Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations
Abstract
:1. Introduction
2. Methods of Estimation of Total Antioxidant Capacity
2.1. Most Popular Methods
2.2. Less Popular Methods
3. Limited Correlation between Results of Various Assays of Total Antioxidant Capacity
4. Why to Assay the Total Antioxidant Capacity of Food Products?
4.1. Comparison of the Antioxidant Value of Food Components: Databases of Food Total Antioxidant Capacity
4.2. Total Antioxidant Capacity as a Measure of Food Origin and Quality
5. Total Antioxidant Capacity of Diet and Diseases
6. Effect of Processing on the Food Total Antioxidant Capacity
7. Is the Measured Total Antioxidant Capacity Only a Top of an Iceberg?
8. How Does the Total Antioxidant Capacity of Food Affect the Antioxidant Status of the Consumers?
9. What Is the Importance of Measurements of Total Antioxidant Capacity of Food Products?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bartosz, G. Total antioxidant capacity. Adv. Clin. Chem. 2003, 37, 219–292. [Google Scholar] [PubMed]
- Sies, H. Total antioxidant capacity: Appraisal of a concept. J. Nutr. 2007, 137, 1493–1495. [Google Scholar] [CrossRef] [Green Version]
- Pompella, A.; Sies, H.; Wacker, R.; Brouns, F.; Grune, T.; Biesalski, H.K.; Frank, J. The use of total antioxidant capacity as surrogate marker for food quality and its effect on health is to be discouraged. Nutrition 2014, 30, 791–793. [Google Scholar] [CrossRef] [PubMed]
- Bartosz, G. Non-enzymatic antioxidant capacity assays: Limitations of use in biomedicine. Free Radic. Res. 2010, 44, 711–720. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kut, K.; Cieniek, B.; Stefaniuk, I.; Bartosz, G.; Sadowska-Bartosz, I. A Modification of the ABTS• Decolorization Method and an Insight into Its Mechanism. Processes 2022, 10, 1288. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- de Menezes, B.B.; Frescura, L.M.; Duarte, R.; Villetti, M.A.; da Rosa, M.B. A critical examination of the DPPH method: Mistakes and inconsistencies in stoichiometry and IC50 determination by UV-Vis spectroscopy. Anal. Chim. Acta 2021, 1157, 338398. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Benzie, I.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yıldız, L.; Karaman, S.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Meth. 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef] [Green Version]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Whitehead, T.P.; Thorpe, G.H.G.; Maxwell, S.R.J. Enhanced chemiluminescent assay for antioxidant capacity in biological fluids. Anal. Chim. Acta 1992, 266, 265–277. [Google Scholar] [CrossRef]
- Fedorova, G.F.; Menshov, V.A.; Trofimov, A.V.; Vasilev, R.F. Facile chemiluminescence assay for antioxidative properties of vegetable lipids: Fundamentals and illustrative examples. Analyst 2009, 134, 2128–2134. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Hernández-Ruiz, J.; García-Cánovas, F.; Acosta, M. Inhibition by L-ascorbic acid and other antioxidants of the 2.2'-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) oxidation catalyzed by peroxidase: a new approach for determining total antioxidant status of foods. Anal. Biochem. 1996, 236, 255–261. [Google Scholar]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [Green Version]
- Durmaz, G. Freeze-dried ABTS+ method: A ready-to-use radical powder to assess antioxidant capacity of vegetable oils. Food Chem. 2012, 133, 1658–1663. [Google Scholar] [CrossRef]
- Özyürek, M.; Güçlü, K.; Apak, R. The main and modified CUPRAC methods of antioxidant measurement. Trends Anal. Chem. 2011, 30, 652–664. [Google Scholar] [CrossRef]
- Schaich, K.M.; Tian, X.; Xie, J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods 2015, 14, 111–125. [Google Scholar] [CrossRef]
- Staško, A.; Brezová, V.; Biskupič, S.; Mišík, V. The potential pitfalls of using 1,1-diphenyl-2-picrylhydrazyl to characterize antioxidants in mixed water solvents. Free Radic. Res. 2007, 41, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Foti, M.C. Use and Abuse of the DPPH• Radical. J. Agric. Food Chem. 2015, 63, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Moore, J.; Yu, L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Femenía-Ríos, M.; García-Pajón, C.M.; Hernández-Galán, R.; Macías-Sánchez, A.J.; Collado, I.G. Synthesis and free radical scavenging activity of a novel metabolite from the fungus Colletotrichum gloeosporioides. Bioorg. Med. Chem. Lett. 2006, 16, 5836–5839. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A procedure to measure the antiradical efficiency of polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Arranz, S.; Tabernero, M.; Díaz-Rubio, M.E.; Serrano, J.; Goñi, I.; Saura-Calixto, F. Updated methodology to determine antioxidant capacity in plant foods, oils and beverages: Extraction, measurement and expression of results. Food Res. Int. 2008, 41, 274–285. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Simultaneous automated measurement of total ‘antioxidant’ (reducing) capacity and ascorbic acid concentration. Redox Rep. 1997, 3, 233–238. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E.; Altun, M. Total antioxidant capacity assay of human serum using copper(II)-neocuproine as chromogenic oxidant: The CUPRAC method. Free Radic Res. 2005, 39, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Berker, K.I.; Güçlü, K.; Demirata, B.; Apak, R. A novel antioxidant assay of ferric reducing capacity measurement using ferrozine as the colour forming complexation reagent. Anal. Meth. 2010, 2, 1770–1778. [Google Scholar] [CrossRef]
- Ozyürek, M.; Bektaşoğlu, B.; Güçlü, K.; Güngör, N.; Apak, R. Simultaneous total antioxidant capacity assay of lipophilic and hydrophilic antioxidants in the same acetone-water solution containing 2% methyl-beta-cyclodextrin using the cupric reducing antioxidant capacity (CUPRAC) method. Anal. Chim. Acta 2008, 630, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.; Wang, Y.J.; Milligan, M.; Li, X.; Ji, J.A. Analysis of 2, 2′-azobis(2-amidinopropane) dihydrochloride degradation and hydrolysis in aqueous solutions. J. Pharm. Sci. 2011, 100, 3307–3315. [Google Scholar] [CrossRef]
- Becker, E.M.; Nissen, L.R.; Skibsted, L.H. Antioxidant evaluation protocols: Food quality or health effects. Eur. Food Res. Technol. 2004, 219, 561–571. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Development and validation of oxygen radical absorbance capacity assay for lipophilic antioxidants using randomly methylated β-cyclodextrin as the solubility enhancer. J. Agric. Food Chem. 2002, 50, 1815–1821. [Google Scholar] [CrossRef]
- Godoy-Navajas, J.; Aguilar Caballos, M.P.; Gomez-Hens, A. Long-wavelength fluorimetric determination of food antioxidant capacity using Nile blue as reagent. J. Agric. Food Chem. 2011, 59, 2235–2240. [Google Scholar] [CrossRef]
- Guclu, K.; Kıbrıslıoğlu, G.; Ozyurek, M.; Apak, R. Development of a fluorescent probe for measurement of peroxyl radical scavenging activity in biological samples. J. Agric. Food Chem. 2014, 62, 1839–1845. [Google Scholar] [CrossRef]
- Nkhili, E.; Brat, P. Reexamination of the ORAC assay: Effect of metal ions. Anal. Bioanal. Chem. 2011, 400, 1451–1458. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Kancheva, V.D.; Dettori, M.A.; Fabbri, D.; Alov, P.; Angelova, S.E.; Slavova-Kazakova, A.K.; Carta, P.; Menshov, V.A.; Yablonskaya, O.I.; Trofimov, A.V.; et al. Natural Chain-Breaking Antioxidants and Their Synthetic Analogs as Modulators of Oxidative Stress. Antioxidants 2021, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.V.; Lungu, L.; Savoiu, M.; Bradu, C.; Dinoiu, V.; Danet, A.F. Total antioxidant activity and phenols and flavonoids content of several plant extracts. Int. J. Food Prop. 2012, 15, 691–701. [Google Scholar] [CrossRef]
- Christodouleas, D.; Papadopoulos, K.; Calokerinos, A.C. Determination of total antioxidant activity of edible oils as well as their aqueous and organic extracts by chemiluminescence. Food Anal. Meth. 2011, 4, 475–484. [Google Scholar] [CrossRef]
- Zargoosh, K.; Ghayeb, Y.; Aeineh, N.; Qandalee, M. Evaluation of antioxidant capacity of hydrophilic and hydrophobic antioxidants using peroxyoxalate chemiluminescence reaction of the novel furandicarboxylate derivative. Food Anal. Meth. 2014, 7, 283–290. [Google Scholar] [CrossRef]
- Iranifam, M.; Al Lawati, H.A. Monitoring the antioxidant capacity in honey and fruit juices using a microfluidic device with a NaHCO3-H2O2-Co2+ chemiluminescence reaction. Food Chem. 2019, 297, 124930. [Google Scholar] [CrossRef]
- Weingerl, V.; Strlič, M.; Kočar, D. Evaluation of the chemiluminometric method for determination of polyphenols in wine. Anal. Lett. 2011, 44, 1310–1322. [Google Scholar] [CrossRef]
- Pegg, R.B.; Amarowicz, R.; Naczk, M.; Shahidi, F. PHOTOCHEM® for determination of antioxidant capacity of plant extracts. In ACS Symposium Series; Shahidi, F., Ho, C.-T., Eds.; Antioxidant Measurement and Applications; American Chemical Society: Washington, DC, USA, 2007; Volume 956, pp. 140–158. [Google Scholar]
- Wesołowska, M.; Dżugan, M. The use of the Photochem device in evaluation of antioxidant activity of Polish honey. Food Anal. Meth. 2017, 10, 1568–1574. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel fluorometric assay for hydroxyl radical prevention capacity using fluorescein as the probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Berker, K.I.; Güçlü, K.; Tor, I.; Apak, R. Comparative evaluation of Fe (III) reducing power-based antioxidant capacity assays in the presence of phenanthroline, batho-phenanthroline, tripyridyltriazine (FRAP), and ferricyanide reagents. Talanta 2007, 72, 1157–1165. [Google Scholar] [CrossRef]
- Berker, K.I.; Güçlü, K.; Tor, İ.; Demirata, B.; Apak, R. Total antioxidant capacity assay using optimized ferricyanide/prussian blue method. Food Anal. Meth. 2010, 3, 154–168. [Google Scholar] [CrossRef]
- Lissi, E.; Salim-Hanna, M.; Pascual, C.; del Castillo, M.D. Evaluation of total antioxidant potential (TRAP) and total antioxidant reactivity from luminol-enhanced chemiluminescence measurements. Free Radic. Biol. Med. 1995, 18, 153–158. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Maiani, G.; Azzini, E.; Ferro-Luzzi, A. A fluorescence-based method for measuring total plasma antioxidant capability. Free Radic. Biol. Med. 1995, 18, 29–36. [Google Scholar] [CrossRef]
- Valkonen, M.; Kuusi, T. Spectrophotometric assay for total peroxyl radical-trapping antioxidant potential in human serum. J. Lipid Res. 1997, 38, 823–833. [Google Scholar] [CrossRef]
- Somogyi, A.; Rosta, K.; Pusztai, P.; Tulassay, Z.; Nagy, G. Antioxidant measurements. Physiol. Meas. 2007, 28, R41. [Google Scholar] [CrossRef]
- Winston, G.W.; Regoli, F.; Dugas, A.J., Jr.; Fong, J.H.; Blanchard, K.A. A rapid gas chromatographic assay for determining oxyradical scavenging capacity of antioxidants and biological fluids. Free Radic. Biol. Med. 1998, 24, 480–493. [Google Scholar] [CrossRef]
- Marco, G.J. A rapid method for evaluation of antioxidants. J. Am. Oil Chem. Soc. 1968, 45, 594–598. [Google Scholar] [CrossRef]
- Prieto, M.A.; Rodríguez-Amado, I.; Vázquez, J.A.; Murado, M.A. β-Carotene assay revisited. application to characterize and quantify antioxidant and prooxidant activities in a microplate. J. Agric. Food Chem. 2012, 60, 8983–8993. [Google Scholar] [CrossRef] [Green Version]
- Lage, M.Á.P.; García, M.A.M.; Álvarez, J.A.V.; Anders, Y.; Curran, T.P. A new microplate procedure for simultaneous assessment of lipophilic and hydrophilic antioxidants and pro-oxidants, using crocin and β-carotene bleaching methods in a single combined assay: Tea extracts as a case study. Food Res. Int. 2013, 53, 836–846. [Google Scholar] [CrossRef] [Green Version]
- Bors, W.; Michel, C.; Saran, M. Inhibition of the bleaching of the carotenoid crocin a rapid test for quantifying antioxidant activity. Biochim. Biophys. Acta 1984, 796, 312–319. [Google Scholar] [CrossRef]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef]
- Kotora, P.; Šeršeň, F.; Filo, J.; Loos, D.; Gregáň, J.; Gregáň, F. The scavenging of DPPH, galvinoxyl and ABTS radicals by imine analogs of resveratrol. Molecules 2016, 21, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciuti, R.; Liguri, G. A novel assay for measuring total antioxidant capacity in whole blood and other biological samples. J. Biomed. Sci. Eng. 2017, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Ozyurt, D.; Demirata, B.; Apak, R. Determination of total antioxidant capacity by a new spectrophotometric method based on Ce (IV) reducing capacity measurement. Talanta 2007, 71, 1155–1165. [Google Scholar] [CrossRef]
- Robaszkiewicz, A.; Bartosz, G. Estimation of antioxidant capacity against pathophysiologically relevant oxidants using Pyrogallol Red. Biochem. Biophys. Res. Commun. 2009, 390, 659–661. [Google Scholar] [CrossRef]
- Robaszkiewicz, A.; Bartosz, G. Estimation of antioxidant capacity against peroxynitrite and hypochlorite with fluorescein. Talanta 2010, 80, 2196–2198. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Ciocalteu, V. On tyrosine and tryptophane determinations in proteins. J. Biol. Chem. 1927, 73, 627–650. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Haque, M.A.; Morozova, K.; Ferrentino, G.; Scampicchio, M. Electrochemical methods to evaluate the antioxidant activity and capacity of foods: A review. Electroanalysis 2021, 33, 1419–1435. [Google Scholar] [CrossRef]
- Cano, A.; Alcaraz, O.; Acosta, M.; Arnao, M.B. On-line antioxidant activity determination: Comparison of hydrophilic and lipophilic antioxidant activity using the ABTS•+ assay. Redox Rep. 2002, 7, 103–109. [Google Scholar] [CrossRef]
- Merola, E.T.; Catherman, A.D.; Yehl, J.B.; Strein, T.G. Determination of total antioxidant capacity of commercial beverage samples by capillary electrophoresis via inline reaction with 2,6-dichlorophenolindophenol. J. Agric. Food Chem. 2009, 57, 6518–6523. [Google Scholar] [CrossRef] [Green Version]
- Kozik, V.; Jarzembek, K.; Jędrzejowska, A.; Bąk, A.; Polak, J.; Bartoszek, M.; Pytlakowska, K. Investigation of Antioxidant Activity of Pomegranate Juices by Means of Electron Paramagnetic Resonance and UV-Vis Spectroscopy. J. AOAC Int. 2015, 98, 866–870. [Google Scholar] [CrossRef]
- Polak, J.; Bartoszek, M.; Lowe, A.R.; Postnikov, E.B.; Chorążewski, M. Antioxidant Properties of Various Alcoholic Beverages: Application of a Semiempirical Equation. Anal. Chem. 2020, 92, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Frasco, T.; Andreescu, D.; Andreescu, S. Portable ceria nanoparticle-based assay for rapid detection of food antioxidants (NanoCerac). Analyst 2013, 138, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Teerasong, S.; Jinnarak, A.; Chaneam, S.; Wilairat, P.; Nacapricha, D. Poly (vinyl alcohol) capped silver nanoparticles for antioxidant assay based on seed-mediated nanoparticle growth. Talanta 2017, 170, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Puangbanlang, C.; Sirivibulkovit, K.; Nacapricha, D.; Sameenoi, Y. A paper-based device for simultaneous determination of antioxidant activity and total phenolic content in food samples. Talanta 2019, 198, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Apichai, S.; Thajee, K.; Pattananandecha, T.; Saenjum, C.; Grudpan, K. A Simple Minimized System Based on Moving Drops for Antioxidant Analysis Using a Smartphone. Molecules 2021, 26, 5744. [Google Scholar] [CrossRef]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.-T.; Li, S.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, P.; Li, S.; Ho, C.-T.; Zhao, H. Antioxidant activity evaluation of dietary phytochemicals using Saccharomyces cerevisiae as a model. J. Funct. Foods 2017, 38, 36–44. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative antioxidant activities of carotenoids measured by ferric reducing antioxidant power (FRAP), ABTS bleaching assay (TEAC). Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
- Milardović, S.; Iveković, D.; Grabarić, B.S. A novel amperometric method for antioxidant activity determination using DPPH free radical. Bioelectrochemistry 2006, 68, 175–180. [Google Scholar] [CrossRef]
- Buettner, G.R. The pecking order of free radicals and antioxidants: Lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biophys. 1993, 300, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Fraga, C.G.; Sadgdicoglu Celep, G.; Galleano, M. Biochemical actions of plant phenolics compounds: Thermodynamic and kinetic aspects. In Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology; Fraga, C.G., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2010; pp. 91–106. [Google Scholar]
- Pyrzynska, K.; Pękal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Meth. 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Fadda, A.; Serra, M.; Molinu, M.G.; Azara, E.; Barberis, A.; Sanna, D. Reaction time and DPPH concentration influence antioxidant activity and kinetic parameters of bioactive molecules and plant extracts in the reaction with the DPPH radical. J. Food Compos. Anal. 2014, 35, 112–119. [Google Scholar] [CrossRef]
- Yu, L.; Gao, B.; Li, Y.; Wang, T.T.; Luo, Y.; Wang, J.; Yu, L.L. Home food preparation techniques impacted the availability of natural antioxidants and bioactivities in kale and broccoli. Food Funct. 2018, 9, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Kerchev, P.; Ivanov, S. Influence of extraction techniques and solvents on the antioxidant capacity of plant material. Biotechnol. Biotechnol. Equip. 2008, 22, 556–559. [Google Scholar] [CrossRef] [Green Version]
- Bartosz, G.; Baran, S.; Grzesik-Pietrasiewicz, M.; Sadowska-Bartosz, I. The Antioxidant capacity and hydrogen peroxide formation by black and orange carrots: Black and orange carrots. Agric. Food Sci. 2022, 31, 71–77. [Google Scholar] [CrossRef]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Saura-Calixto, F. Effect of solvent and certain food constituents on different antioxidant capacity assays. Food Res. Int. 2006, 39, 791–800. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Goñi, I. Antioxidant capacity of the Spanish Mediterranean diet. Food Chem. 2006, 94, 442–447. [Google Scholar] [CrossRef]
- Bravo, L.; Abia, R.; Saura-Calixto, F. Polyphenols as dietary fiber associated compounds. Comparative study on in vivo and in vitro properties. J. Agric. Food Chem. 1994, 42, 1481–1487. [Google Scholar] [CrossRef]
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Carrillo, C.; Barrio, Á.; Del Mar Cavia, M.; Alonso-Torre, S. Global antioxidant response of meat. J. Sci. Food Agric. 2017, 97, 2358–2365. [Google Scholar] [CrossRef]
- Cao, G.; Russell, M.; Lischner, N.; Prior, R.L. Serum antioxidant capacity is increased by consumption of strawberries, spinach, red wine or vitamin C in elderly women. J. Nutr. 1998, 128, 2383–2390. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Perret, J.; Davy, B.; Wilson, J.; Melby, C.L. Antioxidant properties of cereal products. J. Food Sci. 2002, 67, 2600–2603. [Google Scholar] [CrossRef]
- Xu, J.; Yang, F.; Chen, L.; Hu, Y.; Hu, Q. Effect of selenium on increasing the antioxidant activity of tea leaves harvested during the early spring tea producing season. J. Agric. Food Chem. 2003, 51, 1081–1084. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006, 1067, 10–21. [Google Scholar] [CrossRef]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [Green Version]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Cabello-Verrugio, C.; Simon, F.; Trollet, C.; Santibañez, J.F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies 2016. Oxid. Med. Cell. Longev. 2017, 2017, 4310469. [Google Scholar] [CrossRef]
- Senoner, T.; Dichtl, W. Oxidative Stress in Cardiovascular Diseases: Still a Therapeutic Target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Age. Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef]
- Abner, E.L.; Schmitt, F.A.; Mendiondo, M.S.; Marcum, J.L.; Kryscio, R.J. Vitamin E and all-cause mortality: A meta-analysis. Curr. Aging Sci. 2011, 4, 158–170. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef] [Green Version]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [Green Version]
- Berger, R.G.; Lunkenbein, S.; Ströhle, A.; Hahn, A. Antioxidants in food: Mere myth or magic medicine? Crit. Rev. Food Sci. Nutr. 2012, 52, 162–171. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants: Updating a personal view. Nutr. Rev. 2012, 70, 257–265. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barrandon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-carotene supplementation and cancer risk: A systematic review and metaanalysis of randomized controlled trials. Int. J. Cancer 2010, 127, 172–184. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerød, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Carlsen, M.H.; Phillips, K.M.; Bøhn, S.K.; Holte, K.; Jacobs, D.R., Jr.; Blomhoff, R. Content of redox-active compounds (ie, antioxidants) in foods consumed in the United States. Am. J. Clin. Nutr. 2006, 84, 95–135. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Pellegrini, N.; Vitaglione, P.; Granato, D.; Fogliano, V. Twenty-five years of total antioxidant capacity measurement of foods and biological fluids: Merits and limitations. J. Sci. Food Agric. 2020, 100, 5064–5078. [Google Scholar] [CrossRef]
- Reddy, C.V.K.; Sreeramulu, D.; Raghunath, M. Antioxidant activity of fresh and dry fruits commonly consumed in India. Food Res. Int. 2010, 43, 285–288. [Google Scholar] [CrossRef]
- Sreeramulu, D.; Raghunath, M. Antioxidant activity and phenolic content of roots, tubers and vegetables commonly consumed in India. Food Res. Int. 2010, 43, 1017–1020. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.P.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common vegetables in Singapore. Food Chem. 2010, 120, 993–1003. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.L.; Lim, M.T.; Koh, W.P.; Huang, D.; Ong, C.N. Antioxidant activity and profiles of common fruits in Singapore. Food Chem. 2010, 123, 77–84. [Google Scholar] [CrossRef]
- Takebayashi, J.; Oki, T.; Watanabe, J.; Yamasaki, K.; Chen, J.; Sato-Furukawa, M.; Tsubota-Utsugi, M.; Taku, K.; Goto, K.; Matsumoto, T.; et al. Hydrophilic antioxidant capacities of vegetables and fruits commonly consumed in Japan and estimated average daily intake of hydrophilic antioxidants from these foods. J. Food Comp. Anal. 2013, 29, 25–31. [Google Scholar] [CrossRef]
- Rautenbach, F.; Venter, I. Hydrophilic and lipophilic antioxidant capacity of commonly consumed South African fruits, vegetables, grains, legumes, fats/oils and beverages. J. Food Comp. Anal. 2010, 23, 753–761. [Google Scholar] [CrossRef]
- Pellegrini, N.; Serafini, M.; Colombi, B.; Del Rio, D.; Salvatore, S.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003, 133, 2812–2819. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, N.; Serafini, M.; Salvatore, S.; Del Rio, D.; Bianchi, M.; Brighenti, F. Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol. Nutr. Food Res. 2006, 50, 1030–1038. [Google Scholar] [CrossRef]
- Pellegrini, N.; Miglio, C.; Del Rio, D.; Salvatore, S.; Serafini, M.; Brighenti, F. Effect of domestic cooking methods on the total antioxidant capacity of vegetables. Int. J. Food Sci. Nutr. 2009, 60 (Suppl. 2), 2–22. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Peñalver, R.; Ros, G.; Nieto, G. Antioxidant capacity and diet pattern evaluation in a university community in south eastern Spain. Nutr. Hosp. 2021, 38, 1200–1208. [Google Scholar]
- Tanemura, N.; Machii, Y.; Urushihara, H. The first survey of gap between the actual labelling and efficacy information of functional substances in food under the regulatory processes in Japan. J. Funct. Foods 2020, 72, 104047. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [Green Version]
- Wereńska, M.; Okruszek, A.; Haraf, G.; Wołoszyn, J.; Goluch, Z. Impact of frozen storage on oxidation changes of some components in goose meat. Poult. Sci. 2022, 101, 101517. [Google Scholar] [CrossRef]
- Gutierrez, A.M.; Boylston, T.D.; Clark, S. Effects of Pro-Oxidants and Antioxidants on the Total Antioxidant Capacity and Lipid Oxidation Products of Milk During Refrigerated Storage. J. Food Sci. 2018, 83, 275–283. [Google Scholar] [CrossRef]
- Galani, J.H.Y.; Patel, J.S.; Patel, N.J.; Talati, J.G. Storage of Fruits and Vegetables in Refrigerator Increases their Phenolic Acids but Decreases the Total Phenolics, Anthocyanins and Vitamin C with Subsequent Loss of their Antioxidant Capacity. Antioxidants 2017, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.R.; Cho, H.S.; Cho, Y.S.; Kim, D.O. Changes in phenolics, soluble solids, vitamin C, and antioxidant capacity of various cultivars of hardy kiwifruits during cold storage. Food Sci. Biotechnol. 2020, 29, 1763–1770. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D. Changes in Bioactive Compounds, Antioxidant Activity, and Nutritional Quality of Blood Orange Cultivars at Different Storage Temperatures. Antioxidants 2020, 9, 1016. [Google Scholar] [CrossRef]
- Dragišić Maksimović, J.; Poledica, M.; Mutavdžić, D.; Mojović, M.; Radivojević, D.; Milivojević, J. Variation in nutritional quality and chemical composition of fresh strawberry fruit: Combined effect of cultivar and storage. Plant Foods Hum. Nutr. 2015, 70, 77–84. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Blessington, T.; Scheuring, D.C.; Nzaramba, M.N.; Hale, A.L.; Reddivari, L.; Vestal, T.A.; Maxim, J.E.; Miller, J.C., Jr. The use of low-dose electron-beam irradiation and storage conditions for sprout control and their effects on Xanthophylls, antioxidant capacity, and phenolics in the potato cultivar Atlantic. Am. J. Potato Res. 2015, 92, 609–618. [Google Scholar] [CrossRef]
- Gerolis, L.G.L.; Lameiras, F.S.; Krambrock, K.; Neves, M.J. Effect of gamma radiation on antioxidant capacity of green tea, yerba mate, and chamomile tea as evaluated by different methods. Radiat. Phys. Chem. 2017, 130, 177–185. [Google Scholar] [CrossRef]
- Murcia, M.A.; Egea, I.; Romojaro, F.; Parras, P.; Jiménez, A.M.; Martínez-Tomé, M. Antioxidant evaluation in dessert spices compared with common food additives. Influence of irradiation procedure. J. Agric. Food Chem. 2004, 52, 1872–1881. [Google Scholar] [CrossRef]
- Henríquez-Sánchez, P.; Sánchez-Villegas, A.; Ruano-Rodríguez, C.; Gea, A.; Lamuela-Raventós, R.M.; Estruch, R.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Schröder, H.; et al. Dietary total antioxidant capacity and mortality in the PREDIMED study. Eur. J. Nutr. 2016, 55, 227–236. [Google Scholar] [CrossRef]
- Ha, K.; Kim, K.; Sakaki, J.R.; Chun, O.K. Relative Validity of Dietary Total Antioxidant Capacity for Predicting All-Cause Mortality in Comparison to Diet Quality Indexes in US Adults. Nutrients 2020, 12, 1210. [Google Scholar] [CrossRef]
- Parohan, M.; Anjom-Shoae, J.; Nasiri, M.; Khodadost, M.; Khatibi, S.R.; Sadeghi, O. Dietary total antioxidant capacity and mortality from all causes, cardiovascular disease and cancer: A systematic review and dose–response meta-analysis of prospective cohort studies. Eur. J. Nutr. 2019, 58, 2175–2189. [Google Scholar] [CrossRef]
- Bastide, N.; Dartois, L.; Dyevre, V.; Dossus, L.; Fagherazzi, G.; Serafini, M.; Boutron-Ruault, M.C. Dietary antioxidant capacity and all-cause and cause-specific mortality in the E3N/EPIC cohort study. Eur. J. Nutr. 2017, 56, 1233–1243. [Google Scholar] [CrossRef]
- Sheng, L.T.; Jiang, Y.W.; Pan, A.; Koh, W.P. Dietary total antioxidant capacity and mortality outcomes: The Singapore Chinese Health Study. Eur. J. Nutr. 2022, 61, 2375–2382. [Google Scholar] [CrossRef]
- Parohan, M.; Sadeghi, A.; Khatibi, S.R.; Nasiri, M.; Milajerdi, A.; Khodadost, M.; Sadeghi, O. Dietary total antioxidant capacity and risk of cancer: A systematic review and meta-analysis on observational studies. Crit. Rev. Oncol. Hematol. 2019, 138, 70–86. [Google Scholar] [CrossRef]
- Zamani, B.; Daneshzad, E.; Azadbakht, L. Dietary Total Antioxidant Capacity and Risk of Gastrointestinal Cancers: A Systematic Review and Meta-analysis of Observational Studies. Arch. Iran. Med. 2019, 22, 328–335. [Google Scholar]
- Vahid, F.; Rahmani, D.; Davoodi, S.H. Validation of Dietary Antioxidant Index (DAI) and investigating the relationship between DAI and the odds of gastric cancer. Nutr. Metab. 2020, 17, 102. [Google Scholar] [CrossRef]
- Mekary, R.A.; Wu, K.; Giovannucci, E.; Sampson, L.; Fuchs, C.; Spiegelman, D.; Willett, W.C.; Smith-Warner, S.A. Total antioxidant capacity intake and colorectal cancer risk in the Health Professionals Follow-up Study. Cancer Causes Control 2010, 21, 1315–1321. [Google Scholar] [CrossRef] [Green Version]
- Vece, M.M.; Agnoli, C.; Grioni, S.; Sieri, S.; Pala, V.; Pellegrini, N.; Frasca, G.; Tumino, R.; Mattiello, A.; Panico, S.; et al. Dietary Total Antioxidant Capacity and Colorectal Cancer in the Italian EPIC Cohort. PLoS ONE 2015, 10, e0142995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Vecchia, C.; Decarli, A.; Serafini, M.; Parpinel, M.; Bellocco, R.; Galeone, C.; Bosetti, C.; Zucchetto, A.; Polesel, J.; Lagiou, P.; et al. Dietary total antioxidant capacity and colorectal cancer: A large case-control study in Italy. Int. J. Cancer 2013, 133, 1447–1451. [Google Scholar] [CrossRef]
- Amiano, P.; Molina-Montes, E.; Molinuevo, A.; Huerta, J.M.; Romaguera, D.; Gracia, E.; Martín, V.; Castaño-Vinyals, G.; Pérez-Gómez, B.; Moreno, V.; et al. Association study of dietary non-enzymatic antioxidant capacity (NEAC) and colorectal cancer risk in the Spanish Multicase-Control Cancer (MCC-Spain) study. Eur. J. Nutr. 2019, 58, 2229–2242. [Google Scholar] [CrossRef]
- Rafiee, P.; Jafari Nasab, S.; Bahrami, A.; Rezaeimanesh, N.; Jalali, S.; Hekmatdoost, A.; Sadeghi, A.; Naja, F.; Houshyari, M.; Hejazi, E. Dietary total antioxidant capacity and colorectal cancer and colorectal adenomatous polyps: A case-control study. Eur. J. Cancer Prev. 2021, 30, 40–45. [Google Scholar] [CrossRef]
- Vance, T.M.; Wang, Y.; Su, L.J.; Fontham, E.T.; Steck, S.E.; Arab, L.; Bensen, J.T.; Mohler, J.L.; Chen, M.H.; Chun, O.K. Dietary Total Antioxidant Capacity is Inversely Associated with Prostate Cancer Aggressiveness in a Population-Based Study. Nutr. Cancer 2016, 68, 214–224. [Google Scholar] [CrossRef] [Green Version]
- Gifkins, D.; Olson, S.H.; Demissie, K.; Lu, S.E.; Kong, A.N.; Bandera, E.V. Total and individual antioxidant intake and endometrial cancer risk: Results from a population-based case-control study in New Jersey. Cancer Causes Control 2012, 23, 887–895. [Google Scholar] [CrossRef] [Green Version]
- Gifkins, D.; Olson, S.H.; Paddock, L.; King, M.; Demissie, K.; Lu, S.E.; Kong, A.N.; Rodriguez-Rodriguez, L.; Bandera, E.V. Total and individual antioxidant intake and risk of epithelial ovarian cancer. BMC Cancer 2012, 12, 211. [Google Scholar] [CrossRef]
- Rossi, M.; Tavani, A.; Ciociola, V.; Ferraroni, M.; Parpinel, M.; Serafini, M.; Bellocco, R.; Zucchetto, A.; Montella, M.; Serraino, D.; et al. Dietary total antioxidant capacity in relation to endometrial cancer risk: A case-control study in Italy. Cancer Causes Control 2016, 27, 425–431. [Google Scholar] [CrossRef]
- Pantavos, A.; Ruiter, R.; Feskens, E.F.; de Keyser, C.E.; Hofman, A.; Stricker, B.H.; Franco, O.H.; Kiefte-de Jong, J.C. Total dietary antioxidant capacity, individual antioxidant intake and breast cancer risk: The Rotterdam Study. Int. J. Cancer 2015, 136, 2178–2186. [Google Scholar] [CrossRef]
- Sasanfar, B.; Toorang, F.; Maleki, F.; Esmaillzadeh, A.; Zendehdel, K. Association between dietary total antioxidant capacity and breast cancer: A case-control study in a Middle Eastern country. Public Health Nutr. 2021, 24, 965–972. [Google Scholar] [CrossRef]
- Jalali, S.; Heidari, Z.; de Courten, B.; Rashidkhani, B. Dietary Total Antioxidant Capacity and Odds of Breast Cancer: A Case-Control Study. Nutr. Cancer 2022, Aug 16, 1–8. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Fedirko, V.; Trichopoulou, A.; González, C.A.; Bamia, C.; Trepo, E.; Nöthlings, U.; Duarte-Salles, T.; Serafini, M.; Bredsdorff, L.; et al. Dietary flavonoid, lignan and antioxidant capacity and risk of hepatocellular carcinoma in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2013, 133, 2429–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, A.L.; Bosetti, C.; Boffetta, P.; Negri, E.; Tavani, A.; Serafini, M.; Polesel, J.; Serraino, D.; La Vecchia, C.; Rossi, M. Dietary total antioxidant capacity and pancreatic cancer risk: An Italian case-control study. Br. J. Cancer 2016, 115, 102–107. [Google Scholar] [CrossRef] [Green Version]
- Heydari, M.; Shayanfar, M.; Sharifi, G.; Saneei, P.; Sadeghi, O.; Esmaillzadeh, A. The Association between Dietary Total Antioxidant Capacity and Glioma in Adults. Nutr. Cancer 2021, 73, 1947–1956. [Google Scholar]
- Han, D.; Chung, M.; Park, Y. Association of Dietary Total Antioxidant Capacity with Cancer Recurrence and Mortality among Breast Cancer Survivors: A Prospective Cohort Study. Nutr. Cancer 2022, 74, 3253–3262. [Google Scholar] [CrossRef]
- Abshirini, M.; Siassi, F.; Koohdani, F.; Qorbani, M.; Mozaffari, H.; Aslani, Z.; Soleymani, M.; Entezarian, M.; Sotoudeh, G. Dietary total antioxidant capacity is inversely associated with depression, anxiety and some oxidative stress biomarkers in postmenopausal women: A cross-sectional study. Ann. Gen. Psychiatry 2019, 18, 3. [Google Scholar] [CrossRef]
- Devore, E.E.; Feskens, E.; Ikram, M.A.; den Heijer, T.; Vernooij, M.; van der Lijn, F.; Hofman, A.; Niessen, W.J.; Breteler, M.M. Total antioxidant capacity of the diet and major neurologic outcomes in older adults. Neurology 2013, 80, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Devore, E.E.; Kang, J.H.; Stampfer, M.J.; Grodstein, F. Total antioxidant capacity of diet in relation to cognitive function and decline. Am. J. Clin. Nutr. 2010, 92, 1157–1164. [Google Scholar] [CrossRef] [Green Version]
- Rautiainen, S.; Levitan, E.B.; Orsini, N.; Åkesson, A.; Morgenstern, R.; Mittleman, M.A.; Wolk, A. Total antioxidant capacity from diet and risk of myocardial infarction: A prospective cohort of women. Am. J. Med. 2012, 125, 974–980. [Google Scholar] [CrossRef]
- Rautiainen, S.; Levitan, E.B.; Mittleman, M.A.; Wolk, A. Total antioxidant capacity of diet and risk of heart failure: A population-based prospective cohort of women. Am. J. Med. 2013, 126, 494–500. [Google Scholar] [CrossRef]
- Rautiainen, S.; Larsson, S.; Virtamo, J.; Wolk, A. Total antioxidant capacity of diet and risk of stroke: A population-based prospective cohort of women. Stroke 2012, 43, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Del Rio, D.; Agnoli, C.; Pellegrini, N.; Krogh, V.; Brighenti, F.; Mazzeo, T.; Masala, G.; Bendinelli, B.; Berrino, F.; Sieri, S.; et al. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J. Nutr. 2011, 141, 118–123. [Google Scholar] [PubMed] [Green Version]
- Colarusso, L.; Serafini, M.; Lagerros, Y.T.; Nyren, O.; La Vecchia, C.; Rossi, M.; Ye, W.; Tavani, A.; Adami, H.O.; Grotta, A.; et al. Dietary antioxidant capacity and risk for stroke in a prospective cohort study of Swedish men and women. Nutrition 2017, 33, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-Souza, M.A.; Paiva, P.G.; Martino, H.S.D.; Ribeiro, A.Q. Dietary total antioxidant capacity as a tool in health outcomes in middle-aged and older adults: A systematic review. Crit. Rev. Food Sci. Nutr. 2018, 58, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.R.; Affret, A.; Dow, C.; Balkau, B.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia 2018, 61, 308–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashino, I.; Serafini, M.; Kurotani, K.; Akter, S.; Mizoue, T.; Ishihara, J.; Kotemori, A.; Sawada, N.; Inoue, M.; Iwasaki, M.; et al. Japan Public Health Center-based Prospective Study Group. Relationship between dietary non-enzymatic antioxidant capacity and type 2 diabetes risk in the Japan Public Health Center-based Prospective Study. Nutrition 2019, 66, 62–69. [Google Scholar] [CrossRef]
- Puchau, B.; Zulet, M.A.; de Echávarri, A.G.; Hermsdorff, H.H.; Martínez, J.A. Dietary total antioxidant capacity is negatively associated with some metabolic syndrome features in healthy young adults. Nutrition 2010, 26, 534–541. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Golzarand, M.; Mirmiran, P.; Shiva, N.; Azizi, F. Dietary total antioxidant capacity and the occurrence of metabolic syndrome and its components after a 3-year follow-up in adults: Tehran Lipid and Glucose Study. Nutr. Metab. (Lond). 2012, 9, 70. [Google Scholar] [CrossRef]
- Kim, D.; Han, A.; Park, Y. Association of Dietary Total Antioxidant Capacity with Bone Mass and Osteoporosis Risk in Korean Women: Analysis of the Korea National Health and Nutrition Examination Survey 2008–2011. Nutrients 2021, 13, 1149. [Google Scholar] [CrossRef]
- Fateh, H.L.; Mirzaei, N.; Gubari, M.I.M.; Darbandi, M.; Najafi, F.; Pasdar, Y. Association between dietary total antioxidant capacity and hypertension in Iranian Kurdish women. BMC Womens Health 2022, 22, 255. [Google Scholar] [CrossRef]
- Nabavizadeh, R.; Sohouli, M.H.; Santos, H.O.; Roustaei, M.; Fatahi, S.; Ghodoosi, N.; Saeidi, R. Higher dietary total antioxidant capacity is inversely associated with Helicobacter pylori infection among adults: A case-control study. Indian J. Gastroenterol. 2022, 41, 258–265. [Google Scholar] [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Surkan, P.J.; Azadbakht, L. Dietary total antioxidant capacity and cardiovascular disease risk factors: A systematic review of observational studies. J. Am. Coll. Nutr. 2018, 37, 533–545. [Google Scholar] [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Larijani, B.; Surkan, P.J.; Azadbakht, L. Association of dietary total antioxidant capacity to anthropometry in healthy women: A cross-sectional study. Nutrition 2020, 69, 110577. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Joung, H.; Shin, S. Dietary pattern, dietary total antioxidant capacity, and dyslipidemia in Korean adults. Nutr. J. 2019, 18, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermsdorff, H.H.; Puchau, B.; Volp, A.C.; Barbosa, K.B.; Bressan, J.; Zulet, M.Á.; Martínez, J.A. Dietary total antioxidant capacity is inversely related to central adiposity as well as to metabolic and oxidative stress markers in healthy young adults. Nutr. Metab. 2011, 8, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsay, D.G.; Astley, S.B. European research on the functional effects of dietary antioxidants—EUROFEDA. Mol. Aspects Med. 2002, 23, 1–38. [Google Scholar] [CrossRef]
- Stanley, S.; Basile, A.; Ruiz Tejada, A.; Hjelm, E.; Sweazea, K. Effect of Food Processing on Total Antioxidant Capacity. Curr. Dev. Nutr. 2021, 5 (Suppl. 2), 370. [Google Scholar] [CrossRef]
- Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araújo Bezerra, J.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Improvement of the Bioavailability of Amazonian Juices Rich in Bioactive Compounds Using Glow Plasma Technique. Food Bioproc. Technol. 2020, 13, 670–679. [Google Scholar] [CrossRef]
- Ramírez-Anaya Jdel, P.; Samaniego-Sánchez, C.; Castañeda-Saucedo, M.C.; Villalón-Mir, M.; de la Serrana, H.L. Phenols and the antioxidant capacity of Mediterranean vegetables prepared with extra virgin olive oil using different domestic cooking techniques. Food Chem. 2015, 188, 430–438. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Cui, X.; Zhang, Q.; Wang, Y.; Cao, L.; Luo, X.; Xiong, J.; Ruan, R. Assessment of Potential Nitrite Safety Risk of Leafy Vegetables after Domestic Cooking. Foods 2021, 10, 2953. [Google Scholar] [CrossRef]
- Miglio, C.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effects of different cooking methods on nutritional and physicochemical characteristics of selected vegetables. J. Agric. Food Chem. 2008, 56, 139–147. [Google Scholar] [CrossRef]
- Mazzeo, T.; N’Dri, D.; Chiavaro, E.; Visconti, A.; Fogliano, V.; Pellegrini, N. Effect of two cooking procedures on phytochemical compounds, total antioxidant capacity and colour of selected frozen vegetables. Food Chem. 2011, 128, 627–633. [Google Scholar] [CrossRef]
- N’Dri, D.; Mazzeo, T.; Zaupa, M.; Ferracane, R.; Fogliano, V.; Pellegrini, N. Effect of cooking on the total antioxidant capacity and phenolic profile of some whole-meal African cereals. J. Sci. Food Agric. 2013, 93, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Zaupa, M.; Calani, L.; Del Rio, D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly) phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. (2005). The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Natella, F.; Belelli, F.; Ramberti, A.; Scaccini, C. Microwave and traditional cooking methods: Effect of cooking on antioxidant capacity and phenolic compounds content of seven vegetables. J. Food Biochem. 2010, 34, 796–810. [Google Scholar] [CrossRef]
- Ng, Z.X.; Rosman, N.F. In vitro digestion and domestic cooking improved the total antioxidant activity and carbohydrate-digestive enzymes inhibitory potential of selected edible mushrooms. J. Food Sci. Technol. 2019, 56, 865–877. [Google Scholar] [CrossRef]
- Jati, I.R.A.; Darmoatmodjo, L.M.; Suseno, T.I.; Ristiarini, S.; Wibowo, C. Effect of Processing on Bioactive Compounds, Antioxidant Activity, Physicochemical, and Sensory Properties of Orange Sweet Potato, Red Rice, and Their Application for Flake Products. Plants 2022, 11, 440. [Google Scholar] [CrossRef]
- Harasym, J.; Olędzki, R. Comparison of Conventional and Microwave Assisted Heating on Carbohydrate Content, Antioxidant Capacity and Postprandial Glycemic Response in Oat Meals. Nutrients 2018, 10, 207. [Google Scholar] [CrossRef] [Green Version]
- Oduro-Obeng, H.; Fu, B.X.; Beta, T. Influence of cooking duration on carotenoids, physical properties and in vitro antioxidant capacity of pasta prepared from three Canadian durum wheat cultivars. Food Chem. 2021, 363, 130016. [Google Scholar] [CrossRef]
- Serpen, A.; Gökmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Pérez-Lamela, C.; Franco, I.; Falqué, E. Impact of High-Pressure Processing on Antioxidant Activity during Storage of Fruits and Fruit Products: A Review. Molecules 2021, 26, 5265. [Google Scholar] [CrossRef]
- Camiro-Cabrera, M.; Escobedo-Avellaneda, Z.; Salinas-Roca, B.; Martín-Belloso, O.; Welti-Chanes, J. High hydrostatic pressure and temperature applied to preserve the antioxidant compounds of mango pulp (Mangifera indica L.). Food Bioproc. Technol. 2017, 10, 639–649. [Google Scholar] [CrossRef]
- Gao, G.; Ren, P.; Cao, X.; Yan, B.; Liao, X.; Sun, Z.; Wang, Y. Comparing quality changes of cupped strawberry treated by high hydrostatic pressure and thermal processing during storage. Food Bioprod. Process. 2016, 100, 221–229. [Google Scholar] [CrossRef]
- Sravani, V.J.; Ravi, N.; Roopa, N.; Kumar, S.; Pandey, A.K.; Chauhan, O.P. Use of high pressure technology for the development of novel jam and its quality evaluation during storage. J. Food Sci. Technol. 2017, 54, 3562–3568. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.V.; Denoya, G.I.; Jagus, R.J.; Vaudagna, S.R.; Agüero, M.V. Microbiological, antioxidant and physicochemical stability of a fruit and vegetable smoothie treated by high pressure processing and stored at room temperature. LWT 2019, 105, 206–210. [Google Scholar] [CrossRef]
- Miller, D.D.; Schricker, B.R.; Rasmussen, R.R.; Van Campen, D. An in vitro method for estimation of iron availability from meals. Am. J. Clin. Nutr. 1981, 34, 2248–2256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rufián-Henares, J.A.; Delgado-Andrade, C. Effect of digestive process on Maillard reaction indexes and antioxidant properties of breakfast cereals. Food Res. Int. 2009, 42, 394–400. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Valverde-Moya, Á.; Hinojosa-Nogueira, D.; Pastoriza, S.; Rufián-Henares, J.Á. Effect of Cooking Methods on the Antioxidant Capacity of Foods of Animal Origin Submitted to In Vitro Digestion-Fermentation. Antioxidants 2021, 10, 445. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Hinojosa-Nogueira, D.; Douros, K.; Pastoriza, S.; Rufián-Henares, J.Á. The Gut Microbiota of Obese Children Releases Lower Antioxidant Capacity from Food than That of Lean Children. Nutrients 2022, 14, 2829. [Google Scholar] [CrossRef]
- Serafini, M.; Del Rio, D. Understanding the association between dietary antioxidants, redox status and disease: Is the total antioxidant capacity the right tool? Redox Rep. 2004, 9, 145–152. [Google Scholar] [CrossRef]
- Harasym, J.; Olędzki, R. Effect of fruit and vegetable antioxidants on total antioxidant capacity of blood plasma. Nutrition 2014, 30, 511–517. [Google Scholar] [CrossRef]
- Melville, C.A. Red wine, tea, and antioxidants. Lancet 1994, 344, 626. [Google Scholar] [PubMed]
- Serafini, M.; Ghiselli, A.; Ferro-Luzzi, A. Red wine, tea, and antioxidants. Lancet 1994, 344, 626. [Google Scholar] [CrossRef]
- Lotito, S.B.; Frei, B. The increase in human plasma antioxidant capacity after apple consumption is due to the metabolic effect of fructose on urate, not apple-derived antioxidant flavonoids. Free Radic. Biol. Med. 2004, 37, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Lotito, S.B.; Frei, B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: Cause, consequence, or epiphenomenon? Free Radic. Biol. Med. 2006, 41, 1727–1746. [Google Scholar] [CrossRef]
- Tulipani, S.; Romandini, S.; Busco, F.; Bompadre, S.; Mezzetti, B.; Battino, M. Ascorbate, not urate, modulates the plasma antioxidant capacity after strawberry intake. Food Chem. 2009, 117, 181–188. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2016, 60, 203–211. [Google Scholar] [CrossRef] [Green Version]
- Wisnuwardani, R.W.; De Henauw, S.; Forsner, M.; Gottrand, F.; Huybrechts, I.; Kafatos, A.G.; Kersting, M.; Knaze, V.; Manios, Y.; Nova, E.; et al. Adolescents’ dietary polyphenol intake in relation to serum total antioxidant capacity: The HELENA study. Int. J. Food Sci. Nutr. 2022, 73, 71–81. [Google Scholar] [CrossRef]
- Sirotkin, A.V.; Harrath, A.H. Phytoestrogens and their effects. Eur. J. Pharmacol. 2014, 741, 230–236. [Google Scholar]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules, Křížová, L., Dadáková, K., Kašparovská, J., & Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar]
- Forman, H.J.; Davies, K.J.; Ursini, F. How do nutritional antioxidants really work: Nucleophilic tone and para-hormesis versus free radical scavenging in vivo. Free Radic. Biol. Med. 2014, 66, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Oteiza, P.I.; Galleano, M. In vitro measurements and interpretation of total antioxidant capacity. Biochim. Biophys. Acta 2014, 1840, 931–934. [Google Scholar] [CrossRef]
- Alves, A.M.; Dias, T.; Hassimotto, N.M.A.; Naves, M.M.V. Ascorbic acid and phenolic contents, antioxidant capacity and flavonoids composition of Brazilian Savannah native fruits. Food Sci. Technol. 2017, 37, 564–569. [Google Scholar] [CrossRef]
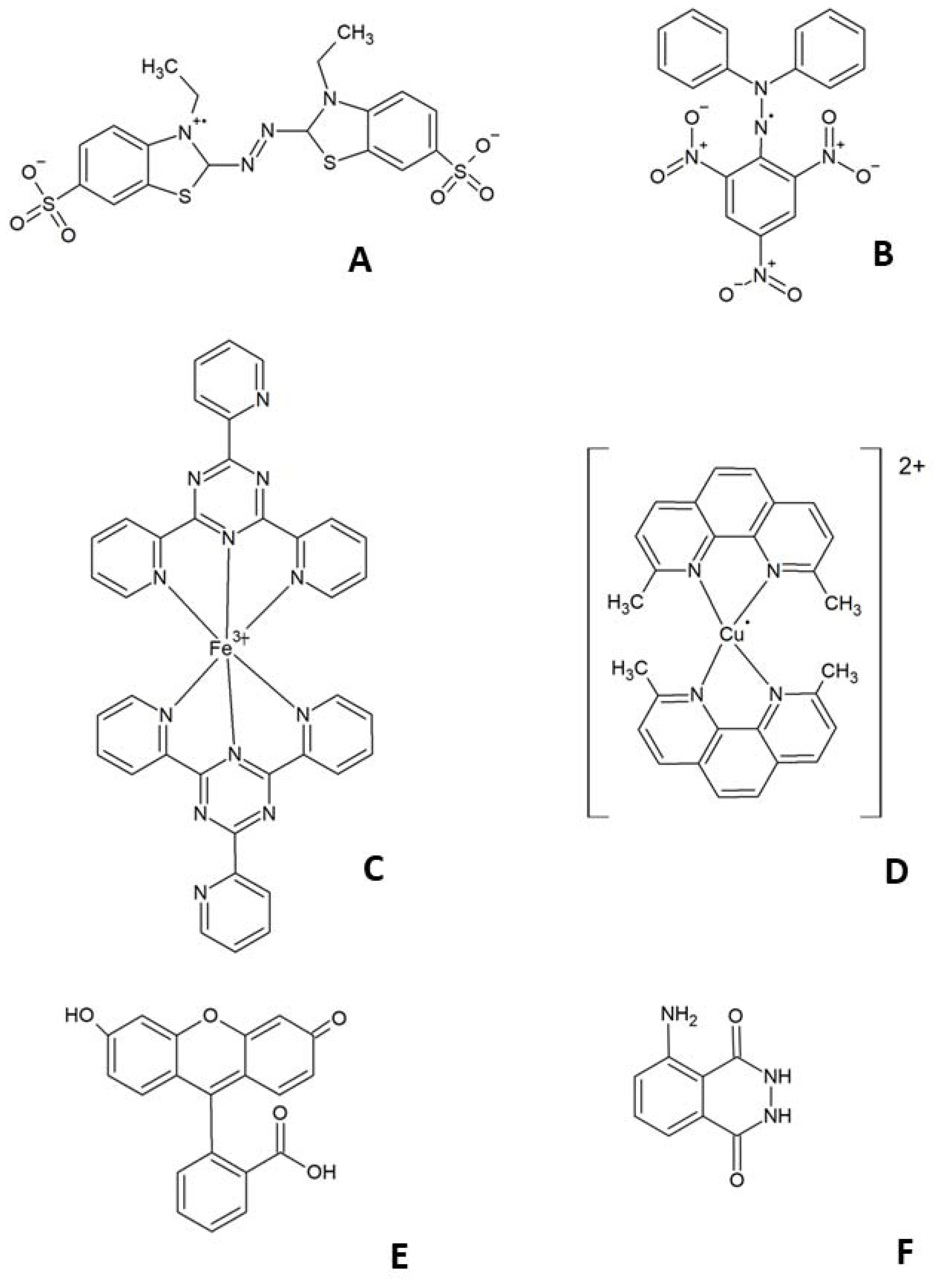
| Method | Principle of Measurement | References |
|---|---|---|
| ABTS● reduction | Decrease in absorbance of solution of pre-formed ABTS● radical (usually at 734 or 414 nm) | [6,7] |
| DPPH● reduction | Decrease in absorbance of solution of the stable DPPH● radical (around 517 nm) | [8,9] |
| FRAP | Increase in absorbance of Fe2+-TPTZ complex upon reduction of Fe3+ to Fe2+ (at 593 nm) | [10,11] |
| CUPRAC | Increase in absorbance of bis(neocuproine)copper(+) upon reduction of Cu2+ to Cu+ (at 540 nm) | [12,13] |
| ORAC CL assay | Inhibition of fluorescence decrease of R-phycoerythrin or fluorescein induced by a source of peroxyl radicals Inhibition of chemiluminescence of a detector induced by an oxidant | [14,15] [16,17] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. https://doi.org/10.3390/pr10102031
Sadowska-Bartosz I, Bartosz G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes. 2022; 10(10):2031. https://doi.org/10.3390/pr10102031
Chicago/Turabian StyleSadowska-Bartosz, Izabela, and Grzegorz Bartosz. 2022. "Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations" Processes 10, no. 10: 2031. https://doi.org/10.3390/pr10102031





