin, P.; Davies, T.; Fae, K.; et al. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: A randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect. Dis. 2017, 17, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Gómez, M.F.; Padilla-Fernã¡Ndez, B.; Garcãa-Cenador, M.B.; Virseda-Rodrãguez, J.; Martãn-Garcãa, I.; Sã¡Nchez-Escudero, A.; Vicente-Arroyo, M.J.; Mirón-Canelo, J.A. Comparison of sublingual therapeutic vaccine with antibiotics for the prophylaxis of recurrent urinary tract infections. Front. Cell. Infect. Microbiol. 2015, 5, 50. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jirillo, E.; Palmirotta, R.; Colella, M.; Santacroce, L. A Bird’s-Eye View of the Pathophysiologic Role of the Human Urobiota in Health and Disease: Can We Modulate It? Pathophysiology 2024, 31, 52-67. https://doi.org/10.3390/pathophysiology31010005
Jirillo E, Palmirotta R, Colella M, Santacroce L. A Bird’s-Eye View of the Pathophysiologic Role of the Human Urobiota in Health and Disease: Can We Modulate It? Pathophysiology. 2024; 31(1):52-67. https://doi.org/10.3390/pathophysiology31010005
Chicago/Turabian StyleJirillo, Emilio, Raffaele Palmirotta, Marica Colella, and Luigi Santacroce. 2024. "A Bird’s-Eye View of the Pathophysiologic Role of the Human Urobiota in Health and Disease: Can We Modulate It?" Pathophysiology 31, no. 1: 52-67. https://doi.org/10.3390/pathophysiology31010005
APA StyleJirillo, E., Palmirotta, R., Colella, M., & Santacroce, L. (2024). A Bird’s-Eye View of the Pathophysiologic Role of the Human Urobiota in Health and Disease: Can We Modulate It? Pathophysiology, 31(1), 52-67. https://doi.org/10.3390/pathophysiology31010005









