Candesartan Normalizes Changes in Retinal Blood Flow and p22phox in the Diabetic Rat Retina
Abstract
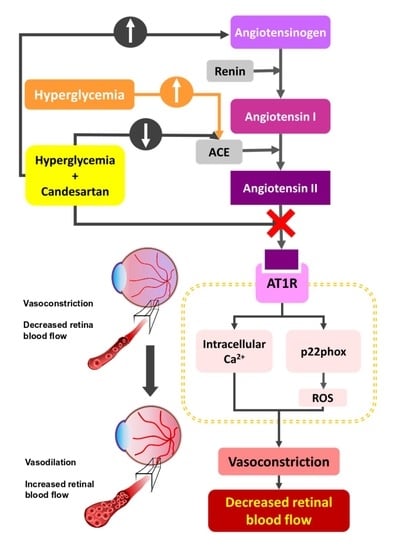
1. Introduction
2. Materials and Methods
2.1. Animal Groups
2.2. Microsphere Measurement of Retinal Blood Flow
2.3. Retinal Tissue Western Blots
2.4. Statistics
3. Results
3.1. Glucose Levels and Body Weights of Experimental Animals
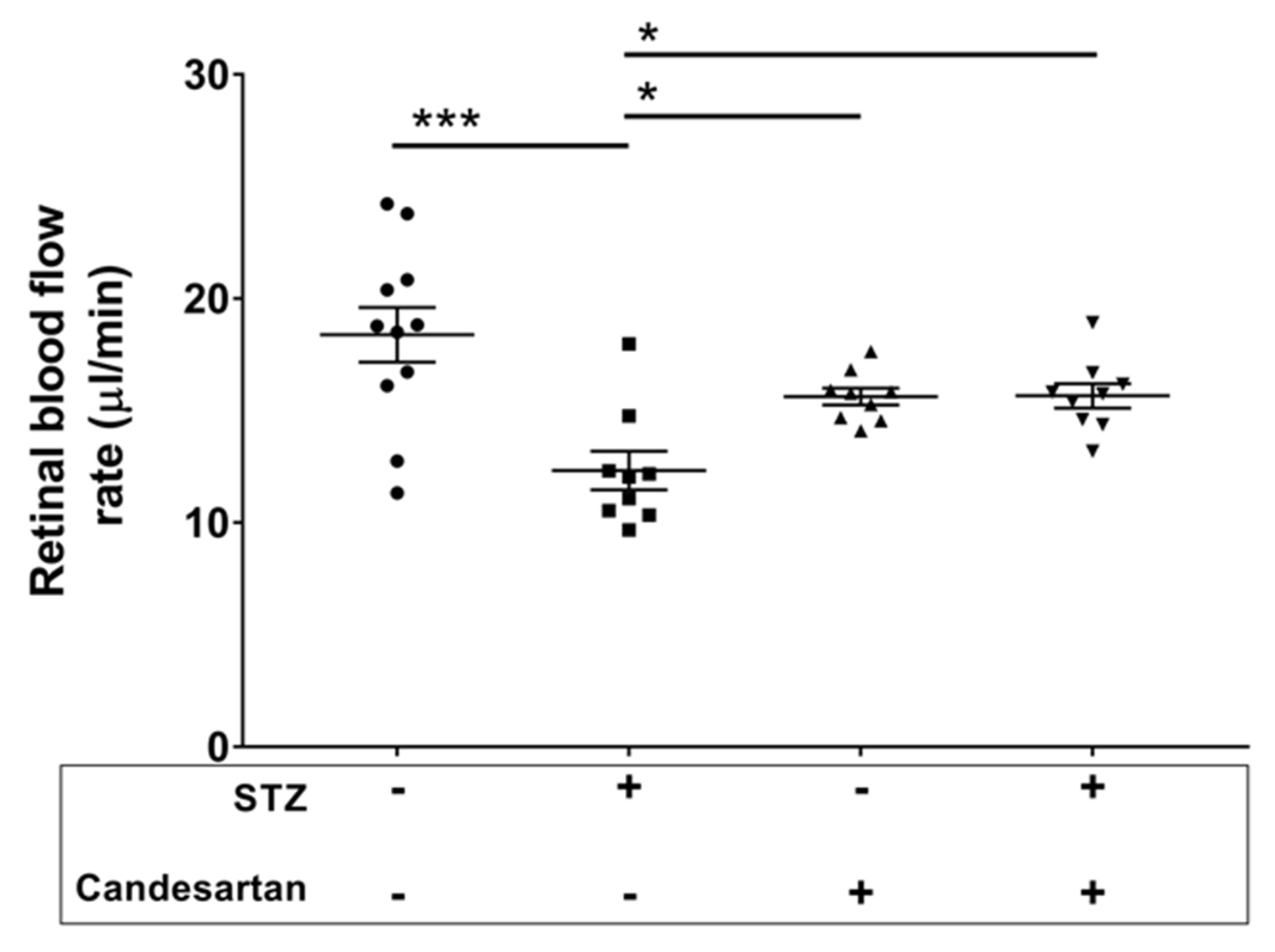
3.2. Retinal Blood Flow Rate
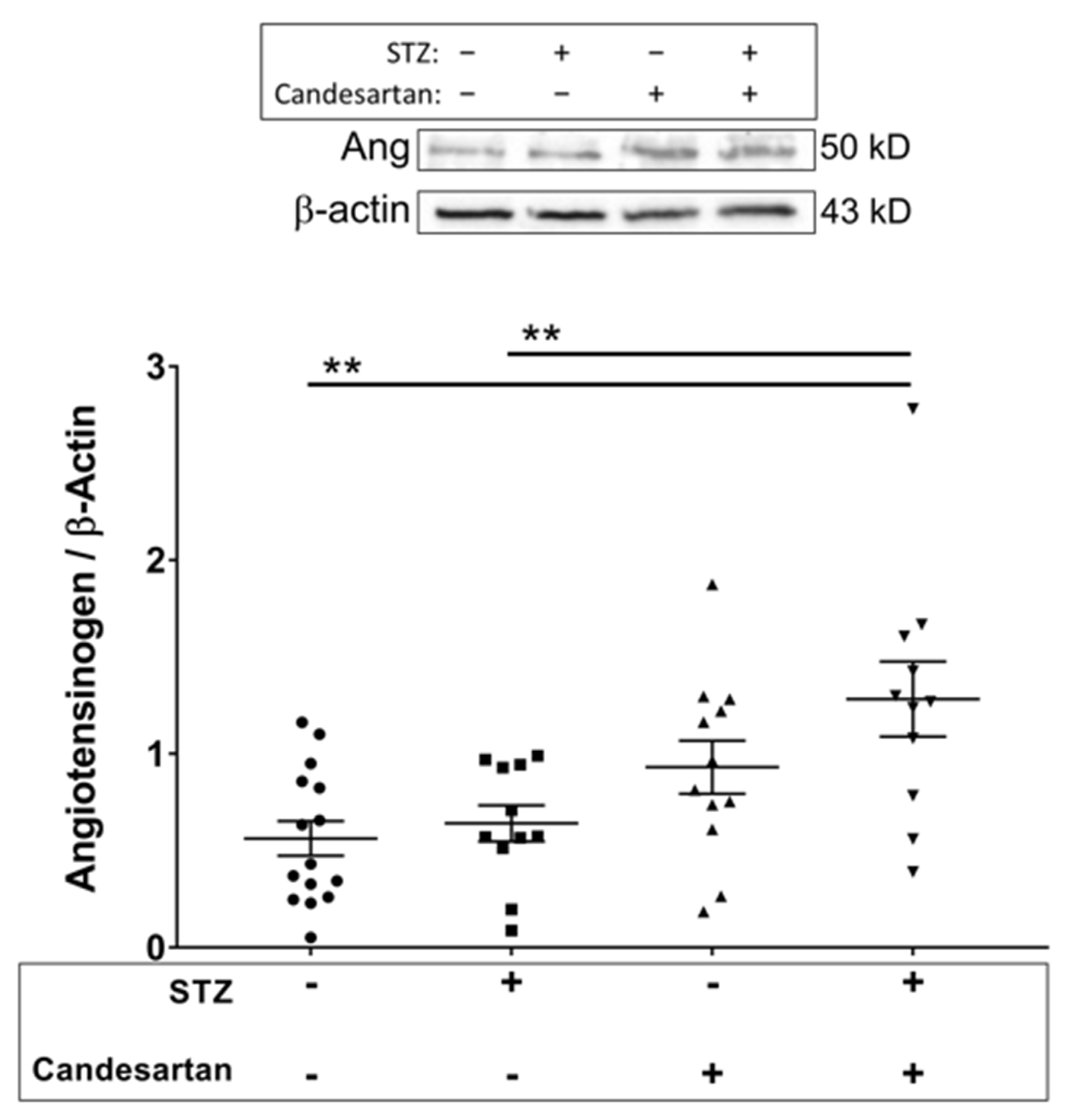
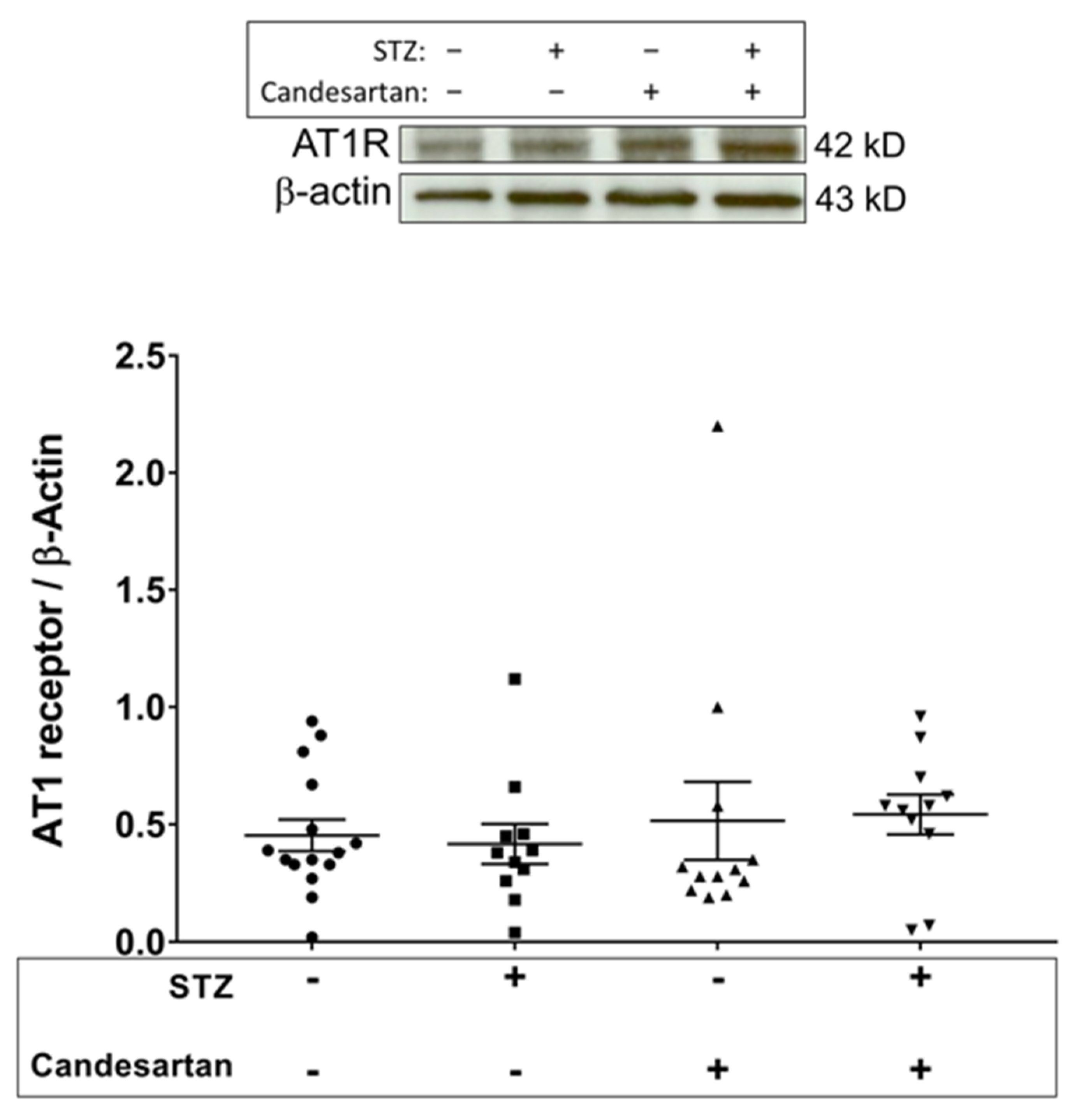
3.3. Angiotensin Pathway
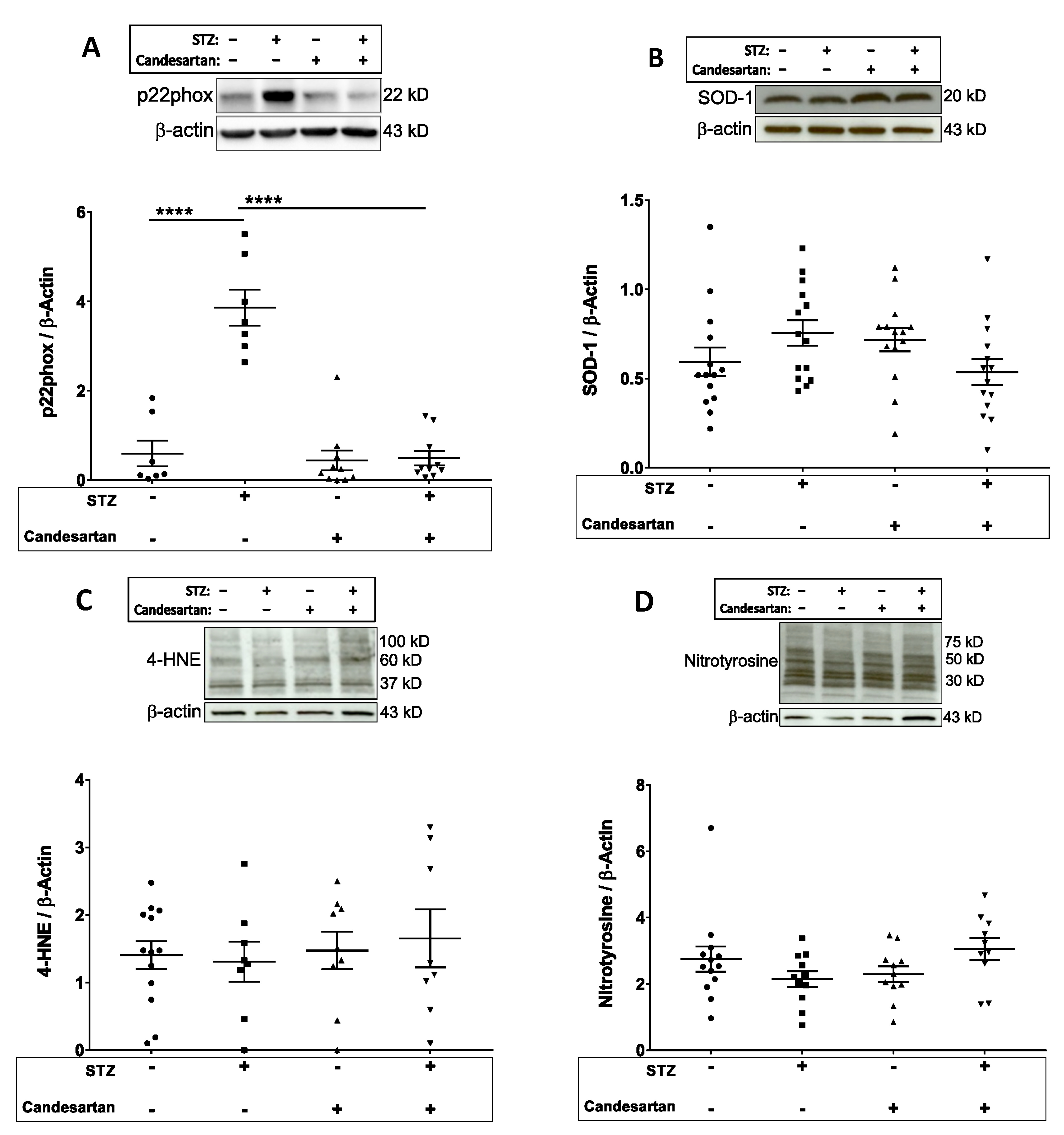
3.4. Oxidative and Nitrosative Stress Markers
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wright, W.S.; Harris, N.R. Ozagrel attenuates early streptozotocin-induced constriction of arterioles in the mouse retina. Exp. Eye Res. 2008, 86, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Harris, N.R. Losartan and Ozagrel Reverse Retinal Arteriolar Constriction in Non-Obese Diabetic Mice. Microcirculation 2008, 15, 379–387. [Google Scholar] [CrossRef]
- Lee, S.; Morgan, G.A.; Harris, N.R. Ozagrel reverses streptozotocin-induced constriction of arterioles in rat retina. Microvasc. Res. 2008, 76, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.S.; Yadav, A.S.; McElhatten, R.M.; Harris, N.R. Retinal blood flow abnormalities following six months of hyperglycemia in the Ins2(Akita) mouse. Exp. Eye Res. 2012, 98, 9–15. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Drel, V.R.; Kumagai, A.K.; Szabo, C.; Pacher, P.; Stevens, M.J. Early diabetes-induced biochemical changes in the retina: Comparison of rat and mouse models. Diabetologia 2006, 49, 2525–2533. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Fathallah, L.; Greene, D.A. Early changes in lipid peroxidation and antioxidative defense in diabetic rat retina: Effect of dl-α-lipoic acid. Eur. J. Pharmacol. 2000, 398, 139–146. [Google Scholar] [CrossRef]
- Agardh, C.D.; Israelsson, B.; Thuesen-Olesen, B.; Agardh, E. Application of quantitative competitive polymerase chain reaction for measurements of mRNA from antioxidative enzymes in the diabetic rat retina and kidney. Metab. Clin. Exp. 2002, 51, 1279–1284. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Kim, J.L.; Lee, H.K.; Ryu, G.W.; Hur, D.Y.; Yun, I.H.; Yang, J.W.; Kim, H.W. Simvastatin suppresses expression of angiogenic factors in the retinas of rats with streptozotocin-induced diabetes. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 389–397. [Google Scholar] [CrossRef]
- Lee, S.G.; Lee, C.G.; Yun, I.H.; Hur, D.Y.; Yang, J.W.; Kim, H.W. Effect of lipoic acid on expression of angiogenic factors in diabetic rat retina. Clin. Exp. Ophthalmol. 2012, 40, e47–e57. [Google Scholar] [CrossRef]
- Fan, J.; Xu, G.; Jiang, T.; Qin, Y. Pharmacologic Induction of Heme Oxygenase-1 Plays a Protective Role in Diabetic Retinopathy in Rats. Investig. Opthalmol. Vis. Sci. 2012, 53, 6541–6556. [Google Scholar] [CrossRef] [PubMed]
- Yar, A.S.; Menevse, S.; Dogan, I.; Alp, E.; Ergin, V.; Cumaoglu, A.; Aricioğlu, A.; Ekmekçi, A.; Menevse, A.; Cumaoǧlu, A. Investigation of Ocular Neovascularization–Related Genes and Oxidative Stress in Diabetic Rat Eye Tissues After Resveratrol Treatment. J. Med. Food 2012, 15, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Wright, W.S.; McElhatten, R.M.; Busu, C.; Amit, S.Y.; Leskova, W.; Aw, T.Y.; Harris, N.R. Influence of Glutathione on the Electroretinogram in Diabetic and Non-diabetic Rats. Curr. Eye Res. 2011, 36, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Leskova, W.; Watts, M.N.; Carter, P.R.; Eshaq, R.S.; Harris, N.R. Measurement of Retinal Blood Flow Rate in Diabetic Rats: Disparity Between Techniques Due to Redistribution of Flow. Investig. Opthalmol. Vis. Sci. 2013, 54, 2992–3018. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fortune, B.; Cull, G.; McElwain, K.M.; Cioffi, G.A. Microspheres method for ocular blood flow measurement in rats: Size and dose optimization. Exp. Eye Res. 2007, 84, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Grant, C.; Fortune, B.; Cioffi, G.A. Retinal and choroidal vasoreactivity to altered PaCO2 in rat measured with a modified microsphere technique. Exp. Eye Res. 2008, 86, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Clermont, A.C.; Aiello, L.P.; Mori, F.; Aiello, L.M.; Bursell, S.E. Vascular Endothelial Growth Factor and Severity of Nonproliferative Diabetic Retinopathy Mediate Retinal Hemodynamics In Vivo: A Potential Role for Vascular Endothelial Growth Factor in the Progression of Nonproliferative Diabetic Retinopathy. Am. J. Ophthalmol. 1997, 124, 433–446. [Google Scholar] [CrossRef]
- Bursell, S.E.; Clermont, A.C.; Kinsley, B.T.; Simonson, D.C.; Aiello, L.M.; Wolpert, H.A. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 1996, 37, 886–897. [Google Scholar]
- Kawagishi, T.; Nishizawa, Y.; Emoto, M.; Konishi, T.; Maekawa, K.; Hagiwara, S.; Okuno, Y.; Inada, H.; Isshiki, G.; Morii, H. Impaired Retinal Artery Blood Flow in IDDM Patients Before Clinical Manifestations of Diabetic Retinopathy. Diabetes Care 1995, 18, 1544–1549. [Google Scholar] [CrossRef]
- Horio, N.; Clermont, A.C.; Abiko, A.; Abiko, T.; Shoelson, B.D.; Bursell, S.-E.; Feener, E.P. Angiotensin AT(1) receptor antagonism normalizes retinal blood flow and acetylcholine-induced vasodilatation in normotensive diabetic rats. Diabetologia 2004, 47, 113–123. [Google Scholar] [CrossRef]
- Small, K.W.; Stefansson, E.; Hatchell, D.L. Retinal blood flow in normal and diabetic dogs. Investig. Ophthalmol. Vis. Sci. 1987, 28, 672–675. [Google Scholar]
- Rimmer, T.; Linsenmeier, R.A. Resistance of diabetic rat electroretinogram to hypoxemia. Investig. Ophthalmol. Vis. Sci. 1993, 34, 3246–3252. [Google Scholar]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of Retinal Neurons in Streptozotocin-Induced Diabetic Mice. Investig. Opthalmol. Vis. Sci. 2004, 45, 3330–3336. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, S.J.; Park, S.H.; Kim, K.Y.; Chung, J.W.; Chun, M.H.; Oh, S.J. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiol. Dis. 2006, 21, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, Y.; Jin, Y.; Ji, F.; Sinclair, S.H.; Luo, Y.; Xu, G.; Lu, L.; Dai, W.; Yanoff, M.; et al. Intravitreal Injection of Erythropoietin Protects both Retinal Vascular and Neuronal Cells in Early Diabetes. Investig. Opthalmol. Vis. Sci. 2008, 49, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.; Zhang, J.; Lei, X.; Xu, G.-T.; Ye, W. Protection of exendin-4 analogue in early experimental diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Ottlecz, A.; Bensaoula, T. Captopril ameliorates the decreased Na+,K(+)-ATPase activity in the retina of streptozotocin-induced diabetic rats. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1633–1641. [Google Scholar]
- Kowluru, R.A.; Tang, J.; Kern, T.S. Abnormalities of Retinal Metabolism in Diabetes and Experimental Galactosemia: VII. Effect of Long-Term Administration of Antioxidants on the Development of Retinopathy. Diabetes 2001, 50, 1938–1942. [Google Scholar] [CrossRef]
- Illing, E.K.B.; Gray, C.H. Retinal metabolism in diabetes: The metabolism of retinae of normal and alloxan-diabetic rabbits. J. Endocrinol. 1951, 7, 242–247. [Google Scholar] [CrossRef]
- Sutherland, F.S.; Stefansson, E.; Hatchell, D.L.; Reiser, H. Retinal oxygen consumption in vitro the effect of diabetes mellitus, oxygen and glucose. Acta Ophthalmol. 2009, 68, 715–720. [Google Scholar] [CrossRef]
- Linsenmeier, R.A.; Braun, R.D.; McRipley, M.A.; Padnick, L.B.; Ahmed, J.; Hatchell, D.L.; McLeod, D.S.; Lutty, G.A. Retinal hypoxia in long-term diabetic cats. Investig. Ophthalmol. Vis. Sci. 1998, 39, 1647–1657. [Google Scholar]
- Mori, H.; Oikawa, M.; Tamagami, T.; Kumaki, H.; Nakaune, R.; Amano, J.; Akinaga, Y.; Fukui, K.; Abe, K.; Urano, S. Oxidized Proteins in Astrocytes Generated in a Hyperbaric Atmosphere Induce Neuronal Apoptosis. J. Alzheimer’s Dis. 2007, 11, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Gerstner, B.; DeSilva, T.M.; Genz, K.; Armstrong, A.; Brehmer, F.; Neve, R.L.; Felderhoff-Mueser, U.; Volpe, J.J.; Rosenberg, P.A. Hyperoxia Causes Maturation-Dependent Cell Death in the Developing White Matter. J. Neurosci. 2008, 28, 1236–1245. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.M. ACE2: More of Ang-(1–7) or less Ang II? Curr. Opin. Nephrol. Hypertens. 2011, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Prestes, T.R.; Rocha, N.P.; Miranda, A.S.; Teixeira, A.L.; Simoes-E-Silva, A.C. The Anti-Inflammatory Potential of ACE2/Angiotensin-(1-7)/Mas Receptor Axis: Evidence from Basic and Clinical Research. Curr. Drug Targets 2017, 18, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Chappell, M.C. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-MAS receptor axis: More than regulation of blood pressure? Hypertension 2007, 50, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Senanayake, P.D.; Bonilha, V.L.; Peterson, J.W.; Yamada, Y.; Karnik, S.S.; Daneshgari, F.; Brosnihan, K.B.; Hollyfield, J.G. Retinal angiotensin II and angiotensin-(1-7) response to hyperglycemia and an intervention with captopril. J. Renin-Angiotensin-Aldosterone Syst. 2018, 19. [Google Scholar] [CrossRef]
- Ferrario, C.M.; Jessup, J.; Chappell, M.C.; Averill, D.B.; Brosnihan, K.; Bridget Tallant, E. Ann Diz Debra, I.; Gallagher Patricia, E. Effect of Angiotensin-Converting Enzyme Inhibition and Angiotensin II Receptor Blockers on Cardiac Angiotensin-Converting Enzyme 2. Circulation 2005, 111, 2605–2610. [Google Scholar] [CrossRef]
- Wang, Y.; Lou, M.F. The Regulation of NADPH Oxidase and Its Association with Cell Proliferation in Human Lens Epithelial Cells. Investig. Opthalmology Vis. Sci. 2009, 50, 2291–2300. [Google Scholar] [CrossRef]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Sedeek, M.; Montezano, A.C.; Hébert, R.L.; Gray, S.P.; Di Marco, E.; Jha, J.C.; Cooper, M.E.; Jandeleit-Dahm, K.; Schiffrin, E.L.; Wilkinson-Berka, J.L.; et al. Oxidative Stress, Nox Isoforms and Complications of Diabetes—Potential Targets for Novel Therapies. J. Cardiovasc. Transl. Res. 2012, 5, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: Implications for retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Ahmed, M.M.; Abuohashish, H.M.; Al-Rejaie, S.S.; Alhomida, A.S. Telmisartan Ameliorates Neurotrophic Support and Oxidative Stress in the Retina of Streptozotocin-Induced Diabetic Rats. Neurochem. Res. 2013, 38, 1572–1579. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.-F.; Zhang, Q.; Liu, X.-Z. Protective effects of curcumin on retinal Muller cell in early diabetic rats. Int. J. Ophthalmol. 2013, 6, 422–424. [Google Scholar] [PubMed]
- Kumar, B.; Gupta, S.K.; Srinivasan, B.; Nag, T.C.; Srivastava, S.; Saxena, R.; Jha, K.A. Hesperetin rescues retinal oxidative stress, neuroinflammation and apoptosis in diabetic rats. Microvasc. Res. 2013, 87, 65–74. [Google Scholar] [CrossRef]
- Si, Y.F.; Wang, J.; Guan, J.; Zhou, L.; Sheng, Y.; Zhao, J. Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br. J. Pharmacol. 2013, 169, 619–631. [Google Scholar] [CrossRef]
- Verma, A.; Shan, Z.; Lei, B.; Yuan, L.; Liu, X.; Nakagawa, T.; Grant, M.B.; Lewin, A.S.; Hauswirth, W.W.; Raizada, M.K.; et al. ACE2 and Ang-(1-7) Confer Protection Against Development of Diabetic Retinopathy. Mol. Ther. J. Am. Soc. Gene Ther. 2012, 20, 28–36. [Google Scholar] [CrossRef]

| Primary Antibody | Secondary Antibody | |||||
|---|---|---|---|---|---|---|
| Host | Dilution | Product | Dilution | Product | ||
| Angiotensin converting enzyme | mouse | 1:5000 | Millipore MAB 4051 | Goat anti-mouse | 1:10,000 | Invitrogen G21040 |
| Angiotensinogen | goat | 1:2000 | Santa Cruz SC-7419 | Donkey anti-goat | 1:10,000 | Santa Cruz SC-2020 |
| Angiotensin II type 1 receptor | mouse | 1:1000 | Abcam ab9391 | Goat anti-mouse | 1:10,000 | Invitrogen G21040 |
| Nitrotyrosine | rabbit | 1:15,000 | Millipore 06-284 | Goat anti-rabbit | 1:10,000 | Invitrogen G21234 |
| Superoxide dismutase-1 | rabbit | 1:15,000 | Origene TA300916 | Goat anti-rabbit | 1:10,000 | Invitrogen G21234 |
| 4-hydroxynonenal | rabbit | 1:1000 | Abcam ab45545 | Goat anti-rabbit | 1:10,000 | Invitrogen G21234 |
| p22phox | rabbit | 1:500 | Santa Cruz SC-20781 | Goat anti-rabbit | 1:10,000 | Invitrogen G21234 |
| Initial Body Weight (g) | Final Body Weight (g) | Initial Plasma Glucose (mg/dL) | Final Plasma Glucose (mg/dL) | ||
|---|---|---|---|---|---|
| Untreated Controls | N = 22 | 147 ± 4 | 461 ± 9 | 151 ± 6 | 160 ± 4 |
| Untreated STZ | N = 18 | 139 ± 4 | 276 ± 10 *** | 146 ± 5 | 529 ± 23 *** |
| Candesartan Controls | N = 20 | 136 ± 4 | 445 ± 6 | 157 ± 6 | 155 ± 7 |
| Candesartan STZ | N = 19 | 138 ± 5 | 287 ± 13 *** | 155 ± 6 | 574 ± 27 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eshaq, R.S.; Watts, M.N.; Carter, P.R.; Leskova, W.; Aw, T.Y.; Alexander, J.S.; Harris, N.R. Candesartan Normalizes Changes in Retinal Blood Flow and p22phox in the Diabetic Rat Retina. Pathophysiology 2021, 28, 86-97. https://doi.org/10.3390/pathophysiology28010008
Eshaq RS, Watts MN, Carter PR, Leskova W, Aw TY, Alexander JS, Harris NR. Candesartan Normalizes Changes in Retinal Blood Flow and p22phox in the Diabetic Rat Retina. Pathophysiology. 2021; 28(1):86-97. https://doi.org/10.3390/pathophysiology28010008
Chicago/Turabian StyleEshaq, Randa S., Megan N. Watts, Patsy R. Carter, Wendy Leskova, Tak Yee Aw, Jonathan Steven Alexander, and Norman R. Harris. 2021. "Candesartan Normalizes Changes in Retinal Blood Flow and p22phox in the Diabetic Rat Retina" Pathophysiology 28, no. 1: 86-97. https://doi.org/10.3390/pathophysiology28010008
APA StyleEshaq, R. S., Watts, M. N., Carter, P. R., Leskova, W., Aw, T. Y., Alexander, J. S., & Harris, N. R. (2021). Candesartan Normalizes Changes in Retinal Blood Flow and p22phox in the Diabetic Rat Retina. Pathophysiology, 28(1), 86-97. https://doi.org/10.3390/pathophysiology28010008