Advantages of Multiposition Scanning in Echocardiographic Assessment of the Severity of Discordant Aortic Stenosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Echocardiography Analysis
2.2. Multiple-View Scanning of the Aortic Valve
2.3. Reproducibility
2.4. Statistical Analysis
3. Results
Reproducibility Assessment
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AS | aortic stenosis |
| ΔPmean | mean pressure gradient |
| Vmax | peak aortic jet velocity |
| AVA | aortic valve area |
| AVAi | indexed aortic valve area |
| EOA | effective orifice area |
| TTE | transthoracic echocardiography |
| AV | aortic valve |
| RPW | right parasternal window |
| BSA | body surface area |
| BMI | body mass index |
| COPD | chronic obstructive pulmonary disease |
| TIA | transient ischemic attack |
| NYHA | New York Heart Association |
| IQR | interquartile range |
| LV | left ventricle |
| ASE | American Society of Echocardiography |
| EACVI | European Association of Cardiovascular Imaging |
| CSALVOT | cross-sectional area of left ventricle outflow tract |
| VTILVOT | the left ventricle outflow tract velocity time integral |
| VTIAV | velocity time integral of transvalvular flow |
| LVOT | left ventricle outflow tract |
| A5C | apical 5-chamber view |
| LA | left atrium |
| Ao | aorta |
| ICC | intraclass correlation coefficient |
| EDILV | end-diastolic volume index of left ventricle |
| ESILV | end-systolic volume index of left ventricle |
| SILV | stroke index of left ventricle |
| EFLV | ejection fraction of left ventricle |
| ΔPmax | peak pressure gradient |
| TAVR | transcatheter aortic valve replacement |
| MACE | major adverse cardiovascular events |
References
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492, Erratum in Circulation 2014, 129, e650. [Google Scholar] [CrossRef] [PubMed]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632, Erratum in Eur. Heart J. 2022, 43, 2022. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.A.; Burwash, I.G.; Pibarot, P. Cardiac Imaging for Assessing Low-Gradient Severe Aortic Stenosis. JACC Cardiovasc. Imaging 2017, 10, 185–202. [Google Scholar] [CrossRef]
- Pazos-López, P.; Paredes-Galán, E.; Peteiro-Vázquez, J.; López-Rodríguez, E.; García-Rodríguez, C.; Bilbao-Quesada, R.; Blanco-González, E.; González-Ríos, C.; Calvo-Iglesias, F.; Íñiguez-Romo, A. Value of non-apical echocardiographic views in the up-grading of patients with aortic stenosis. Scand. Cardiovasc. J. 2021, 55, 279–286. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P., 3rd; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500, Erratum in J. Am. Coll. Cardiol. 2021, 77, 1276. [Google Scholar] [CrossRef]
- Pibarot, P.; Messika-Zeitoun, D.; Ben-Yehuda, O.; Hahn, R.T.; Burwash, I.G.; Van Mieghem, N.M.; Spitzer, E.; Leon, M.B.; Bax, J.; Otto, C.M. Moderate Aortic Stenosis and Heart Failure with Reduced Ejection Fraction: Can Imaging Guide Us to Therapy? JACC Cardiovasc. Imaging 2019, 12, 172–184. [Google Scholar] [CrossRef]
- Shah, P.K. Should severe aortic stenosis be operated on before symptom onset? Severe aortic stenosis should not be operated on before symptom onset. Circulation 2012, 126, 118–125. [Google Scholar] [CrossRef]
- Carabello, B.A. Should severe aortic stenosis be operated on before symptom onset? Aortic valve replacement should be operated on before symptom onset. Circulation 2012, 126, 112–117. [Google Scholar] [CrossRef]
- Généreux, P.; Stone, G.W.; O’Gara, P.T.; Marquis-Gravel, G.; Redfors, B.; Giustino, G.; Pibarot, P.; Bax, J.J.; Bonow, R.O.; Leon, M.B. Natural History, Diagnostic Approaches, and Therapeutic Strategies for Patients with Asymptomatic Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2016, 67, 2263–2288. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.J.; Lee, S.A.; Lee, S.; Kim, D.H.; Kim, H.K.; Yun, S.C.; Hong, G.R.; Song, J.M.; Chung, C.H.; et al. Early Surgery or Conservative Care for Asymptomatic Aortic Stenosis. N. Engl. J. Med. 2020, 382, 111–119. [Google Scholar] [CrossRef]
- Banovic, M.; Putnik, S.; Penicka, M.; Doros, G.; Deja, M.A.; Kockova, R.; Kotrc, M.; Glaveckaite, S.; Gasparovic, H.; Pavlovic, N.; et al. Aortic Valve Replacement Versus Conservative Treatment in Asymptomatic Severe Aortic Stenosis: The AVATAR Trial. Circulation 2022, 145, 648–658, Erratum in Circulation 2022, 145, e761. [Google Scholar] [CrossRef]
- Wei, C.; Li, Z.; Xu, C.; Yin, T.; Zhao, C. Timing of surgery for asymptomatic patients with severe aortic valve stenosis: An updated systematic review and meta-analysis. Hellenic. J. Cardiol. 2021, 62, 270–277. [Google Scholar] [CrossRef]
- Ullah, W.; Gowda, S.N.; Khan, M.S.; Sattar, Y.; Al-Khadra, Y.; Rashid, M.; Mohamed, M.O.; Alkhouli, M.; Kapadia, S.; Bagur, R.; et al. Early intervention or watchful waiting for asymptomatic severe aortic valve stenosis: A systematic review and meta-analysis. J. Cardiovasc. Med. 2020, 21, 897–904. [Google Scholar] [CrossRef]
- Everett, R.J.; Tastet, L.; Clavel, M.A.; Chin, C.W.L.; Capoulade, R.; Vassiliou, V.S.; Kwiecinski, J.; Gomez, M.; van Beek, E.J.R.; White, A.C.; et al. Progression of Hypertrophy and Myocardial Fibrosis in Aortic Stenosis: A Multicenter Cardiac Magnetic Resonance Study. Circ. Cardiovasc. Imaging 2018, 11, e007451. [Google Scholar] [CrossRef]
- Kvaslerud, A.B.; Santic, K.; Hussain, A.I.; Auensen, A.; Fiane, A.; Skulstad, H.; Aaberge, L.; Gullestad, L.; Broch, K. Outcomes in asymptomatic, severe aortic stenosis. PLoS ONE 2021, 16, e0249610. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow-up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef]
- Durko, A.P.; Osnabrugge, R.L.; Van Mieghem, N.M.; Milojevic, M.; Mylotte, D.; Nkomo, V.T.; Pieter Kappetein, A. Annual number of candidates for transcatheter aortic valve implantation per country: Current estimates and future projections. Eur. Heart J. 2018, 39, 2635–2642. [Google Scholar] [CrossRef]
- Michalski, B.; Dweck, M.R.; Marsan, N.A.; Cameli, M.; D’Andrea, A.; Carvalho, R.F.; Holte, E.; Podlesnikar, T.; Manka, R.; Haugaa, K.H. The evaluation of aortic stenosis, how the new guidelines are implemented across Europe: A survey by EACVI. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 357–362, Erratum in Eur. Heart J. Cardiovasc. Imaging 2020, 21, 804. [Google Scholar] [CrossRef]
- Benfari, G.; Gori, A.M.; Rossi, A.; Papesso, B.; Vassanelli, C.; Zito, G.B.; Nistri, S. Feasibility and relevance of right parasternal view for assessing severity and rate of progression of aortic valve stenosis in primary care. Int. J. Cardiol. 2017, 240, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, T.; Izumo, M.; Sato, Y.; Shiokawa, N.; Uenomachi, N.; Miyauchi, M.; Miyamoto, J.; Kikuchi, H.; Shinoda, J.; Okamura, T.; et al. Additive value of the right parasternal view for the assessment of aortic stenosis. Echocardiography 2022, 39, 1338–1343. [Google Scholar] [CrossRef]
- Tavli, T.; Ammar, A.; Wong, M. Doppler-derived aortic valve gradients: Imaging versus non-imaging techniques. J. Heart Valve Dis. 1993, 2, 253–256. [Google Scholar] [PubMed]
- De Monchy, C.C.; Lepage, L.; Boutron, I.; Leye, M.; Detaint, D.; Hyafil, F.; Brochet, E.; Iung, B.; Vahanian, A.; Messika-Zeitoun, D. Usefulness of the right parasternal view and non-imaging continuous-wave Doppler transducer for the evaluation of the severity of aortic stenosis in the modern area. Eur. J. Echocardiogr. 2009, 10, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Thaden, J.J.; Nkomo, V.T.; Lee, K.J.; Oh, J.K. Doppler Imaging in Aortic Stenosis: The Importance of the Nonapical Imaging Windows to Determine Severity in a Contemporary Cohort. J. Am. Soc. Echocardiogr. 2015, 28, 780–785. [Google Scholar] [CrossRef]
- Cho, E.J.; Kim, S.M.; Park, S.J.; Lee, S.C.; Park, S.W. Identification of Factors that Predict whether the Right Parasternal View Is Required for Accurate Evaluation of Aortic Stenosis Severity. Echocardiography 2016, 33, 830–837. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2009, 10, 1–25, Erratum in Eur. J. Echocardiogr. 2009, 10, 479. [Google Scholar] [CrossRef]
- Mirea, O.; Maffessanti, F.; Gripari, P.; Tamborini, G.; Muratori, M.; Fusini, L.; Claudia, C.; Fiorentini, C.; Plesea, I.E.; Pepi, M. Effects of aging and body size on proximal and ascending aorta and aortic arch: Inner edge-to-inner edge reference values in a large adult population by two-dimensional transthoracic echocardiography. J. Am. Soc. Echocardiogr. 2013, 26, 419–427. [Google Scholar] [CrossRef]
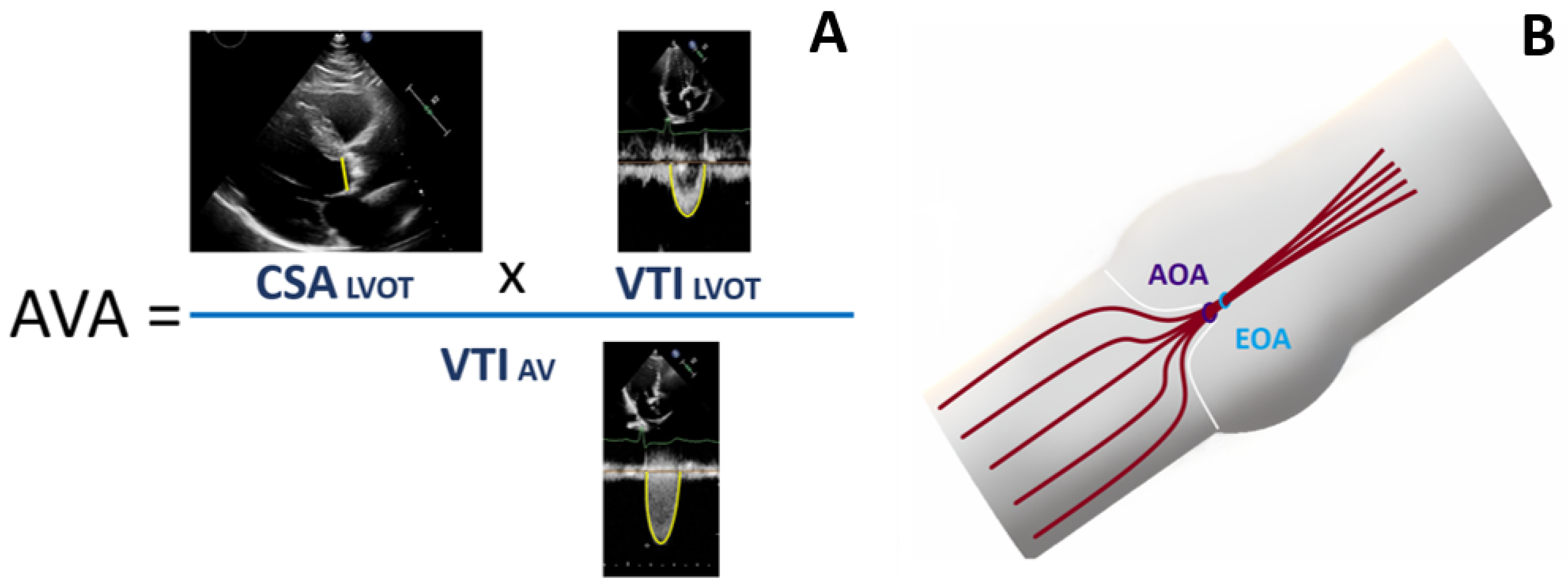

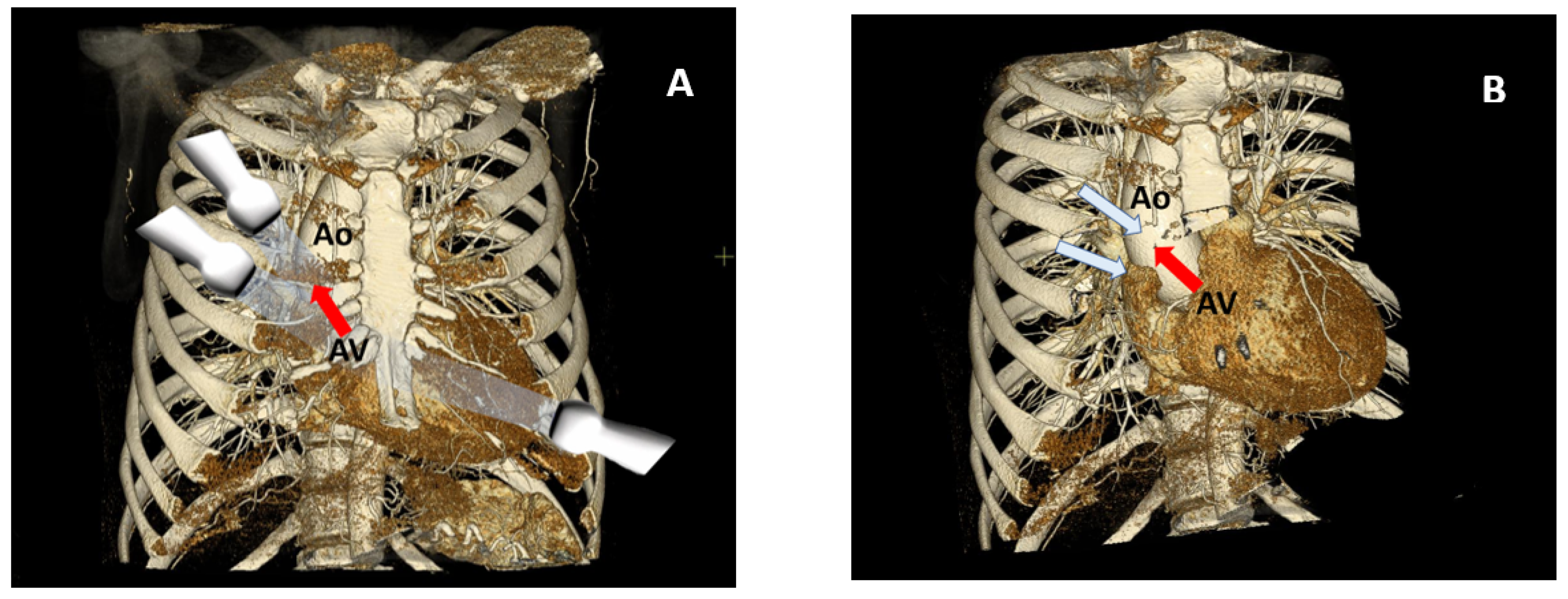
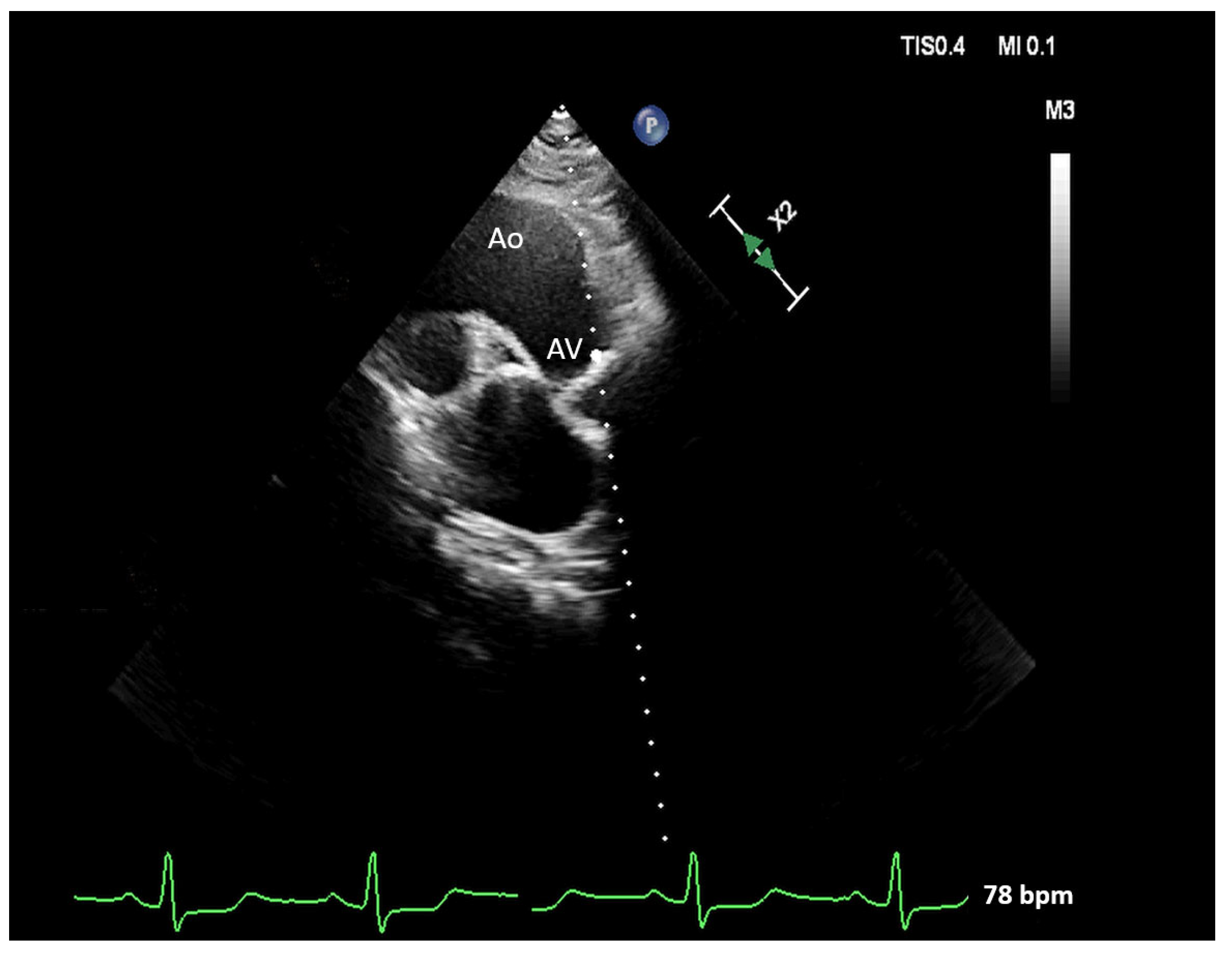

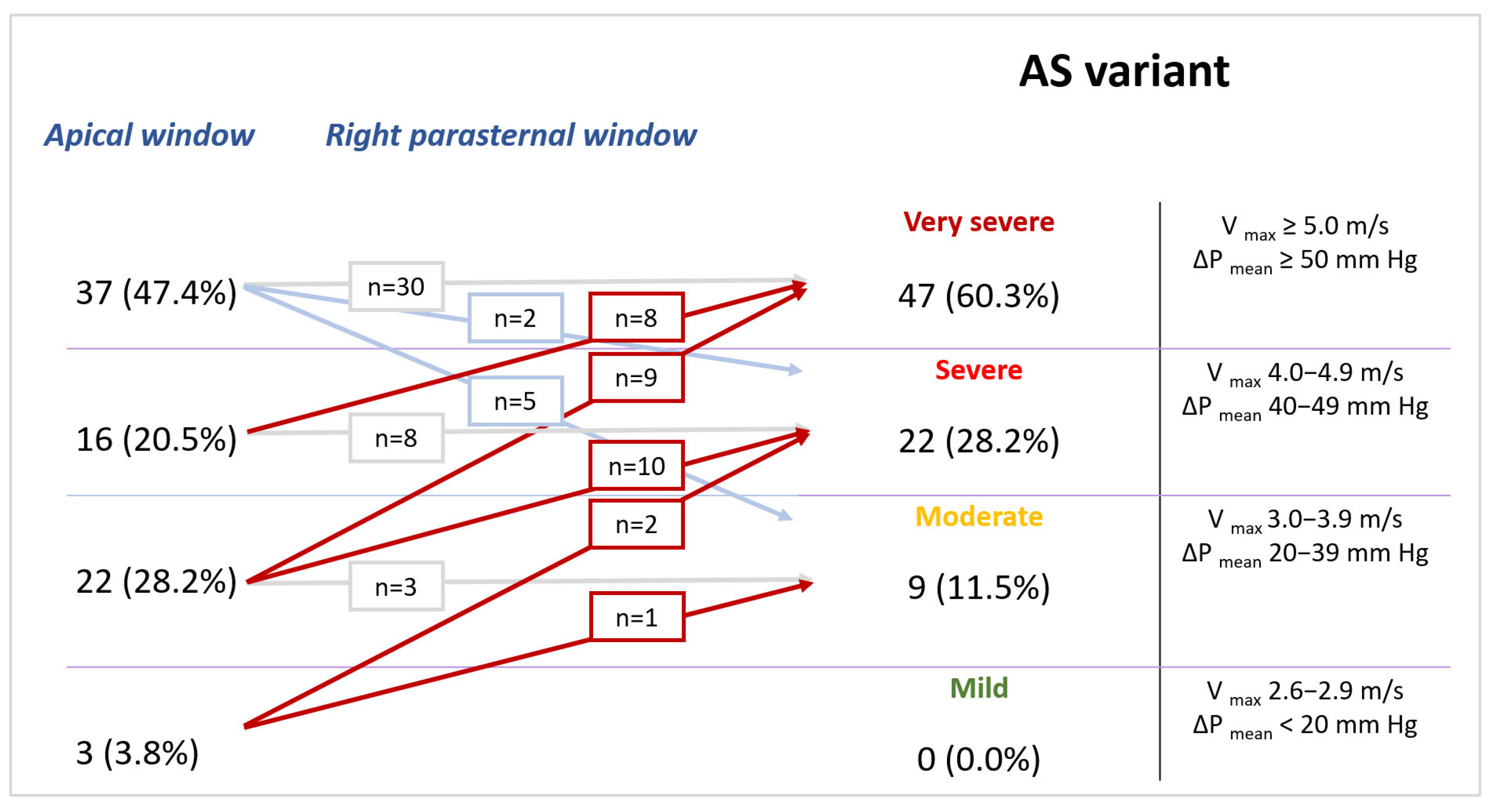
| Parameters | Baseline | Min | Max | |
|---|---|---|---|---|
| Age, years | 64 [55; 70] | 20 | 81 | |
| Gender: | -Male -Female | 38 (48.7) | ||
| 40 (51.3) | ||||
| BSA (m2) | 1.94 [1.81; 2.07] | 1.49 | 2.72 | |
| BMI (kg/m2) | 28.1 [24.6; 31.2] | 16.9 | 45.3 | |
| Rhythm: | -Sinus -Paroxysmal atrial fibrillation -Persistent atrial fibrillation | 73 (93.6) | ||
| 1 (1.3) | ||||
| 4 (5.1) | ||||
| Concomitant pathology | ||||
| Arterial hypertension | I grade II grade III grade | 4 (5.1) | ||
| 8 (10.3) | ||||
| 41 (52.6) | ||||
| Atherosclerotic disease of great vessels | 19 (24.3) | |||
| Atherosclerotic disease of peripheral vessels | 19 (24.3) | |||
| COPD | 10 (12.8) | |||
| Bronchial asthma | 1 (1.3) | |||
| Diabetes mellitus | 8 (10.3) | |||
| Chronic kidney disease | 4 (5.1) | |||
| History of cerebral stroke/TIA | 1 (1.3) | |||
| Coronary artery disease (stenosis ≥ 65%) | 8 (10.3) | |||
| History of myocardial infarction | 4 (5.1) | |||
| Functional class | NYHA II NYHA III NYHA IV | 13 (16.7) | ||
| 63 (46.2) | ||||
| 2 (2.6) | ||||
| EuroScore II, (%) | 1 [1; 2] | 1 | 7 | |
| Parameters | Concordant AS 56 (71.8) | Discordant AS 22 (28.2) | p |
|---|---|---|---|
| Left ventricle | |||
| EDILV, mL/m2 | 49.4 [41.0; 55.0] | 52.7 [45.9; 68.1] | 0.228 |
| ESILV, mL/m2 | 19.2 [16.0; 29.8] | 18.9 [16.2; 23.8] | 0.787 |
| SILV, mL/m2 | 30.6 [24.7; 35.7] | 34.1 [29.0; 43.9] | 0.082 |
| EFLV, % | 59 [55; 64] | 65 [60; 68] | 0.007 * |
| E/A | 0.85 [0.70; 1.21] | 0.83 [0.67; 1.22] | 1.000 |
| E/e’ | 11.0 [8.5; 13.6] | 8.0 [6.6; 12.2] | 0.072 |
| Parameters of the aorta and aortic valve | |||
| VTILVOT, cm | 21.7 [18.7; 25.6] | 23.2 [19.7; 25.7] | 0.702 |
| AV annulus diameter, mm | 21 [20; 23] | 22 [20; 23] | 0.788 |
| LVOT diameter, mm | 21 [20; 22] | 21 [20; 24] | 0.207 |
| Valsalva sinus diameter | 33 [31; 35] | 35 [30; 38] | 0.506 |
| Thickness of septum at basal level, mm | 18 [17; 20] | 18 [15; 20] | 0.426 |
| Aortoseptal angle, ° | 124 [118; 132] | 114 [110; 117] | <0.001 * |
| Doppler intercept angle in A5C, ° | 18.8 [12.6; 26.0] | 30.6 [27.5; 34.6] | <0.001 * |
| Aortic regurgitation, grade | 1.0 [1.0; 1.5] | 1.0 [1.0; 2.0] | 0.651 |
| Parameters | View | Concordance | p | |
|---|---|---|---|---|
| Concordant AS 56 (71.8) | Discordant AS 22 (28.2) | |||
| ΔPmax, mm Hg | A5C | 87 [76; 108] | 48 [39; 55] | <0.001 * |
| RPW | 93 [75; 109] | 76 [68; 95] | 0.067 | |
| p | 0.324 | 0.324 | <0.001 * ↑ (100.0%), ↓ (0.0%) | |
| Vmax AV, cm/s | A5C | 467 [428; 520] | 346 [310; 367] | <0.001 * |
| RPW | 471 [431; 521] | 439 [412; 487] | 0.119 | |
| p | 0.429 | 0.429 | <0.001 * ↑ (100.0%), ↓ (0.0%) | |
| ΔPmean, mm Hg | A5C | 53 [44; 70] | 29 [25; 33] | <0.001 * |
| RPW | 52 [39; 63] | 43 [38; 56] | 0.175 | |
| p | 0.183 | 0.183 | <0.001 * ↑ (95.5%), ↓ (4.5%) | |
| VTI AV, cm | A5C | 118 [103; 136] | 80 [72; 82] | <0.001 * |
| RPW | 114 [101; 130] | 106 [97; 109] | 0.029 * | |
| p | 0.325 | 0.325 | <0.001 * ↑ (90.9%), ↓ (9.1%) | |
| AVA (VTI), cm2 | A5C | 0.61 [0.52; 0.81] | 1.19 [1.02; 1.27] | <0.001 * |
| RPW | 0.63 [0.52; 0.82] | 0.84 [0.62; 0.92] | 0.099 | |
| p | 0.244 | 0.244 | <0.001 * ↑ (9.1%), ↓ (90.9%) | |
| AVAi, cm2/m2 | A5C | 0.33 [0.27; 0.40] | 0.60 [0.58; 0.65] | <0.001 * |
| RPW | 0.35 [0.28; 0.42] | 0.42 [0.29; 0.46] | 0.131 | |
| p | 0.251 | 0.251 | <0.001 * ↑ (9.1%), ↓ (90.9%) | |
| Variable | ICC (95% Confidence Interval) | ||
|---|---|---|---|
| Intra-Observer | Inter-Observer | Test–Retest | |
| Vmax AV | 0.98 (0.99–1.0) | 0.93 (0.82–0.98) | 0.98 (0.94–0.99) |
| ΔPmean AV | 0.97 (0.95–0.98) | 0.96 (0.90–0.97) | 0.97 (0.91–0.99) |
| VTI AV | 0.96 (0.9–0.99) | 0.95 (0.87–0.98) | 0.96 (0.90–0.99) |
| AV annulus | 0.99 (0.99–1.0) | 0.96 (0.89–0.98) | 0.99 (0.97–0.99) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golukhova, E.Z.; Slivneva, I.V.; Farulova, I.Y.; Skopin, I.I.; Marapov, D.I.; Murysova, D.V.; Pirushkina, Y.D.; Volkovskaya, I.V. Advantages of Multiposition Scanning in Echocardiographic Assessment of the Severity of Discordant Aortic Stenosis. Pathophysiology 2023, 30, 174-185. https://doi.org/10.3390/pathophysiology30020015
Golukhova EZ, Slivneva IV, Farulova IY, Skopin II, Marapov DI, Murysova DV, Pirushkina YD, Volkovskaya IV. Advantages of Multiposition Scanning in Echocardiographic Assessment of the Severity of Discordant Aortic Stenosis. Pathophysiology. 2023; 30(2):174-185. https://doi.org/10.3390/pathophysiology30020015
Chicago/Turabian StyleGolukhova, Elena Zelikovna, Inessa Viktorovna Slivneva, Inga Yur’evna Farulova, Ivan Ivanovich Skopin, Damir Ildarovich Marapov, Dar’ya Vladimirovna Murysova, Yuliya Dmitrievna Pirushkina, and Irina Vasilyevna Volkovskaya. 2023. "Advantages of Multiposition Scanning in Echocardiographic Assessment of the Severity of Discordant Aortic Stenosis" Pathophysiology 30, no. 2: 174-185. https://doi.org/10.3390/pathophysiology30020015
APA StyleGolukhova, E. Z., Slivneva, I. V., Farulova, I. Y., Skopin, I. I., Marapov, D. I., Murysova, D. V., Pirushkina, Y. D., & Volkovskaya, I. V. (2023). Advantages of Multiposition Scanning in Echocardiographic Assessment of the Severity of Discordant Aortic Stenosis. Pathophysiology, 30(2), 174-185. https://doi.org/10.3390/pathophysiology30020015





