Duration of SARS-CoV-2 RNA Shedding Is Significantly Influenced by Disease Severity, Bilateral Pulmonary Infiltrates, Antibiotic Treatment, and Diabetic Status: Consideration for Isolation Period
Abstract
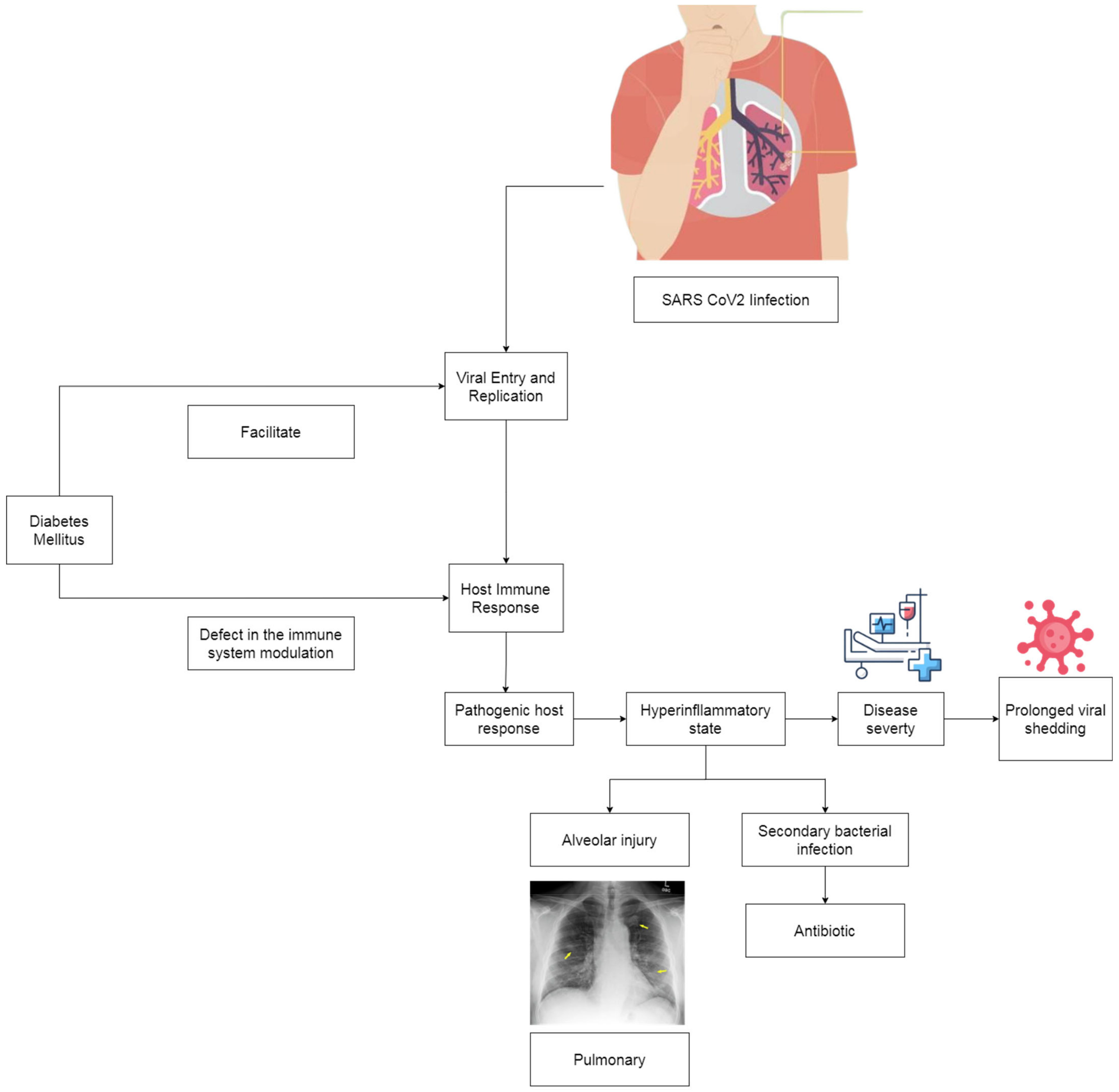
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Selection
- (1)
- Asymptomatic case.
- (2)
- Mild disease: symptomatic patients without evidence of viral pneumonia or hypoxia. Symptoms might appear as fever; cough; fatigue; anorexia; shortness of breath; myalgia; sore throat; nasal congestion; headache; diarrhea; nausea and vomiting; anosmia; or loss of appetite.
- (3)
- Moderate disease: patients with clinical signs of pneumonia (fever, cough, shortness of breath, rapid breathing) but no symptoms of severe pneumonia, including oxygen saturation >93% on room air.
- (4)
- Severe disease: patients with clinical signs of pneumonia (fever, cough, shortness of breath) plus respiratory rate >30 times/minute, severe respiratory distress, or SpO2 < 93% on room air.
- (5)
- Critical disease: patients with acute respiratory distress syndrome (ARDS), sepsis, and septic shock.
2.2. Definition
2.3. Statistical Analysis
2.4. Ethical Clearance
3. Results
3.1. Baseline Characteristics
3.2. Bivariate Analysis
3.3. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weekly Epidemiological Update on COVID-19—1 March 2023. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---1-march-2023 (accessed on 1 March 2023).
- Infeksi Emerging Kementerian Kesehatan RI. Available online: https://infeksiemerging.kemkes.go.id/dashboard/covid-19 (accessed on 12 March 2023).
- Li, T.Z.; Cao, Z.H.; Chen, Y.; Cai, M.T.; Zhang, L.Y.; Xu, H.; Zhang, J.-Y.; Ma, C.-H.; Liu, Y.; Gao, L.-J.; et al. Duration of SARS-CoV-2 RNA shedding and factors associated with prolonged viral shedding in patients with COVID-19. J. Med. Virol. 2020, 93, 506–512. [Google Scholar] [CrossRef]
- Zhou, B.; She, J.; Wang, Y.; Ma, X. Duration of Viral Shedding of Discharged Patients With Severe COVID-19. Clin. Infect. Dis. 2020, 71, 2240–2242. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Sang, L.; Ye, F.; Ruan, S.; Zhong, B.; Song, T.; Alshukairi, A.N.; Chen, R.; Zhang, Z.; et al. Kinetics of viral load and antibody response in relation to COVID-19 severity. J. Clin. Investig. 2020, 130, 5235–5244. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Han, P.; Zhu, R.; Bai, T.; Yi, J.; Zhao, X.; Tao, M.; Quan, R.; Chen, C.; Zhang, Y.; et al. Risk factors for viral RNA shedding in COVID-19 patients. Eur. Respir. J. 2020, 56, 2001190. [Google Scholar] [CrossRef] [PubMed]
- Karia, R.; Nagraj, S. A Review of Viral Shedding in Resolved and Convalescent COVID-19 Patients. SN Compr. Clin. Med. 2020, 2, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.F.; Chorlton, S.; Tyson, J.; Al-Rawahi, G.N.; Jassem, A.N.; Prystajecky, N.; Masud, S.; Deans, G.D.; Chapman, M.G.; Mirzanejad, Y.; et al. COVID-19 in an immunocompromised host: Persistent shedding of viable SARS-CoV-2 and emergence of multiple mutations: A case report. Int. J. Infect. Dis. 2021, 114, 178–182. [Google Scholar] [CrossRef]
- Chen, C.F.; Tsai, T.Y.; Yu, C.H.; Cheng, H.L.; Yeh, T.Y. Prolonged viral shedding and new mutations of COVID-19 could complicate the control of the pandemic. Access Microbiol. 2020, 2, acmi000133. [Google Scholar] [CrossRef]
- Wibisono, E.; Hadi, U.; Bramantono Arfijanto, M.V.; Rusli, M.; Rahman, B.E.; Asmarawati, T.P.; Choirunnisa, M.L.; Rahayu, D.R.P. National early warning score (NEWS) 2 predicts hospital mortality from COVID-19 patients. Ann. Med. Surg. 2022, 76, 103462. [Google Scholar] [CrossRef] [PubMed]
- Nugroho, C.W.; Suryantoro, S.D.; Yuliasih, Y.; Rosyid, A.N.; Asmarawati, T.P.; Andrianto, L.; Setiawan, H.W.; Mahdi, B.A.; Windradi, C.; Agustin, E.D.; et al. Optimal use of tocilizumab for severe and critical COVID-19: A systematic review and meta-analysis. F1000Research 2021, 10, 73. [Google Scholar] [CrossRef]
- Soegiarto, G.; Wulandari, L.; Purnomosari, D.; Dhia Fahmita, K.; Ikhwan Gautama, H.; Tri Hadmoko, S.; Prasetyo, M.E.; Mahdi, B.A.; Arafah, N.; Prasetyaningtyas, D.; et al. Hypertension is asso-ciated with antibody response and breakthrough infection in health care workers following vaccination with inactivated SARS-CoV-2. Vaccine 2022, 40, 4046–4056. [Google Scholar] [CrossRef]
- Asmarawati, T.P.; Suryantoro, S.D.; Rosyid, A.N.; Marfiani, E.; Windradi, C.; Mahdi, B.A.; Sutanto, H. Predictive Value of Sequential Organ Failure Assessment, Quick Sequential Organ Failure Assessment, Acute Physiology and Chronic Health Evaluation II, and New Early Warning Signs Scores Estimate Mortality of COVID-19 Patients Requiring Intensive Care Unit. Indian J. Crit. Care Med. 2022, 26, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Asmarawati, T.P.; Rosyid, A.N.; Suryantoro, S.D.; Mahdi, B.A.; Windradi, C.; Wulaningrum, P.A.; Arifianto, M.V.; Bramantono, B.; Triyono, E.A.; Rusli, M.; et al. The clinical impact of bacterial co-infection among moderate, severe and critically ill COVID-19 patients in the second referral hospital in Surabaya. F1000Research 2021, 10, 1–16. [Google Scholar] [CrossRef]
- Burhan, E.; Susanto, A.D.; Isbaniah, F.; Sally, A.N.; Eka, G.; Ceva, W.P.; Susilo, A.; Firdaus, I.; Santoso, A.; Juzar, D.A.; et al. Pedoman Tatalaksana COVID-19, 3rd ed.; PDPI; PERKI; PAPDI; PERDATIN; IDAI: Jakarta, Indonesia, 2020; pp. 36–37. [Google Scholar]
- Qi, L.; Yang, Y.; Jiang, D.; Tu, C.; Wan, L.; Chen, X.; Li, Z. Factors associated with the duration of viral shedding in adults with COVID-19 outside of Wuhan, China: A retrospective cohort study. Int. J. Infect. Dis. 2020, 96, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Chen, Y.; Yuan, J.; Yi, P.; Ding, C.; Wu, W.; Li, Y.; Ni, Q.; Zou, R.; Li, X.; et al. Factors Associated With Prolonged Viral RNA Shedding in Patients with Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 799–806. [Google Scholar] [CrossRef]
- Feng, Z.; Li, J.; Yao, S.; Yu, Q.; Zhou, W.; Mao, X.; Li, H.; Kang, W.; Ouyang, X.; Mei, J.; et al. Clinical Factors Associated with Progression and Prolonged Viral Shedding in COVID-19 Patients: A Multicenter Study. Aging Dis. 2020, 11, 1069–1081. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Aly, M.H.; Rahman, S.S.; Ahmed, W.A.; Alghamedi, M.H.; Al Shehri, A.A.; Alkalkami, A.M.; Hassan, M.H. Indicators of Critical Illness and Predictors of Mortality in COVID-19 Patients. Infect. Drug Resist. 2020, 13, 1995–2000. [Google Scholar] [CrossRef]
- Zhang, G.; Hu, C.; Luo, L.; Fang, F.; Chen, Y.; Li, J.; Peng, Z.; Pan, H. Clinical features and short-term outcomes of 221 patients with COVID-19 in Wuhan, China. J. Clin. Virol. 2020, 127, 104364. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, T.; Ren, H.; Sun, S.; Yu, X.; Sheng, J.; Shi, Y.; Zhao, H. Impact of age on duration of viral RNA shedding in patients with COVID-19. Aging 2020, 12, 22399–22404. [Google Scholar] [CrossRef]
- Pradhan, A.; Olsson, P.-E. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol. Sex Differ. 2020, 11, 53. [Google Scholar] [CrossRef]
- Muhamad, S.A.; Ugusman, A.; Kumar, J.; Skiba, D.; Hamid, A.A.; Aminuddin, A. COVID-19 and Hypertension: The What, the Why, and the How. Front. Physiol. 2021, 12, 665064. [Google Scholar] [CrossRef] [PubMed]
- Buetti, N.; Trimboli, P.; Mazzuchelli, T.; Priore, E.L.; Balmelli, C.; Trkola, A.; Conti, M.; Martinetti, G.; Elzi, L.; Ceschi, A.; et al. Diabetes mellitus is a risk factor for prolonged SARS-CoV-2 viral shedding in lower respiratory tract samples of critically ill patients. Endocrine 2020, 70, 454–460. [Google Scholar] [CrossRef]
- Codo, A.C.; Davanzo, G.G.; de Brito Monteiro, L.; de Souza, G.F.; Muraro, S.P.; Virgilio-Da-Silva, J.V.; Prodonoff, J.S.; Carregari, V.C.; de Biagi Junior, C.A.O.; Crunfli, F.; et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020, 32, 437–446.e5. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, H.; Ye, H.; Hu, Y.; Zheng, N.; Huang, Z.; Xiong, Z.; Fu, L.; Cai, T. Risk factors for prolonged virus shedding of respiratory tract and fecal in adults with severe acute respiratory syndrome coronavirus-2 infection. J. Clin. Lab. Anal. 2021, 35, e23923. [Google Scholar] [CrossRef]
- Cunha, M.D.P.; Vilela, A.P.P.; Molina, C.V.; Acuna, S.M.; Muxel, S.M.; Barroso, V.D.M.; Baroni, S.; de Oliveira, L.G.; de Souza, A.Y.; Peron, J.P.S.; et al. Atypical Prolonged Viral Shedding With Intra-Host SARS-CoV-2 Evolution in a Mildly Affected Symptomatic Patient. Front. Med. 2021, 8, 1–11. [Google Scholar] [CrossRef]
- Menghua, W.; Xin, Z.; Jianwei, L.; Yu, Z.; Qinwei, Y. Case report: One case of coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV with a normal CD4+ T cell count. AIDS Res. Ther. 2020, 17, 46. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Hameed, M.; Alsoub, H.; Khatib, M.; Jamal, W.; Ahmad, M. COVID-19: Prolonged viral shedding in an HIV patient with literature review of risk factors for prolonged viral shedding and its implications for isolation strategies. Clin. Case Rep. 2021, 9, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Askanase, A.D.; Khalili, L.; Buyon, J.P. Thoughts on COVID-19 and autoimmune diseases. Lupus Sci. Med. 2020, 7, e000396. [Google Scholar] [CrossRef] [PubMed]
- Aghili, S.M.M.; Ebrahimpur, M.; Arjmand, B.; Shadman, Z.; Pejman Sani, M.; Qorbani, M.; Larijani, B.; Payab, M. Obesity in COVID-19 era, implications for mechanisms, comorbidities, and prognosis: A review and meta-analysis. Int. J. Obes. 2021, 45, 998–1016. [Google Scholar] [CrossRef]
- De Leeuw, A.J.M.; Oude Luttikhuis, M.A.M.; Wellen, A.C.; Müller, C.; Calkhoven, C.F. Obesity and its impact on COVID-19. J. Mol. Med. 2021, 99, 899–915. [Google Scholar] [CrossRef]
- Bitker, L.; Dhelft, F.; Chauvelot, L.; Frobert, E.; Folliet, L.; Mezidi, M.; Trouillet-Assant, S.; Belot, A.; Lina, B.; Wallet, F.; et al. Protracted viral shedding and viral load are associated with ICU mortality in Covid-19 patients with acute respiratory failure. Ann. Intensiv. Care 2020, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, S.M.; Kim, W.Y.; Sohn, C.H.; Seo, D.W.; Oh, B.J.; Lee, J.H.; Lee, Y.S.; Lim, K.S. Factors promoting the prolonged shedding of the pandemic (H1N1) 2009 influenza virus in patients treated with oseltamivir for 5 days. Influenza Other Respir. Viruses 2012, 7, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Langford, B.J.; So, M.; Raybardhan, S.; Leung, V.; Westwood, D.; MacFadden, D.R.; Soucy, J.-P.R.; Daneman, N. Bacterial co-infection and secondary infection in patients with COVID-19: A living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020, 26, 1622–1629. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Total (n = 162) |
|---|---|
| Sex, n (%) | |
| Male | 83 (51.23) |
| Female | 79 (48.76) |
| Age, n (%) | |
| ≥60 years | 40 (24.69) |
| <60 years | 122 (75.31) |
| Clinical symptoms, n (%) | |
| Fever | 83 (51.23) |
| Cough | 85 (52.47) |
| Expectorations | 35 (21.60) |
| Hemoptysis | 1 (0.62) |
| Dyspnea | 80 (49.38) |
| Disease severity, n (%) | |
| Mild | 71 (43.83) |
| Moderate | 34 (20.99) |
| Severe | 43 (26.54) |
| Critical | 14 (8.64) |
| Length of hospitalization, mean ± SD | 7.41 ± 8.82 |
| Chest X-ray, n (%) | |
| Normal | 32 (19.75) |
| Unilateral infiltrate | 15 (9.26) |
| Bilateral infiltrates | 110 (67.90) |
| Atypical | 5 (3.09) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 55 (33.95) |
| DM with chronic complication | 12 (7.41) |
| DM without chronic complication | 43 (26.54) |
| Hypertension | 46 (28.40) |
| HIV/AIDS | 11 (6.79) |
| Malignancy | 17 (10.49) |
| Liver cirrhosis | 11 (6.79) |
| Autoimmune disease | 7 (4.32) |
| Obesity | 9 (5.55) |
| Treatment, n (%) | |
| Antiviral therapy: | |
| Lopinavir/ritonavir | 34 (20.99) |
| Favipiravir | 37 (22.84) |
| Remdesivir | 44 (27.16) |
| Oseltamivir | 25 (15.43) |
| Corticosteroid | 63 (38.89) |
| Convalescent plasma | 6 (3.70) |
| Antibiotic | 77 (47.53) |
| Oxygen therapy | 89 (54.94) |
| Output, n (%) | |
| Death | 11 (6.79) |
| Cured | 151 (93.21) |
| Duration of viral shedding (days) (Mean ± SD) | 13 ± 8.44 |
| Characteristics | Total (n = 162) | Duration of Viral Shedding | p-Value | |
|---|---|---|---|---|
| <13 days (n = 99) | ≥13 days (n = 63) | |||
| Sex | ||||
| Male | 83 | 50 (50.5%) | 33 (52.4%) | 0.816 |
| Female | 79 | 49 (49.5%) | 30 (47.6%) | |
| Age | ||||
| ≥60 years | 40 | 20 (20.2%) | 20 (31.7%) | 0.097 * |
| <60 years | 122 | 79 (79.8%) | 43 (68.3%) | |
| Clinical symptoms | ||||
| Fever | 83 | 45 (45.5%) | 38 (60.3%) | 0.065 * |
| Cough | 85 | 48 (48.5%) | 37 (58.7%) | 0.203 * |
| Expectoration | 35 | 18 (18.2%) | 17 (27.0%) | 0.184 * |
| Hemoptysis | 1 | 1 (1.0%) | 0 (0%) | 0.484 |
| Dyspnea | 80 | 41 (41.4%) | 39 (61.9%) | 0.011 * |
| Disease severity | ||||
| Mild | 71 | 50 (50.5%) | 21 (33.3%) | 0.012 * |
| Moderate | 34 | 24 (24.2%) | 10 (15.9%) | |
| Severe | 43 | 19 (19.2%) | 24 (38.1%) | |
| Critically ill | 14 | 6 (6.1%) | 8 (12.7%) | |
| Chest X-ray | ||||
| No infiltrate | 32 | 26 (26.3%) | 6 (9.5%) | 0.009 * |
| Unilateral infiltrate | 15 | 12 (12.1%) | 3 (4.8%) | 0.115 * |
| Bilateral infiltrate | 110 | 56 (56.6%) | 54 (85.7%) | 0.00 * |
| Atypical | 5 | 4 (4.0%) | 1 (1.6%) | 0.379 |
| Comorbidities | ||||
| Diabetes Mellitus | 55 | 24 (24.2%) | 31 (49.2%) | 0.001 * |
| 12 | 5 (6.1%) | 7 (9.5%) | 0.151 * |
| 43 | 18 (18.2%) | 25 (39.7%) | 0.003 * |
| Hypertension | 46 | 22 (22.2%) | 24 (38.1%) | 0.029 * |
| HIV/AIDS | 11 | 5 (5.1%) | 6 (9.5%) | 0.27 |
| Malignancy | 17 | 11 (11.1%) | 6 (9.5%) | 0.748 |
| Liver cirrhosis | 11 | 5 (5.1%) | 6 (9.5%) | 0.27 |
| Autoimmune disease | 7 | 6 (6.1%) | 1 (1.6%) | 0.172 * |
| Obesity | 9 | 5 (5.1%) | 4 (6.3%) | 0.725 |
| Treatment | ||||
| Lopinavir/ritonavir | 34 | 10 (10.1%) | 24 (38.1%) | 0.00 * |
| Favipiravir | 37 | 29 (29.3%) | 8 (12.7%) | 0.014 * |
| Remdesivir | 44 | 27 (27.3%) | 17 (27.0%) | 0.968 |
| Oseltamivir | 25 | 17 (17.2%) | 8 (12.7%) | 0.442 |
| Corticosteroid | 63 | 32 (32.3%) | 31 (49.2%) | 0.032 |
| Convalescent plasma | 6 | 2 (2.0%) | 4 (6.3%) | 0.155 * |
| Antibiotic | 77 | 36 (36.4%) | 41 (65.1%) | 0.00 * |
| Oxygen therapy | 89 | 44 (44.4%) | 45 (71.4%) | 0.001 * |
| Outcome | ||||
| Death | 12 | 7 (7.1%) | 4 (6.3%) | 0.859 |
| Cured | 151 | 92 (92.9%) | 59 (93.7%) | |
| Variable | Multivariate Analysis | Stepwise Analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | aOR | 95% CI | p-Value | ||
| ≥60 years | 1.88 | 0.89–3.78 | 0.09 | ||||
| Disease severity | 3.05 | 1.56–5.97 | 0.001 * | 2.94 | 1.34–6.44 | 0.007 * | |
| Fever | 1.82 | 0.96–3.46 | 0.065 | ||||
| Dyspnea | 2.29 | 1.20–4.39 | 0.011 * | ||||
| Cough | 1.51 | 0.79–2.86 | 0.203 | ||||
| Expectoration | 1.66 | 0.78–3.54 | 0.184 | ||||
| No infiltrate | 0.299 | 0.11–0.76 | 0.009 * | ||||
| Unilateral infiltrate | 0.363 | 0.09–1.34 | 0.115 | ||||
| Bilateral pulmonary infiltrates | 4.61 | 2.05–10.35 | 0.000 * | 2.79 | 1.14–6.84 | 0.025 * | |
| Hypertension | 2.15 | 1.08–4.32 | 0.029 * | ||||
| Diabetes mellitus | 3.027 | 1.54–5.94 | 0.001 * | 2.17 | 1.02–4.63 | 0.046 * | |
| Autoimmune disease | 0.25 | 0.03–2.13 | 0.172 | ||||
| Oxygen therapy | 3.13 | 1.59–6.14 | 0.001 * | ||||
| Antibiotic | 3.26 | 1.69–6.31 | 0.000 * | 3.66 | 1.74–7.71 | 0.001 * | |
| Oral antiviral | 1.36 | 0.70–2.62 | 0.36 | 1.96 | 0.89–4.29 | 0.093 | |
| Corticosteroid | 2.03 | 1.06–3.88 | 0.032 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arfijanto, M.V.; Asmarawati, T.P.; Bramantono, B.; Rusli, M.; Rachman, B.E.; Mahdi, B.A.; Nasronudin, N.; Hadi, U. Duration of SARS-CoV-2 RNA Shedding Is Significantly Influenced by Disease Severity, Bilateral Pulmonary Infiltrates, Antibiotic Treatment, and Diabetic Status: Consideration for Isolation Period. Pathophysiology 2023, 30, 186-198. https://doi.org/10.3390/pathophysiology30020016
Arfijanto MV, Asmarawati TP, Bramantono B, Rusli M, Rachman BE, Mahdi BA, Nasronudin N, Hadi U. Duration of SARS-CoV-2 RNA Shedding Is Significantly Influenced by Disease Severity, Bilateral Pulmonary Infiltrates, Antibiotic Treatment, and Diabetic Status: Consideration for Isolation Period. Pathophysiology. 2023; 30(2):186-198. https://doi.org/10.3390/pathophysiology30020016
Chicago/Turabian StyleArfijanto, Muhammad Vitanata, Tri Pudy Asmarawati, Bramantono Bramantono, Musofa Rusli, Brian Eka Rachman, Bagus Aulia Mahdi, Nasronudin Nasronudin, and Usman Hadi. 2023. "Duration of SARS-CoV-2 RNA Shedding Is Significantly Influenced by Disease Severity, Bilateral Pulmonary Infiltrates, Antibiotic Treatment, and Diabetic Status: Consideration for Isolation Period" Pathophysiology 30, no. 2: 186-198. https://doi.org/10.3390/pathophysiology30020016
APA StyleArfijanto, M. V., Asmarawati, T. P., Bramantono, B., Rusli, M., Rachman, B. E., Mahdi, B. A., Nasronudin, N., & Hadi, U. (2023). Duration of SARS-CoV-2 RNA Shedding Is Significantly Influenced by Disease Severity, Bilateral Pulmonary Infiltrates, Antibiotic Treatment, and Diabetic Status: Consideration for Isolation Period. Pathophysiology, 30(2), 186-198. https://doi.org/10.3390/pathophysiology30020016






