Abstract
Background: The hypothalamic paraventricular nucleus (PVN) is an important nucleus in the brain that plays a key role in regulating sympathetic nerve activity (SNA) and blood pressure. Silent mating-type information regulation 2 homolog-1 (sirtuin1, SIRT1) not only protects cardiovascular function but also reduces inflammation and oxidative stress in the periphery. However, its role in the central regulation of hypertension remains unknown. It is hypothesized that SIRT1 activation by resveratrol may reduce SNA and lower blood pressure through the regulation of intracellular reactive oxygen species (ROS) and neurotransmitters in the PVN. Methods: The two-kidney one-clip (2K1C) method was used to induce renovascular hypertension in male Sprague-Dawley rats. Then, bilaterally injections of vehicle (artificial cerebrospinal fluid, aCSF, 0.4 μL) or resveratrol (a SIRT1 agonist, 160 μmol/L, 0.4 μL) into rat PVN were performed for four weeks. Results: PVN SIRT1 expression was lower in the hypertension group than the sham surgery (SHAM) group. Activated SIRT1 within the PVN lowered systolic blood pressure and plasma norepinephrine (NE) levels. It was found that PVN of 2K1C animals injected with resveratrol exhibited increased expression of SIRT1, copper-zinc superoxide dismutase (SOD1), and glutamic acid decarboxylase (GAD67), as well as decreased activity of nuclear factor-kappa B (NF-κB) p65 and NAD(P)H oxidase (NOX), particularly NOX4. Treatment with resveratrol also decreased expression of ROS and tyrosine hydroxylase (TH). Conclusion: Resveratrol within the PVN attenuates hypertension via the SIRT1/NF-κB pathway to decrease ROS and restore the balance of excitatory and inhibitory neurotransmitters.
1. Introduction
Hypertension is a serious threat to human health due to its high morbidity and mortality rate [1]. The hypothalamic paraventricular nucleus (PVN) influences cardiovascular activity by secreting various neural and humoral factors [2,3]. The PVN is crucial in controlling sympathetic nerve activity (SNA) and contributes to blood pressure regulation [4]. Resveratrol is a kind of polyphenol compound, which mostly exists in grapes and red wine [5,6]. Resveratrol exhibits obvious antioxidant and anti-inflammatory effects in the treatment of hypertension [7,8,9]. Recent studies have also confirmed that resveratrol is an effective activator of silent mating-type information regulation 2 homolog-1 (sirtuin1, SIRT1) [10,11]. SIRT1, a deacetylase, functions as a silent information regulator in mammals [12]. It is a highly conserved protein with myocardial protective effects, such as improved endothelial function, enhanced cardiac function, and reduced atherosclerosis [13,14]. However, it remains unclear whether SIRT1 has any effect on hypertension in the central nervous system.
An increase in oxidative stress is not only a signature pathological event in cardiovascular disease, but also an important factor affecting SNA in hypertension [15]. One of the main characteristics of oxidative stress is massive production of reactive oxygen species (ROS) [16]. The redox pathway related to the NAD(P)H oxidase (NOX) family is a critical mechanism leading to increased ROS levels in chronic cardiovascular diseases [17]. NOX2 and NOX4 are the two main components of the NOX family [18]. The authors of [19] found that ROS production in the PVN and sympathetic activity increased in Aldo/NaCl induced hypertensive mice, whereas injection of AdsiRNA-NOX2 or AdsiRNA-NOX4 into the PVN significantly lowered blood pressure. Zhu found that microinjection of ROS scavengers or NOX inhibitors into the PVN attenuated SNA and thereby lowered blood pressure [20]. Similarly, our laboratory found that administration of tempol via the PVN significantly repressed renal sympathetic nerve activity (RSNA), and lowered plasma norepinephrine (NE) and arterial pressure [21].
Nuclear factor κB (NF-κB) is an extensively studied nuclear transcription factor [22], which includes five subunits: C-Rel, NF-κB1 (p50/p105), NF-κB2 (p52/p100), RelA (p65), and RelB [23]. The activity of NF-κB in the PVN is closely related to the enhancement of SNA. Inhibiting NF-κB activities greatly reduced ROS and SNA in hypertension [24] and myocardial infarction rat models [25,26]. The authors of [27] found that NF-κB activity increased in myeloid-specific SIRT1 knockout mice, suggesting that SIRT1 in bone marrow may inhibit NF-κB activation. In addition, the authors of [28] found that SIRT1 can interfere with cell apoptosis and improve cell survival by deacetylate NF-κB, and thus play a neuroprotective role in the development of neurodegenerative diseases. For the purposes of this study, we hypothesized that, in cases of hypertension, SIRT1 would regulate ROS levels through the NF-κB pathway in the PVN.
Tyrosine hydroxylase (TH) and glutamate decarboxylase 67 (GAD67) are the rate-limiting enzymes for the production of excitatory and inhibitory neurotransmitters in the central nervous system [29,30]. The production of excitatory neurotransmitters, such as dopamine and NE in the brain, depends on TH [31]. GAD67 promotes the degradation of glutamate (Glu) and accelerates the production of inhibitory neurotransmitter γ-aminobutyric acid (GABA) [30]. Our laboratory studies and those of others have shown increased TH expression and decreased GAD67 expression in the PVN during hypertension [32] and heart failure [33], causing an imbalance between excitatory and inhibitory neurotransmitters [34]. In addition, suppressing NF-κB activities in the PVN reduces RSNA during heart failure by restoring the balance of excitatory and inhibitory neurotransmitters [35].
In summary, this study explored the hypothesis that administration of resveratrol in the PVN would attenuate high blood pressure via the SIRT1/NF-κB pathway, by regulating ROS and neurotransmitters during hypertension.
2. Materials and Methods
2.1. Animals
Healthy male Sprague-Dawley rats weighing 275–300 g were provided from the animal center of Xi’an Jiaotong University. The Animal Care and Use Committee of the same institution approved the animal protocols (No. 2020-63). All rats were kept in a room with constant temperature and humidity, and a 12-hour light-dark cycle, and allowed access to normal rat chow and tap water ad libitum. Procedures involved in this study were performed in accordance with the Guide for Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication, 8th edition, 2011).
2.2. PVN Cannula Implantation
All rats were implanted bilaterally with PVN with sterilizing cannulas after anesthetizing with isoflurane in line with methods previously cited in the literature [21].
2.3. Preparation and Grouping of Animal Models
After the bilateral PVN cannulas were implanted, all animals were allowed to convalesce for seven days. All rats were then shaved at the surgical site, gas anesthetized with isoflurane, and placed in the left decubitus position. The abdominal surgery was performed to isolate the right renal artery. A silk thread (2-0 specification) was passed through the right renal artery, and a 0.25 mm silver acupuncture needle was placed above the isolated artery, tightening the artery with the needle and causing immediate ischemia of the right kidney. The ischemic state gradually recovered after we carefully withdrew the acupuncture needles. Suturing layer by layer with surgical sutures resulted in stenosis of the right renal artery, constructing a two-kidney one-clip (2K1C) hypertensive animal model. The rats in the sham surgery (SHAM) group were threaded but not ligated, and the surgical mouth was directly sutured. The rats were assigned to four groups at random: (i) SHAM + PVN vehicle; (ii) SHAM + PVN resveratrol; (iii) 2K1C + PVN vehicle; and (iv) 2K1C + PVN resveratrol. Vehicle (artificial cerebrospinal fluid, aCSF, 0.4 μL) or resveratrol (a SIRT1 agonist, 160 μmol/L, 0.4 μL) [36] was then injected into the bilateral PVN of rats each day for four consecutive weeks. Penicillin was administered for three days in doses according to weight, and the animals were observed daily.
2.4. Measurement of Blood Pressure
In line with previously cited methods [37], the non-anesthetized rats were placed on a thermostatically controlled heating plate and heated up to an ambient temperature of 36 ℃ for 15 min, and systolic blood pressure (SBP) was recorded by a non-invasive tail-cuff instrument (BP-300, Chengdu Techman Software Co., Ltd., Chengdu, China) every four days. A seven-day pre-training session before the experiment was necessary for all rats to fully adapt to the measurement procedure.
2.5. Collection of Blood and Tissue Samples
Animals were anesthetized with isoflurane at the end of 28th day. Plasma specimens and brain tissue were collected and stored at −80 °C for future analysis [38].
2.6. Immunofluorescence Staining
According to the specific location of the PVN in the rat brain atlas, transverse sections with a thickness of 18 μm were obtained and immunofluorescence staining was performed [34]. The primary antibodies were SIRT1 (#7475S, CST, MA, USA, 1:400 dilution), NOX4 (ab-133303, Abcam, Cambridge, UK, 1:1000 dilution), TH (sc-25269, Santa Cruz, TX, USA, 1:50 dilution), and GAD67 (ab-26116, Abcam, Cambridge, UK, 1:200 dilution). Dihydroethidium (DHE, Molecular Probes, Eugene, OR, USA) was used to examine ROS generation.
2.7. Western Blotting
In line with previously cited methods [32], the PVN of rats was fragmented by ultrasound and corresponding tissue proteins were extracted for Western blotting detection. The primary antibodies for SIRT1 (#7475S, CST, MA, USA, 1:1000 dilution), copper-zinc superoxide dismutase (SOD1, WL01846, Wanleibio, Shenyang, China, 1:1000 dilution), and β-actin (sc-8432, Santa Cruz, TX, USA, 1:2000 dilution) were bought. Using the wet transfer method, the related proteins were transferred to a PVDF membrane. The strips were then sealed with skimmed milk (4%) and incubated with primary antibodies at four degrees overnight. The following day, they were combined with the corresponding secondary antibody (GB23301, GB23303, Servicebio, Wuhan, China, 1:5000 dilution), then added with chemiluminescence reagent, and protein content was detected.
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
Levels of plasma NE (H096, Nanjing Jiancheng, Nanjing, China), NOX (ab186031, Abcam, Cambridge, UK), and NF-κB p65 (R0674c, elabscience, Wuhan, China) in rat PVN were quantitatively detected using an ELISA kit at 460 nm [32]. Values were then calculated using a spectrophotometer.
2.9. Statistical Analysis
Our data were described in terms of mean ± SEM and two-way ANOVA were performed followed by a post-hoc Tukey test. Blood pressure data were analyzed with repeated measures ANOVA. p values lower than 0.05 were regarded as statistically significant.
3. Result
3.1. Blood Pressure
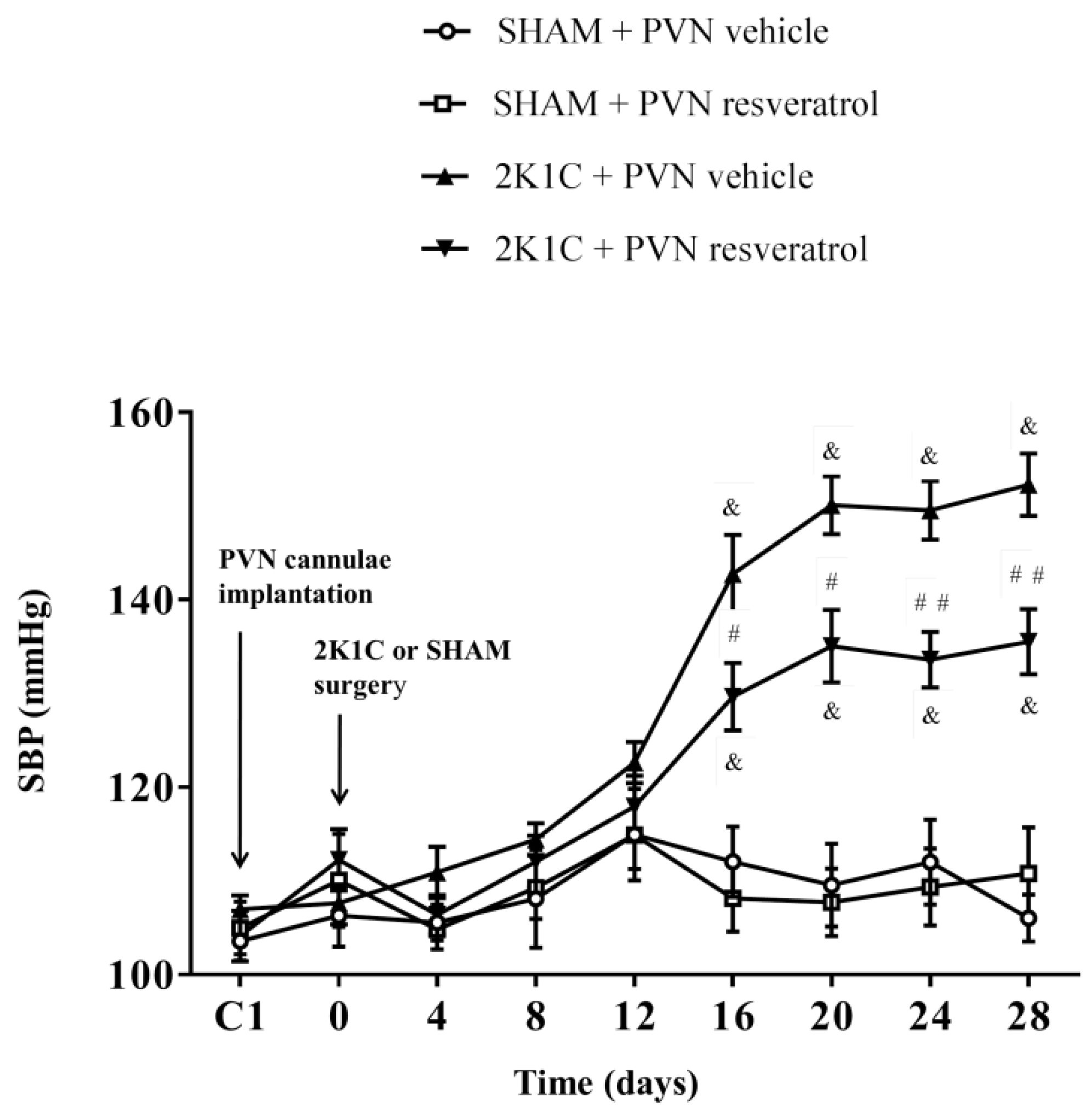
As shown in Figure 1, in contrast to SHAM rats, SBP in 2K1C rats significantly increased from the 16th day and remained increased (& p < 0.001). However, compared with 2K1C + PVN vehicle rats, SBP was significantly reduced from the 16th day to the end in 2K1C + PVN resveratrol rats (Figure 1, # p < 0.05, ## p < 0.01). The basal SBP in each group was similar.
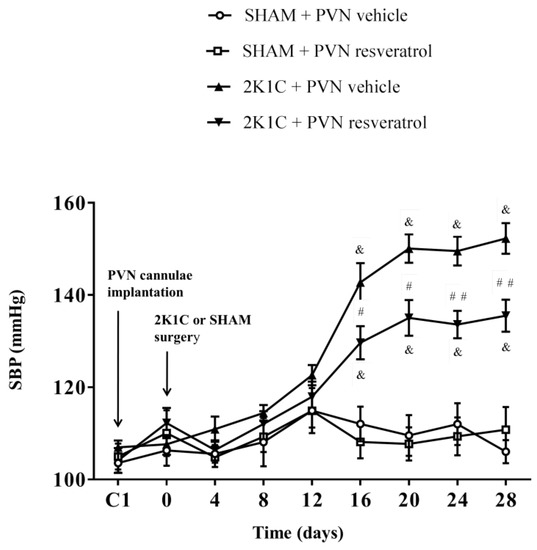
Figure 1.
The effects of resveratrol on SBP. Values are the mean ± SEM. & p < 0.001 vs. SHAM groups; # p < 0.05 or ## p < 0.01, relatively to 2K1C + PVN vehicle, n = 5.
3.2. Plasma NE
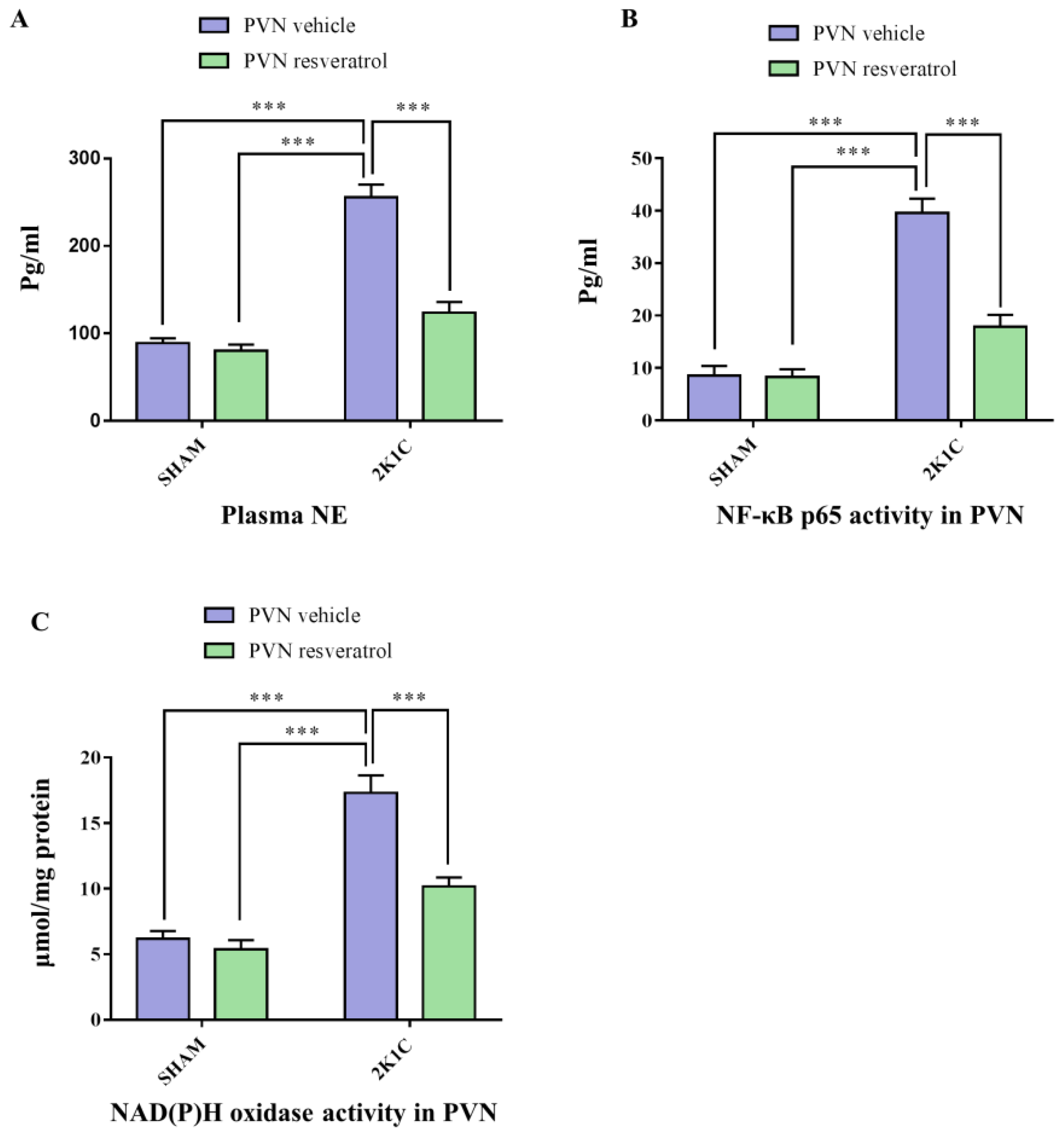
As shown in Figure 2A, in contrast with SHAM animals, hypertensive rats presented a higher level of plasma NE (p < 0.001). After four weeks of infusion of resveratrol, compared with 2K1C + PVN vehicle rats, 2K1C + PVN resveratrol rats had an attenuated level of plasma NE (Figure 2A, p < 0.001).
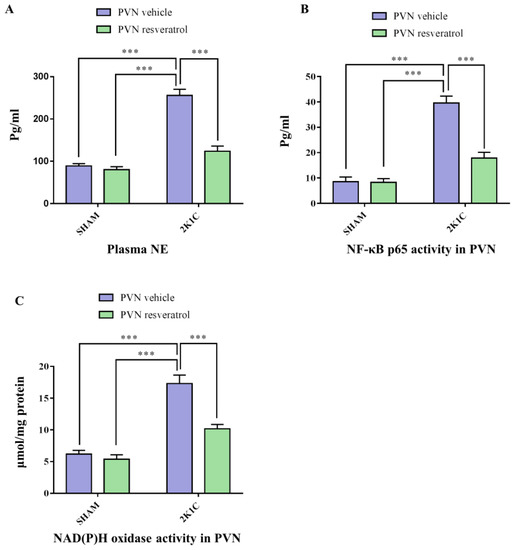
Figure 2.
The effects of resveratrol on the plasma levels of NE, and PVN levels of NOX and NF-κB activation. (A) Statistical analysis of NE; (B) Statistical analysis of NF-κB p65 activity; and (C) Statistical analysis of NOX. Values are the mean ± SEM. *** p < 0.001, n = 4–5.
3.3. SIRT1 Expression in PVN
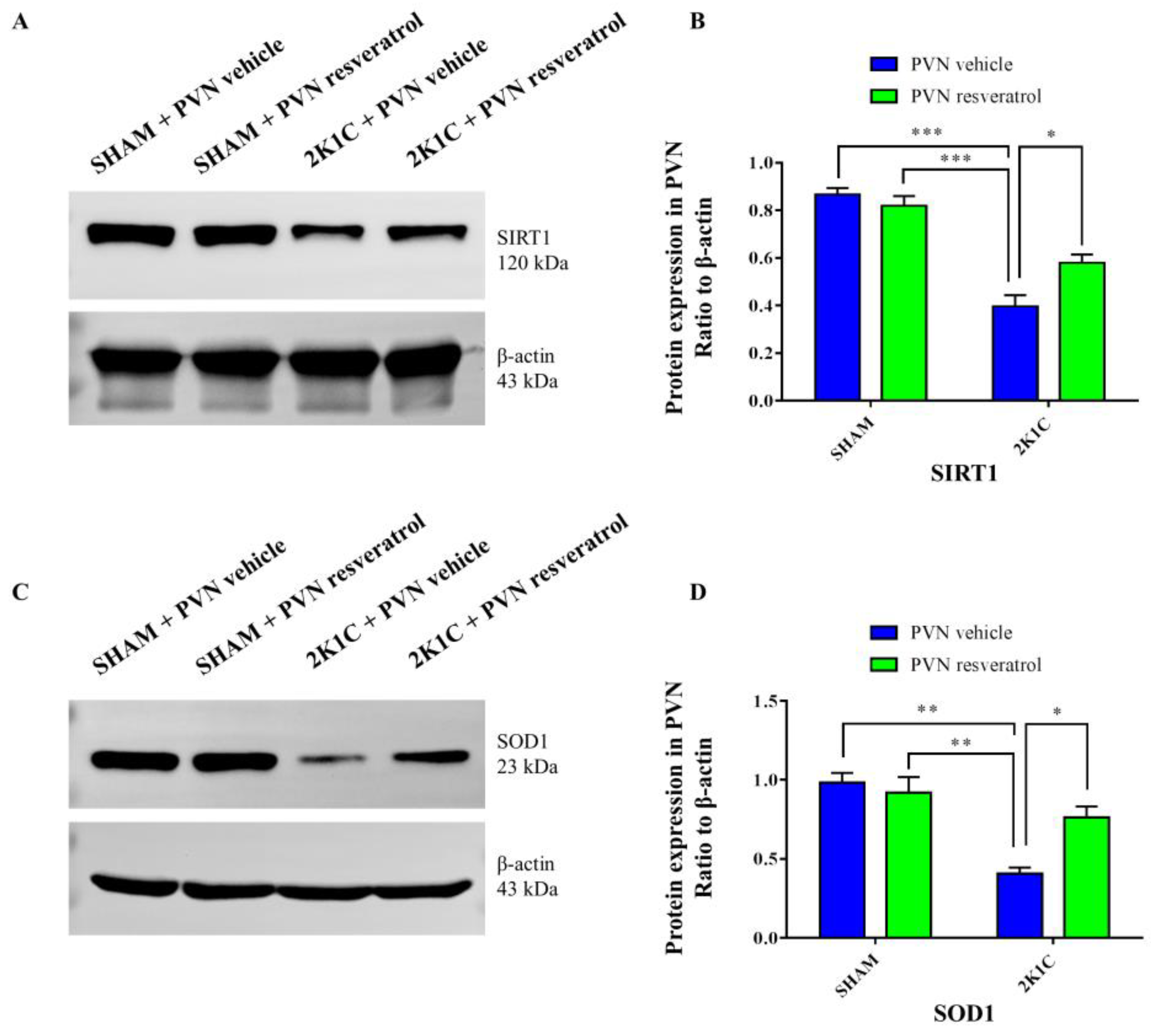
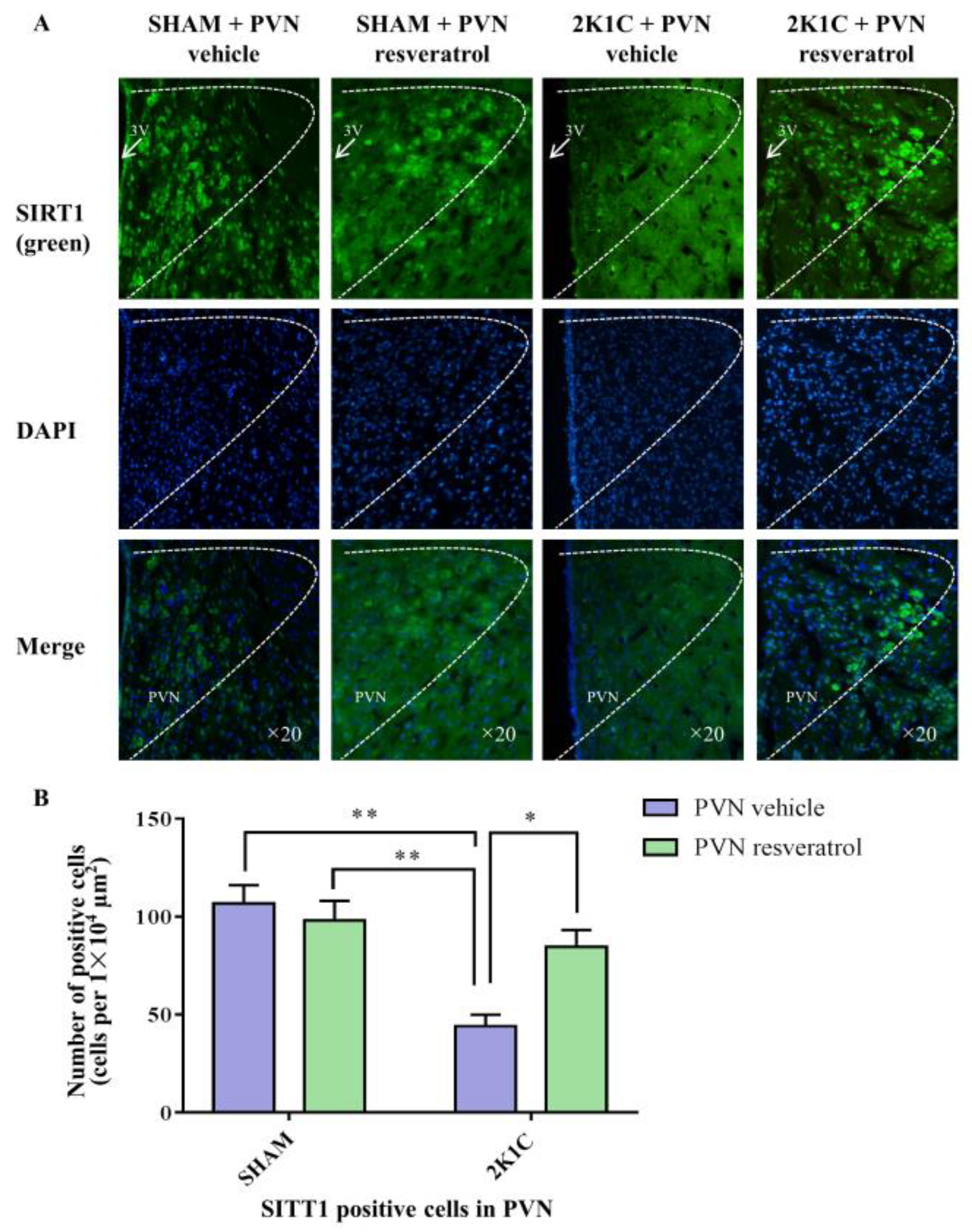
As shown in Figure 3A,B and Figure S1, in contrast with the SHAM rats, SIRT1 protein expression in 2K1C rats were reduced significantly (p < 0.001). After four weeks of PVN infusion of resveratrol, SIRT1 protein expression was increased (Figure 3A,B and Figure S1, p < 0.05). As shown in Figure 4A,B, in contrast with the SHAM rats, the number of SIRT1 positive cells of hypertensive rats was significantly reduced (Figure 4A,B, p < 0.01). Four weeks of PVN infusion of resveratrol effectively increased the number of SIRT1 positive cells (p < 0.05).
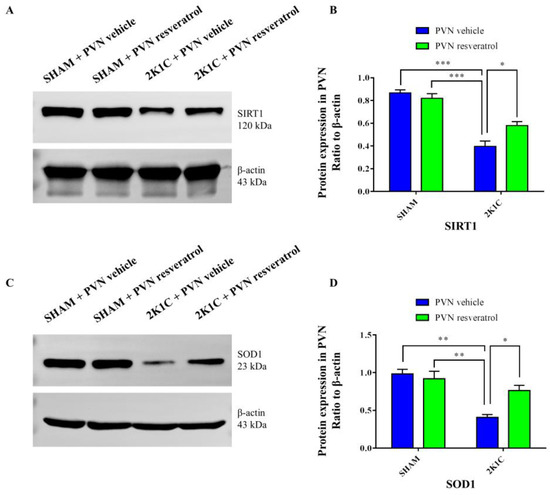
Figure 3.
The effects of resveratrol on SIRT1 and SOD1 protein expression in PVN. (A) Representative immunoblots of SIRT1 and β-actin; (B) densitometry protein expression of SIRT1; (C) immunoreactive bands of SOD1 and β-actin; and (D) statistic of SOD1 protein expression. Values are the mean ± SEM. * p < 0.01, ** p < 0.01, *** p < 0.001, n = 3.
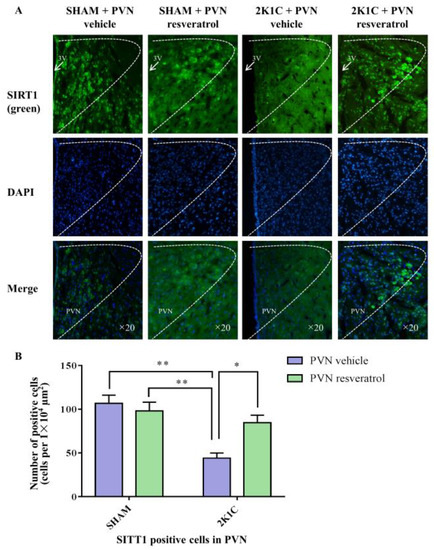
Figure 4.
The effects of resveratrol on SIRT1 expression in PVN. (A) Immunofluorescence staining of SIRT1; (B) statistics of SIRT1 positive cells. 3V: third ventricle. Values are the mean ± SEM. * p < 0.05, ** p < 0.01, n = 4.
3.4. NF-κB Activity in the PVN
3.5. NAD(P)H Oxidase Activity in the PVN
3.6. ROS Production in PVN
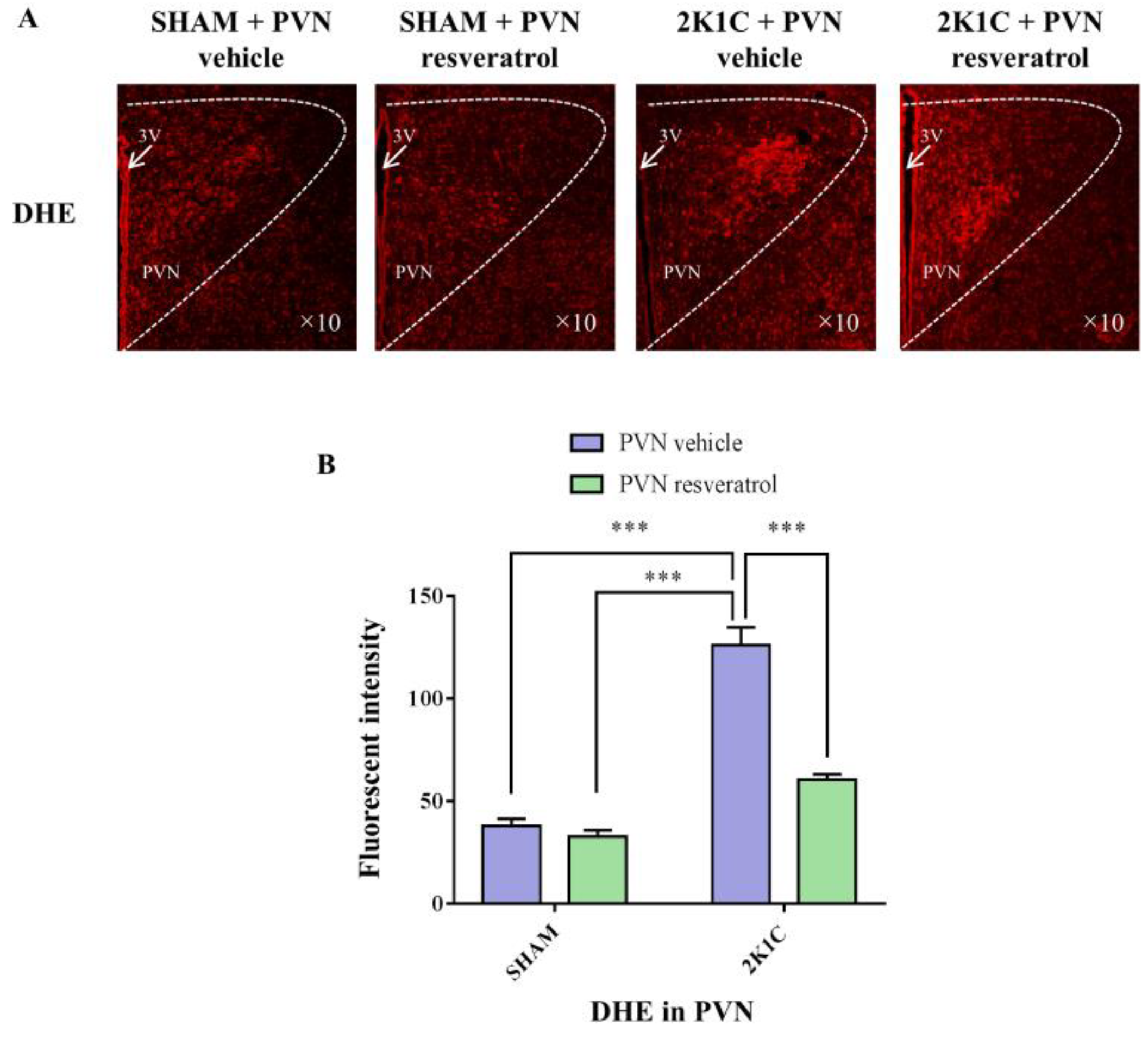
As shown in Figure 5, ROS levels in the hypertensive rats were higher than in the SHAM rats (p < 0.001). When rats were infused with the SIRT1 agonist, resveratrol, for 28 days, the level of ROS was reduced (Figure 5, p < 0.001).
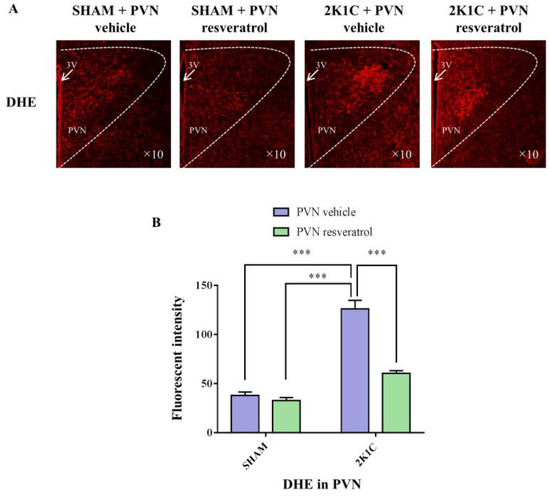
Figure 5.
The effects of resveratrol on ROS in PVN. (A) Immunofluorescence staining of reactive oxygen species (red fluorescence, ×10) and (B) densitometric analysis of DHE staining. 3V: third ventricle. Values are the mean ± SEM. *** p < 0.001, n = 4.
3.7. SOD1 Protein Expression in the PVN
3.8. NOX4 Expression in PVN
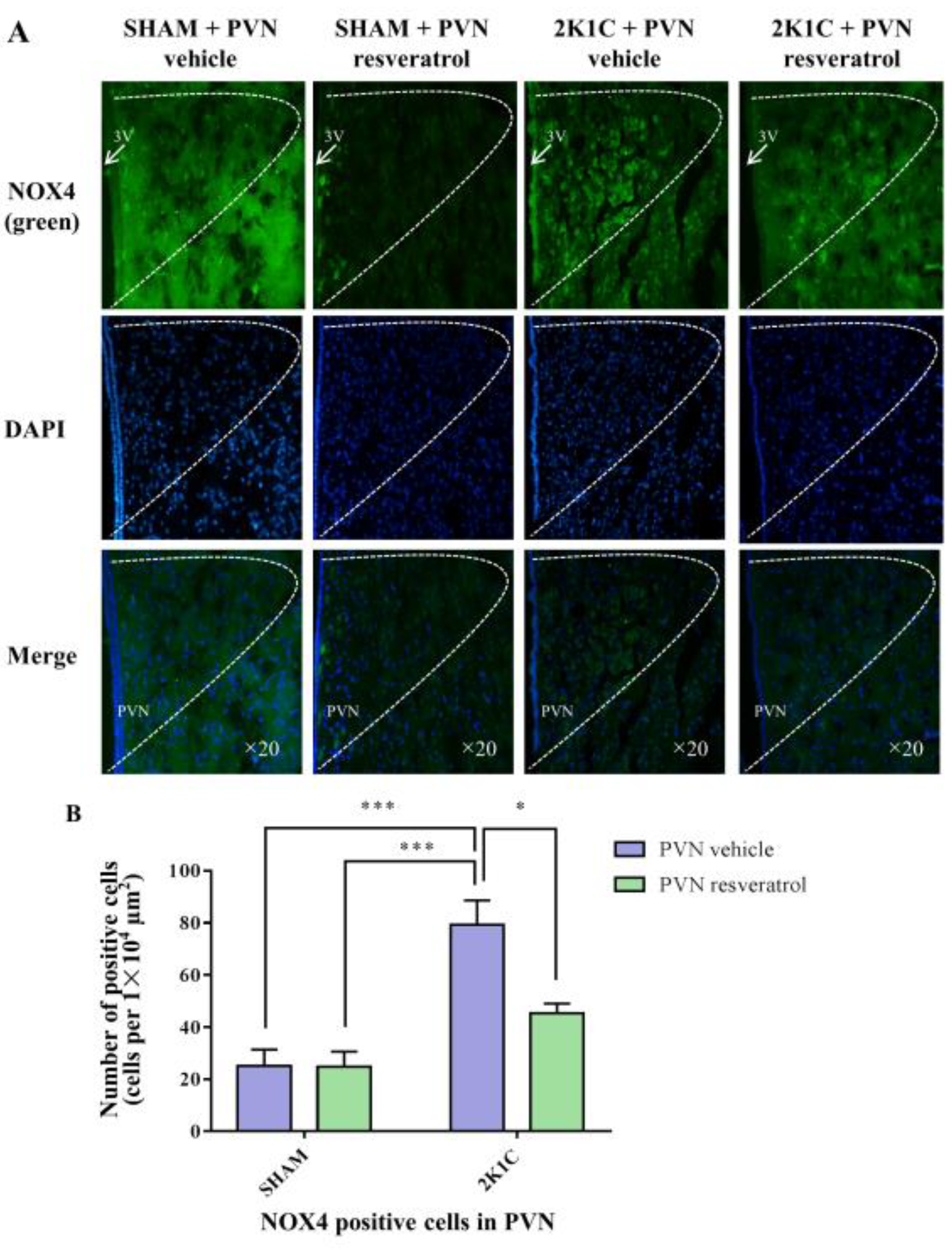
As shown in Figure 6, in contrast with the SHAM rats, the number of NOX4 positive cells in 2K1C rats significantly increased (p < 0.001). When rats were infused with resveratrol for four weeks, the number of NOX4 positive cells was reduced (Figure 6, p = 0.0161).
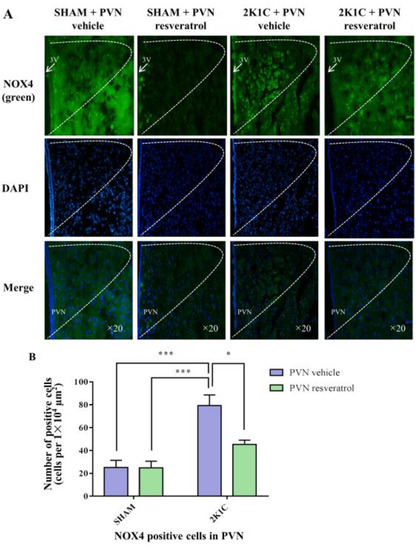
Figure 6.
The effects of PVN resveratrol on NOX4 expression. (A) Immunofluorescence staining of NOX4 (green fluorescence, ×20) and (B) statistics of NOX4 positive cells. 3V: third ventricle. Values are the mean ± SEM. * p < 0.05, *** p < 0.001, n = 4.
3.9. TH Expression in the PVN
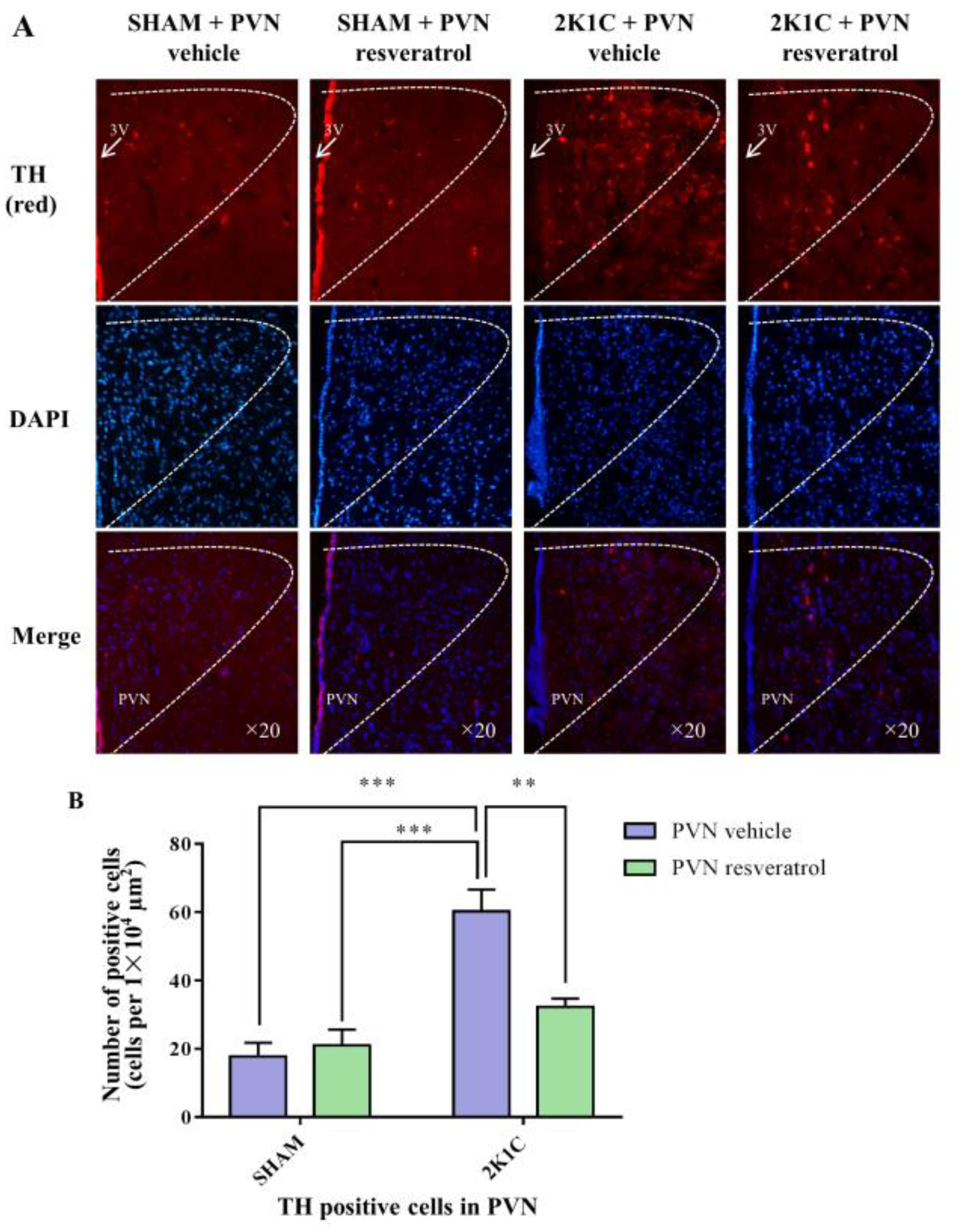
As shown in Figure 7, in contrast with the SHAM rats, the number of TH positive cells in 2K1C rats significantly increased (p < 0.001). When rats were infused with resveratrol for 28 days, the number of TH positive-cells was reduced (Figure 7, p = 0.0048).
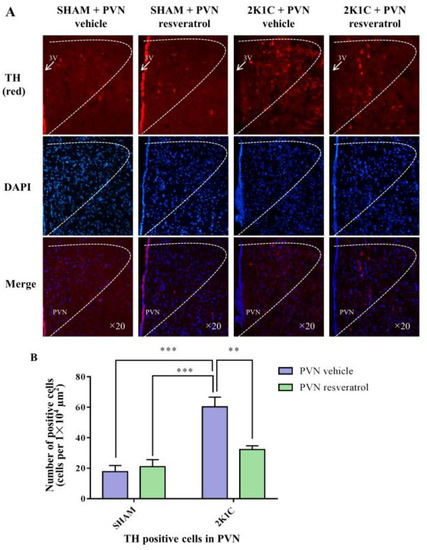
Figure 7.
The effects of PVN resveratrol on TH expression. (A) Immunofluorescence staining of TH (red fluorescence, ×20) and (B) statistics of TH positive cells. 3V: third ventricle. Values are the mean ± SEM. ** p < 0.01, *** p < 0.001, n = 4.
3.10. GAD67 Expression in the PVN
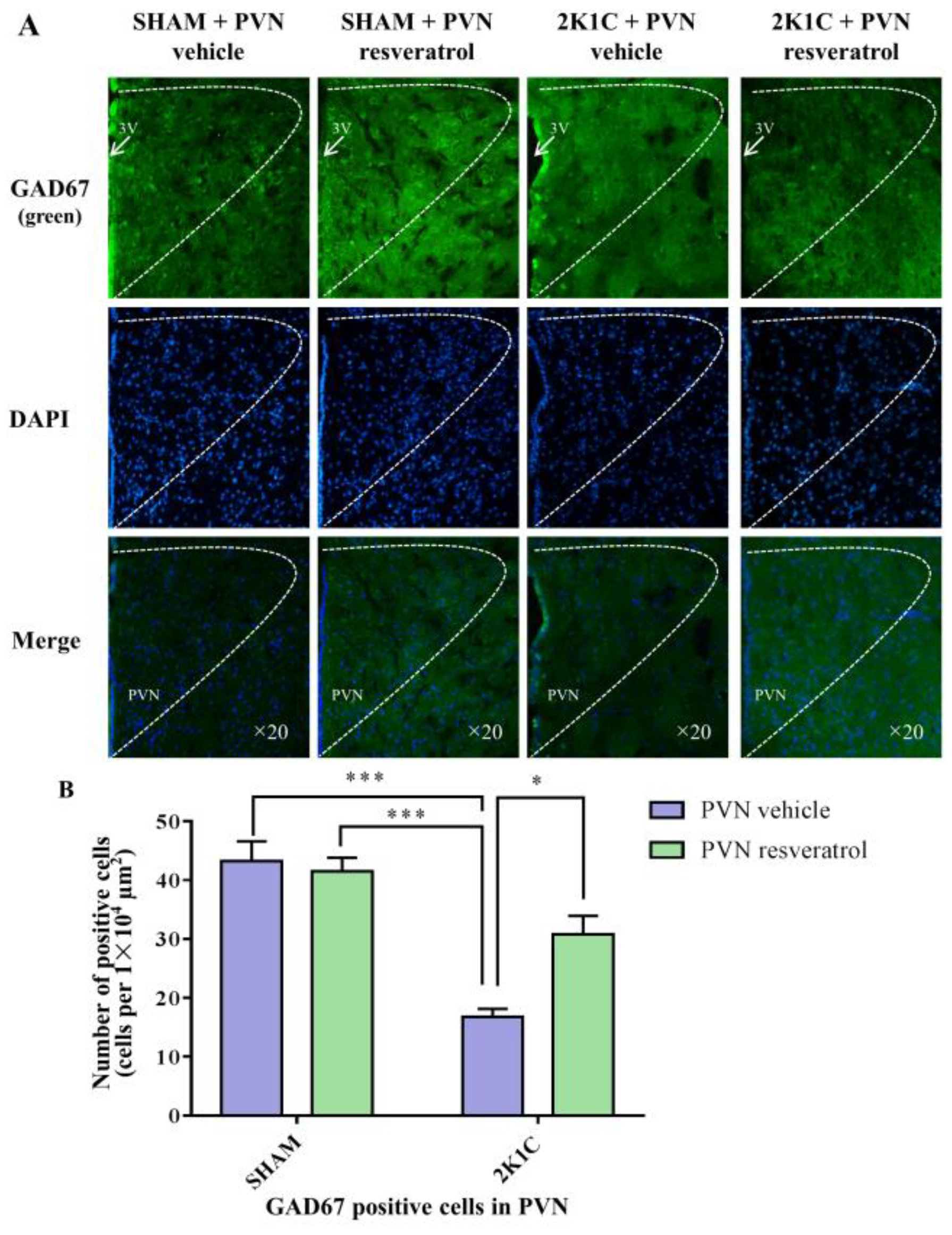
In comparison with the SHAM rats, the number of GAD67 positive cells in 2K1C rats significantly decreased (Figure 8, p < 0.001). When rats were infused with resveratrol for 28 days, the number of GAD67 positive cells was increased (Figure 8, p = 0.0142).
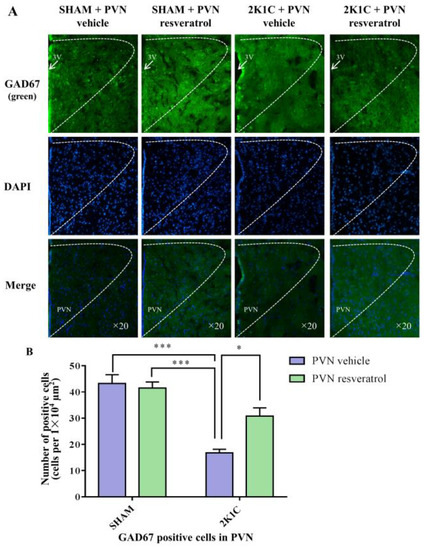
Figure 8.
The effects of PVN resveratrol on GAD67 expression. (A) Immunofluorescence staining of GAD67 (green fluorescence, ×20) and (B) statistics of GAD67 positive cells. 3V: third ventricle. Values are the mean ± SEM. * p < 0.05, *** p < 0.001, n = 4.
4. Discussion
A number of recent studies have reported that ROS in the PVN is crucial to the pathogenesis of cardiovascular diseases [25,39]. High ROS levels in the PVN lead to overstimulation of peripheral SNA [40]. Activation of the NOX family, particularly NOX2 and NOX4, is closely associated with ROS production in the PVN [41]. SOD1 is an important antioxidant enzyme which inhibits ROS production [32]. Some studies have shown that elevated NOX activity and ROS production in the PVN stimulates abnormal peripheral SNA in high-salt or Ang II-induced hypertension rats [38,41]. Blocking the excessive production of ROS in PVN can inhibit SNA in hypertensive rats [40]. Zhou found that inhibiting SIRT1 increased ROS production in the aortic endothelial cells and aortic vascular smooth muscle cells of mice [42].
The authors of [43] found evidence that resveratrol, an anti-inflammatory and antioxidant substance, is an effective activator of SIRT1 [10]. In addition, SIRT1 overexpression in the rostral ventrolateral medulla (RVLM), another central region that regulates sympathetic activity, led to lower blood pressure and reduced sympathetic outflow by lowering ROS levels in spontaneously hypertensive rats [44,45]. In accordance with these findings, our study found that PVN infusion of resveratrol increased SIRT1 and SOD1 protein expression and decreased the expression of NOX4 and ROS in 2K1C hypertensive rats, suggesting that activated SIRT1 exerts anti-oxidative stress effects in other central regions besides the RVLM. However, a study by Liu et al. showed that SIRT1 expression was increased in the arcuate nucleus of the hypothalamus (ARC) in obese hypertensive rats, and knockdown of SIRT1 in the ARC could decrease the RSNA and blood pressure in leptin-induced obese hypertension [46]. The above results suggest conflicting roles of SIRT1 in PVN and ARC in different hypertensive models. Further investigation is needed to fully understand the effects of SIRT1 mechanisms upon blood pressure regulation in different nuclei of the brain.
Researchers have also found that NF-κB activities in the PVN are closely associated with the intensity of SNA in hypertensive rats [24,47]. NF-κB and oxidative stress in PVN play crucial roles in the pathogenesis of hypertension [38,48]. SIRT1 can directly act on NF-κB and reduce the acetylation level of p65 subunit Lys310, thereby inhibiting the transcription activity of NF-κB [49]. Knockout of SIRT1 can lead to excessive NF-κB activity [50]. SIRT1 can also suppress NF-κB-mediated oxidative stress, including NOX, whereas loss of SIRT1 can lead to the hyperacetylation of NF-κB and the enhancement of oxidative stress [51]. Our study revealed that PVN injection with resveratrol lowered levels of NF-κB p65 activity, and reduced NOX4 and ROS expression in 2K1C hypertensive rats.
NE, Glu, and GABA are important neurotransmitters in the PVN that regulate SNA [52]. GABA has been shown to elicit sympatho-inhibitory responses in the PVN [32]. NE is a neurotransmitter secreted from the terminals of noradrenergic nerve fibers. Its content in peripheral blood can reflect the excited state of sympathetic nerves, which is increased in both peripheral and central systems in heart failure rat models [53]. Glu is a major excitatory neurotransmitter, and l-glutamate microinjected into the PVN can cause increased blood pressure [54]. Megan et al. found that injection of the GABA-A receptor agonist muscarine into the PVN significantly reduced RSNA and mean arterial pressure of Ang II- and salt-induced hypertensive rats [55]. An accumulating body of evidence suggests that the loss of balance in neurotransmitter levels in the PVN contributes to abnormal sympathetic activity in heart failure [33,53] and hypertension [34]. Previous studies of our group showed that NF-κB activation and TH expression increased in the PVN of hypertension [32,39] or heart failure rat models, whereas inhibition of NF-κB decreased TH expression in the PVN of heart failure rats [35]. In this study, PVN injection with resveratrol reduced TH levels but increased GAD67 levels. These data suggest that SIRT1 activation in the PVN restores balance in neurotransmitter expressions by acting on NF-κB, thereby affecting SNA and blood pressure.
5. Conclusions
Our results showed that administration of resveratrol in the PVN decreased ROS expression, restored neurotransmitter balance, and subsequently attenuated high blood pressure in 2K1C rats via the SIRT1/NF-κB pathway.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14194177/s1, Figure S1: Resveratrol increased SIRT1 protein expression of 2K1C rats; Figure S2: Resveratrol increased SOD1 protein expression of 2K1C rats.
Author Contributions
Conceptualization, J.Q., H.T. and Y.-M.K.; Data curation, L.-Y.F., K.-L.L., R.-J.L. and J.-A.Q.; Funding acquisition, J.Q., X.-J.Y., H.T. and Y.-M.K.; Investigation, J.Q., L.-Y.F., K.-L.L., R.-J.L., J.-A.Q., X.-J.Y., J.-Y.Y., Z.-P.F., Q.-Y.Y., H.J. and H.-L.G.; Writing—original draft, J.Q., Y.L. and H.T.; Writing—review & editing, J.Q., L.-Y.F., K.-L.L., R.-J.L. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (Nos. 82170451, 81500313, 82070440, 82070439) and Research Program of Xi’an Children’s Hospital (No. 2020A02).
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of Xi’an Jiaotong University (protocol code No. 2020-63 and date of approval 1 April 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PVN | Hypothalamic Paraventricular Nucleus |
| SNA | sympathetic nerve activity |
| SIRT1 | Silent Mating type information regulation 2 homolog-1 |
| ROS | reactive oxygen species |
| NOX | NAD(P)H oxidase |
| RSNA | renal sympathetic nerve activity |
| NF-κB | nuclear factor-kappa B |
| TH | Tyrosine hydroxylase |
| GAD67 | Glutamate decarboxylase 67 |
| NE | norepinephrine |
| Glu | glutamate |
| GABA | γ-aminobutyric acid |
| 2K1C | two-kidney one-clamp |
| SHAM | sham operation |
| aCSF | artificial cerebrospinal fluid |
| SBP | systolic blood pressure |
| DHE | Dihydroethidium |
| RVLM | rostral ventrolateral medulla |
| ARC | arcuate nucleus of the hypothalamus |
References
- Richards, E.M.; Li, J.; Stevens, B.R.; Pepine, C.J.; Raizada, M.K. Gut Microbiome and Neuroinflammation in Hypertension. Circ. Res. 2022, 130, 401–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.L.; Bo, J.H.; Zheng, F.; Zhang, F.; Chen, Q.; Li, Y.H.; Kang, Y.M.; Zhu, G.Q. Salusin-beta in Intermediate Dorsal Motor Nucleus of the Vagus Regulates Sympathetic-Parasympathetic Balance and Blood Pressure. Biomedicines 2021, 9, 1118. [Google Scholar] [CrossRef] [PubMed]
- Cayupe, B.; Morgan, C.; Puentes, G.; Valladares, L.; Burgos, H.; Castillo, A.; Hernandez, A.; Constandil, L.; Rios, M.; Saez-Briones, P.; et al. Hypertension in Prenatally Undernourished Young-Adult Rats Is Maintained by Tonic Reciprocal Paraventricular-Coerulear Excitatory Interactions. Molecules 2021, 26, 3568. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Pachuau, J.; Li, D.P.; Chen, S.R.; Pan, H.L. Group III metabotropic glutamate receptors regulate hypothalamic presympathetic neurons through opposing presynaptic and postsynaptic actions in hypertension. Neuropharmacology 2020, 174, 108159. [Google Scholar] [CrossRef]
- Zhang, K.; Li, W.; Ju, Y.; Wang, X.; Sun, X.; Fang, Y.; Chen, K. Transcriptomic and Metabolomic Basis of Short- and Long-Term Post-Harvest UV-C Application in Regulating Grape Berry Quality Development. Foods 2021, 10, 625. [Google Scholar] [CrossRef]
- Gutierrez Aguilar, G.F.; Alquisiras-Burgos, I.; Franco-Perez, J.; Pineda-Ramirez, N.; Ortiz-Plata, A.; Torres, I.; Pedraza-Chaverri, J.; Aguilera, P. Resveratrol Prevents GLUT3 Up-Regulation Induced by Middle Cerebral Artery Occlusion. Brain Sci. 2020, 10, 651. [Google Scholar] [CrossRef]
- Astley, C.; Houacine, C.; Zaabalawi, A.; Wilkinson, F.; Lightfoot, A.P.; Alexander, Y.; Whitehead, D.; Singh, K.K.; Azzawi, M. Nanostructured Lipid Carriers Deliver Resveratrol, Restoring Attenuated Dilation in Small Coronary Arteries, via the AMPK Pathway. Biomedicines 2021, 9, 1852. [Google Scholar] [CrossRef]
- Grujic-Milanovic, J.; Jacevic, V.; Miloradovic, Z.; Jovovic, D.; Milosavljevic, I.; Milanovic, S.D.; Mihailovic-Stanojevic, N. Resveratrol Protects Cardiac Tissue in Experimental Malignant Hypertension Due to Antioxidant, Anti-Inflammatory, and Anti-Apoptotic Properties. Int. J. Mol. Sci. 2021, 22, 5006. [Google Scholar] [CrossRef]
- Hsu, C.N.; Hou, C.Y.; Chang-Chien, G.P.; Lin, S.; Yang, H.W.; Tain, Y.L. Perinatal Resveratrol Therapy Prevents Hypertension Programmed by Maternal Chronic Kidney Disease in Adult Male Offspring: Implications of the Gut Microbiome and Their Metabolites. Biomedicines 2020, 8, 567. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The Role of Resveratrol in Mammalian Reproduction. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Kung, H.C.; Lin, K.J.; Kung, C.T.; Lin, T.K. Oxidative Stress, Mitochondrial Dysfunction, and Neuroprotection of Polyphenols with Respect to Resveratrol in Parkinson’s Disease. Biomedicines 2021, 9, 918. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Deng, J.; Liu, X.; Li, Q.; Zhang, J.; Bai, H.; Zhang, J. Echinacoside reverses myocardial remodeling and improves heart function via regulating SIRT1/FOXO3a/MnSOD axis in HF rats induced by isoproterenol. J. Cell. Mol. Med. 2021, 25, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Sun, L.; Wu, W.; Wu, J.; Sun, Z.; Ren, J. USP22 Protects against Myocardial Ischemia-Reperfusion Injury via the SIRT1-p53/SLC7A11-Dependent Inhibition of Ferroptosis-Induced Cardiomyocyte Death. Front. Physiol. 2020, 11, 551318. [Google Scholar] [CrossRef] [PubMed]
- Nishi, E.E.; Lopes, N.R.; Gomes, G.N.; Perry, J.C.; Sato, A.Y.S.; Naffah-Mazzacoratti, M.G.; Bergamaschi, C.T.; Campos, R.R. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens. Res. 2019, 42, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Zhang, Y.; Murugesan, P.; Huang, K.; Cai, H. NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 2020, 17, 170–194. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Hartlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Xue, B.; Beltz, T.G.; Johnson, R.F.; Guo, F.; Hay, M.; Johnson, A.K. PVN adenovirus-siRNA injections silencing either NOX2 or NOX4 attenuate aldosterone/NaCl-induced hypertension in mice. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H733–H741. [Google Scholar] [CrossRef]
- Sun, H.J.; Zhou, H.; Feng, X.M.; Gao, Q.; Ding, L.; Tang, C.S.; Zhu, G.Q.; Zhou, Y.B. Superoxide anions in the paraventricular nucleus mediate cardiac sympathetic afferent reflex in insulin resistance rats. Acta Physiol. 2014, 212, 267–282. [Google Scholar] [CrossRef]
- Bai, J.; Yu, X.J.; Liu, K.L.; Wang, F.F.; Jing, G.X.; Li, H.B.; Zhang, Y.; Huo, C.J.; Li, X.; Gao, H.L.; et al. Central administration of tert-butylhydroquinone attenuates hypertension via regulating Nrf2 signaling in the hypothalamic paraventricular nucleus of hypertensive rats. Toxicol. Appl. Pharmacol. 2017, 333, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Schraivogel, D.; Kuhn, T.M.; Rauscher, B.; Rodriguez-Martinez, M.; Paulsen, M.; Owsley, K.; Middlebrook, A.; Tischer, C.; Ramasz, B.; Ordonez-Rueda, D.; et al. High-speed fluorescence image-enabled cell sorting. Science 2022, 375, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.S.; Fuloria, N.K.; Fuloria, S.; Rahman, S.B.; Al-Malki, W.H.; Javed Shaikh, M.A.; Thangavelu, L.; Singh, S.K.; Rama Raju Allam, V.S.; Jha, N.K.; et al. Nuclear factor-kappa B and its role in inflammatory lung disease. Chem. Biol. Interact. 2021, 345, 109568. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Fan, Y.; He, Q.; Li, Y.; Wu, D.; Jiang, E. Exogenous H2S Ameliorates High Salt-Induced Hypertension by Alleviating Oxidative Stress and Inflammation in the Paraventricular Nucleus in Dahl S Rats. Cardiovasc. Toxicol. 2022, 22, 477–491. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, H.; Yin, J.; Shi, Y.; Tan, J.; Zheng, L.; Wang, C.; Li, X.; Xue, M.; Liu, J.; et al. TLR4 participates in sympathetic hyperactivity Post-MI in the PVN by regulating NF-kappaB pathway and ROS production. Redox Biol. 2019, 24, 101186. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, J.; Yin, J.; Hu, H.; Shi, Y.; Wang, Y.; Xue, M.; Li, X.; Liu, J.; Li, Y.; et al. Targeting blockade of nuclear factor-kappaB in the hypothalamus paraventricular nucleus to prevent cardiac sympathetic hyperinnervation post myocardial infarction. Neurosci. Lett. 2019, 707, 134319. [Google Scholar] [CrossRef]
- Schug, T.T.; Xu, Q.; Gao, H.; Peres-da-Silva, A.; Draper, D.W.; Fessler, M.B.; Purushotham, A.; Li, X. Myeloid deletion of SIRT1 induces inflammatory signaling in response to environmental stress. Mol. Cell. Biol. 2010, 30, 4712–4721. [Google Scholar] [CrossRef]
- Deng, H.J.; Zhou, C.H.; Huang, L.T.; Wen, L.B.; Zhou, M.L.; Wang, C.X. Activation of silent information regulator 1 exerts a neuroprotective effect after intracerebral hemorrhage by deacetylating NF-kappaB/p65. J. Neurochem. 2021, 157, 574–585. [Google Scholar] [CrossRef]
- Ramlan, H.; Damanhuri, H.A. Attenuation of the Counter-Regulatory Glucose Response in CVLM C1 Neurons: A Possible Explanation for Anorexia of Aging. Biomolecules 2022, 12, 449. [Google Scholar] [CrossRef]
- Frerker, B.; Rohde, M.; Muller, S.; Bien, C.G.; Kohling, R.; Kirschstein, T. Distinct Effects of Stereotactically Injected Human Cerebrospinal Fluid Containing Glutamic Acid Decarboxylase Antibodies into the Hippocampus of Rats on the Development of Spontaneous Epileptic Activity. Brain Sci. 2020, 10, 123. [Google Scholar] [CrossRef]
- Idalencio, R.; Lopes, T.M.; Soares, S.M.; Pompermaier, A.; de Alcantara Barcellos, H.H.; Kalichak, F.; Fagundes, M.; de Oliveira, C.M.; Barcellos, L.J.G. Effect of levodopa/carbidopa on stress response in zebrafish. J. Comp. Physiol. A Neuroethol. Sens. Neural Behav. Physiol. 2021, 207, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Yu, X.J.; Wang, X.M.; Li, H.B.; Li, Y.; Bai, J.; Qi, J.; Zhang, N.; Liu, K.L.; Zhang, Y.; et al. Bilateral Paraventricular Nucleus Upregulation of Extracellular Superoxide Dismutase Decreases Blood Pressure by Regulation of the NLRP3 and Neurotransmitters in Salt-Induced Hypertensive Rats. Front. Pharmacol. 2021, 12, 756671. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Sun, B.; He, R. Effect of the changes of NMDA receptor in hypothalamic paraventricular nucleus on cardiac function and sympathetic nervous activity in rats with heart failure. Biochem. Biophys. Res. Commun. 2017, 493, 1336–1341. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Yu, X.J.; Yang, Q.; Wang, X.M.; Xia, W.J.; Li, H.B.; Liu, K.L.; Yi, Q.Y.; Kang, Y.M. Inhibition of Maternal c-Src Ameliorates the Male Offspring Hypertension by Suppressing Inflammation and Neurotransmitters in the Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 820–834. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Gao, F.; Li, H.H.; Cardinale, J.P.; Elks, C.; Zang, W.J.; Yu, X.J.; Xu, Y.Y.; Qi, J.; Yang, Q.; et al. NF-kappaB in the paraventricular nucleus modulates neurotransmitters and contributes to sympathoexcitation in heart failure. Basic Res. Cardiol. 2011, 106, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Cao, Y.K.; Liu, Y.X.; Wang, R.; Wu, Y.M. Microinjection of resveratrol into rostral ventrolateral medulla decreases sympathetic vasomotor tone through nitric oxide and intracellular Ca2+ in anesthetized male rats. Acta Pharmacol. Sin. 2008, 29, 906–912. [Google Scholar] [CrossRef]
- Li, Y.; Yu, X.J.; Xiao, T.; Chi, H.L.; Zhu, G.Q.; Kang, Y.M. Nrf1 Knock-Down in the Hypothalamic Paraventricular Nucleus Alleviates Hypertension Through Intervention of Superoxide Production-Removal Balance and Mitochondrial Function. Cardiovasc. Toxicol. 2021, 21, 472–489. [Google Scholar] [CrossRef]
- Su, Q.; Yu, X.J.; Wang, X.M.; Peng, B.; Bai, J.; Li, H.B.; Li, Y.; Xia, W.J.; Fu, L.Y.; Liu, K.L.; et al. Na(+)/K(+)-ATPase Alpha 2 Isoform Elicits Rac1-Dependent Oxidative Stress and TLR4-Induced Inflammation in the Hypothalamic Paraventricular Nucleus in High Salt-Induced Hypertension. Antioxidants 2022, 11, 288. [Google Scholar] [CrossRef]
- Gao, H.L.; Yu, X.J.; Zhang, Y.; Wang, C.L.; Lei, Y.M.; Yu, J.Y.; Zong, D.M.; Liu, K.L.; Zhang, D.D.; Li, Y.; et al. Astaxanthin Ameliorates Blood Pressure in Salt-Induced Prehypertensive Rats Through ROS/MAPK/NF-kappaB Pathways in the Hypothalamic Paraventricular Nucleus. Cardiovasc. Toxicol. 2021, 21, 1045–1057. [Google Scholar] [CrossRef]
- Ding, L.; Kang, Y.; Dai, H.B.; Wang, F.Z.; Zhou, H.; Gao, Q.; Xiong, X.Q.; Zhang, F.; Song, T.R.; Yuan, Y.; et al. Adipose afferent reflex is enhanced by TNFalpha in paraventricular nucleus through NADPH oxidase-dependent ROS generation in obesity-related hypertensive rats. J. Transl. Med. 2019, 17, 256. [Google Scholar] [CrossRef]
- Kang, Y.; Ding, L.; Dai, H.; Wang, F.; Zhou, H.; Gao, Q.; Xiong, X.; Zhang, F.; Song, T.; Yuan, Y.; et al. Intermedin in Paraventricular Nucleus Attenuates Ang II-Induced Sympathoexcitation through the Inhibition of NADPH Oxidase-Dependent ROS Generation in Obese Rats with Hypertension. Int. J. Mol. Sci. 2019, 20, 4217. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, S.; Bolor-Erdene, E.; Wang, L.; Tian, D.; Mei, Y. NAMPT/SIRT1 Attenuate Ang II-Induced Vascular Remodeling and Vulnerability to Hypertension by Inhibiting the ROS/MAPK Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 1974265. [Google Scholar] [CrossRef] [PubMed]
- Castrejon-Tellez, V.; Villegas-Romero, M.; Rubio-Ruiz, M.E.; Perez-Torres, I.; Carreon-Torres, E.; Diaz-Diaz, E.; Guarner-Lans, V. Effect of a Resveratrol/Quercetin Mixture on the Reversion of Hypertension Induced by a Short-Term Exposure to High Sucrose Levels Near Weaning and a Long-Term Exposure That Leads to Metabolic Syndrome in Rats. Int. J. Mol. Sci. 2020, 21, 2231. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.Z.; Wu, Z.T.; Wang, W.; Tan, X.; Yang, Y.H.; Wang, Y.K.; Li, M.L.; Wang, W.Z. SIRT1 exerts anti-hypertensive effect via FOXO1 activation in the rostral ventrolateral medulla. Free Radic. Biol. Med. 2022, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.F.; Zhang, H.; Zheng, P.; Chen, S.; Gu, Z.; Zhou, J.J.; Phaup, J.G.; Chang, H.M.; Yeh, E.T.H.; Pan, H.L.; et al. Impaired Kv7 channel activity in the central amygdala contributes to elevated sympathetic outflow in hypertension. Cardiovasc. Res. 2022, 118, 585–596. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, H. Modulation of Sirt1 and FoxO1 on Hypothalamic Leptin-Mediated Sympathetic Activation and Inflammation in Diet-Induced Obese Rats. J. Am. Heart Assoc. 2021, 10, e020667. [Google Scholar] [CrossRef]
- Qi, J.; Yu, X.J.; Fu, L.Y.; Liu, K.L.; Gao, T.T.; Tu, J.W.; Kang, K.B.; Shi, X.L.; Li, H.B.; Li, Y.; et al. Exercise Training Attenuates Hypertension Through TLR4/MyD88/NF-kappaB Signaling in the Hypothalamic Paraventricular Nucleus. Front. Neurosci. 2019, 13, 1138. [Google Scholar] [CrossRef]
- Xu, M.L.; Yu, X.J.; Zhao, J.Q.; Du, Y.; Xia, W.J.; Su, Q.; Du, M.M.; Yang, Q.; Qi, J.; Li, Y.; et al. Calcitriol ameliorated autonomic dysfunction and hypertension by down-regulating inflammation and oxidative stress in the paraventricular nucleus of SHR. Toxicol. Appl. Pharmacol. 2020, 394, 114950. [Google Scholar] [CrossRef]
- Matsushita, T.; Sasaki, H.; Takayama, K.; Ishida, K.; Matsumoto, T.; Kubo, S.; Matsuzaki, T.; Nishida, K.; Kurosaka, M.; Kuroda, R. The overexpression of SIRT1 inhibited osteoarthritic gene expression changes induced by interleukin-1beta in human chondrocytes. J. Orthop. Res. 2013, 31, 531–537. [Google Scholar] [CrossRef]
- Meng, F.; Yang, M.; Chen, Y.; Chen, W.; Wang, W. miR-34a induces immunosuppression in colorectal carcinoma through modulating a SIRT1/NF-kappaB/B7-H3/TNF-alpha axis. Cancer Immunol. Immunother. 2021, 70, 2247–2259. [Google Scholar] [CrossRef]
- Dasgupta, A.; Shukla, S.K.; Vernucci, E.; King, R.J.; Abrego, J.; Mulder, S.E.; Mullen, N.J.; Graves, G.; Buettner, K.; Thakur, R.; et al. SIRT1-NOX4 signaling axis regulates cancer cachexia. J. Exp. Med. 2020, 217, e20190745. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Zhang, A.Q.; Zhao, X.F.; Cardinale, J.P.; Elks, C.; Cao, X.M.; Zhang, Z.W.; Francis, J. Paraventricular nucleus corticotrophin releasing hormone contributes to sympathoexcitation via interaction with neurotransmitters in heart failure. Basic Res. Cardiol. 2011, 106, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.B.; Hristova, K.; Fedacko, J.; El-Kilany, G.; Cornelissen, G. Chronic heart failure: A disease of the brain. Heart Fail. Rev. 2019, 24, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Gingerich, S.; Krukoff, T.L. Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO. Hypertension 2006, 48, 1130–1136. [Google Scholar] [CrossRef]
- Bardgett, M.E.; Holbein, W.W.; Herrera-Rosales, M.; Toney, G.M. Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-alpha. Hypertension 2014, 63, 527–534. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).