Acute Moderate-Intensity Aerobic Exercise under High PM2.5 Levels Does Not Influence the Pulmonary Function and Lung Diffusion Capacity in Healthy Young Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. PM2.5 Concentration
2.4. Height, Body Mass and Body Composition
2.5. Pulmonary Function
2.6. Statistical Analysis
3. Results
3.1. Participants’ Characteristics and PM2.5 Concentration
3.2. Pulmonary Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, L.; Chuang, C.C.; Zuo, L. Fine particulate matter in acute exacerbation of COPD. Front. Physiol. 2015, 6, 294. [Google Scholar] [CrossRef] [PubMed]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Ostro, B.; Broadwin, R.; Green, S.; Feng, W.-Y.; Lipsett, M. Fine particulate air pollution and mortality in nine California counties: Results from CALFINE. Environ. Health Perspect. 2006, 114, 29–33. [Google Scholar] [CrossRef]
- Schwartz, J.; Dockery, D.W.; Neas, L.M. Is daily mortality associated specifically with fine particles? J. Air Waste Manag. Assoc. 1996, 46, 927–939. [Google Scholar] [CrossRef]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Human: Outdoor Air Pollution; International Agency for Research on Cancer: Lyon, France, 2016; Volume 109. [Google Scholar]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Krebs, B.; Burney, J.; Zivin, J.G.; Neidell, M. Using Crowd-Sourced Data to Assess the Temporal and Spatial Relationship between Indoor and Outdoor Particulate Matter. Environ. Sci. Technol. 2021, 55, 6107–6115. [Google Scholar] [CrossRef] [PubMed]
- Miller, F.J.; Asgharian, B.; Schroeter, J.D.; Price, O. Improvements and additions to the Multiple Path Particle Dosimetry model. J. Aerosol. Sci. 2016, 99, 14–26. [Google Scholar] [CrossRef]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed. Res. Int. 2013, 2013, 279371. [Google Scholar] [CrossRef] [PubMed]
- EPA, U.S. Integrated Science Assessment (ISA) for Particulate Matter (Final Report, Dec 2019); U.S. Environmental Protection Agency: Washington, DC, USA, 2019. [Google Scholar]
- Hansen, E.S.H.; Pitzner-Fabricius, A.; Toennesen, L.L.; Rasmusen, H.K.; Hostrup, M.; Hellsten, Y.; Backer, V.; Henriksen, M. Effect of aerobic exercise training on asthma in adults: A systematic review and meta-analysis. Eur. Respir. J. 2020, 56, 2000146. [Google Scholar] [CrossRef]
- Giles, L.V.; Carlsten, C.; Koehle, M.S. The effect of pre-exercise diesel exhaust exposure on cycling performance and cardio-respiratory variables. Inhal. Toxicol. 2012, 24, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Angane, E.Y.; Navare, A.A. Effects of aerobic exercise on pulmonary function tests in healthy adults. Int. J. Res. Med. Sci. 2017, 4, 2059–2063. [Google Scholar] [CrossRef][Green Version]
- Munibuddin, A.; Khan, S.; Choudhari, S.; Doiphode, R. Effect of traditional aerobic exercises versus sprint interval training on pulmonary function tests in young sedentary males: A randomised controlled trial. J. Clin. Diagn. Res. 2013, 7, 1890. [Google Scholar] [CrossRef]
- McCafferty, W.B.; Horvath, S. Air Pollution and Athletic Performance; Thomas: Springfield, IL, USA, 1981. [Google Scholar]
- Daigle, C.C.; Chalupa, D.C.; Gibb, F.R.; Morrow, P.E.; Oberdorster, G.; Utell, M.J.; Frampton, M.W. Ultrafine particle deposition in humans during rest and exercise. Inhal. Toxicol. 2003, 15, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Giles, L.V.; Carlsten, C.; Koehle, M.S. The pulmonary and autonomic effects of high-intensity and low-intensity exercise in diesel exhaust. Environ. Health 2018, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Sinharay, R.; Gong, J.; Barratt, B.; Ohman-Strickland, P.; Ernst, S.; Kelly, F.J.; Zhang, J.J.; Collins, P.; Cullinan, P.; Chung, K.F. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: A randomised, crossover study. Lancet 2018, 391, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.R.; Brandley, D.C. Exercise in Thermal Inversions: PM2.5 Air Pollution Effects on Pulmonary Function and Aerobic Performance. Wilderness Environ. Med. 2020, 31, 16–22. [Google Scholar] [CrossRef]
- Wagner, D.R.; Clark, N.W. Effects of ambient particulate matter on aerobic exercise performance. J. Exerc. Sci. Fit. 2018, 16, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, L.H.; Devlin, R.B.; Rappold, A.G.; Case, M.W.; Diaz-Sanchez, D. Low levels of fine particulate matter increase vascular damage and reduce pulmonary function in young healthy adults. Part Fibre Toxicol. 2020, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Quanjer, P.H.; Stanojevic, S.; Cole, T.J.; Baur, X.; Hall, G.L.; Culver, B.H.; Enright, P.L.; Hankinson, J.L.; Ip, M.S.; Zheng, J.; et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur. Respir J. 2012, 40, 1324–1343. [Google Scholar] [CrossRef] [PubMed]
- Stefani, L.; Mascherini, G.; Galanti, G. Aerobic threshold for exercise prescription. Int. J. Clin. Med. 2010, 1, 6–9. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Ghio, A.J.; Kim, C.; Devlin, R.B. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. Am. J. Resp. Crit. Care 2000, 162, 981–988. [Google Scholar] [CrossRef]
- Kocot, K.; Baranski, K.; Melaniuk-Wolny, E.; Zajusz-Zubek, E.; Kowalska, M. Acute FeNO and Blood Pressure Responses to Air Pollution Exposure in Young Adults during Physical Activity. Int. J. Env. Res. Pub. Health 2020, 17, 9012. [Google Scholar] [CrossRef]
- Riva, D.R.; Magalhaes, C.B.; Lopes, A.A.; Lancas, T.; Mauad, T.; Malm, O.; Valenca, S.S.; Saldiva, P.H.; Faffe, D.S.; Zin, W.A. Low dose of fine particulate matter (PM2.5) can induce acute oxidative stress, inflammation and pulmonary impairment in healthy mice. Inhal. Toxicol. 2011, 23, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, C.; Zhang, B.; Ge, Y.; Wang, W.; Cai, J.; Kan, H. Acute effects of personal exposure to fine particulate matter on salivary and urinary biomarkers of inflammation and oxidative stress in healthy adults. Chemosphere 2021, 272, 129906. [Google Scholar] [CrossRef]
- Peixoto, M.S.; Galvao, M.F.D.; de Medeiros, S.R.B. Cell death pathways of particulate matter toxicity. Chemosphere 2017, 188, 32–48. [Google Scholar] [CrossRef]
- Bousquet, J.; Jeffery, P.K.; Busse, W.W.; Johnson, M.; Vignola, A.M. Asthma—From bronchoconstriction to airways inflammation and remodeling. Am. J. Resp. Crit. Care 2000, 161, 1720–1745. [Google Scholar] [CrossRef] [PubMed]
- MacNee, W.; Rahman, I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol. Med. 2001, 7, 55–62. [Google Scholar] [CrossRef]
- Pun, V.C.; Kazemiparkouhi, F.; Manjourides, J.; Suh, H.H. Long-Term PM2.5 Exposure and Respiratory, Cancer, and Cardiovascular Mortality in Older US Adults. Am. J. Epidemiol. 2017, 186, 961–969. [Google Scholar] [CrossRef]
- Gong, H.; Linn, W.S.; Clark, K.W.; Anderson, K.R.; Sioutas, C.; Alexis, N.E.; Cascio, W.E.; Devlin, R.B. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in los angeles. Inhal. Toxicol. 2008, 20, 533–545. [Google Scholar] [CrossRef]
- Park, H.Y.; Gilbreath, S.; Barakatt, E. Respiratory outcomes of ultrafine particulate matter (UFPM) as a surrogate measure of near-roadway exposures among bicyclists. Environ. Health. Glob. 2017, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Medicine, A.C.o.S. ACSM’s Health-Related Physical Fitness Assessment Manual; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Child, R.; Wilkinson, D.; Fallowfield, J. Resting serum antioxidant status is positively correlated with peak oxygen uptake in endurance trained runners. J. Sports Med. Phys. Fit. 1999, 39, 282. [Google Scholar]
- de Camargo, A.A.; de Castro, R.A.S.; Vieira, R.P.; Oliveira-Júnior, M.C.; Araujo, A.A.d.; De Angelis, K.; Rached, S.Z.; Athanazio, R.A.; Stelmach, R.; Corso, S.D. Systemic Inflammation and Oxidative Stress in Adults with Bronchiectasis: Association with Clinical and Functional Features. Clinics 2021, 76, e2474. [Google Scholar] [CrossRef]
- Pietropaoli, A.P.; Frampton, M.W.; Hyde, R.W.; Morrow, P.E.; Oberdorster, G.; Cox, C.; Speers, D.M.; Frasier, L.M.; Chalupa, D.C.; Huang, L.S.; et al. Pulmonary function, diffusing capacity, and inflammation in healthy and asthmatic subjects exposed to ultrafine particles. Inhal. Toxicol. 2004, 16 (Suppl. S1), 59–72. [Google Scholar] [CrossRef]
- Brook, R.D.; Brook, J.R.; Urch, B.; Vincent, R.; Rajagopalan, S.; Silverman, F. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 2002, 105, 1534–1536. [Google Scholar] [CrossRef] [PubMed]
- Cutrufello, P.T.; Rundell, K.W.; Smoliga, J.M.; Stylianides, G.A. Inhaled whole exhaust and its effect on exercise performance and vascular function. Inhal. Toxicol. 2011, 23, 658–667. [Google Scholar] [CrossRef] [PubMed]
- Batalha, J.R.; Saldiva, P.H.; Clarke, R.W.; Coull, B.A.; Stearns, R.C.; Lawrence, J.; Murthy, G.G.; Koutrakis, P.; Godleski, J.J. Concentrated ambient air particles induce vasoconstriction of small pulmonary arteries in rats. Environ. Health Perspect. 2002, 110, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
| Group | Participants (n = 9) |
|---|---|
| Age (years) | 24.6 ± 0.4 |
| Height (cm) | 177.4 ± 1.5 |
| Body mass (kg) | 77.9 ± 1.6 |
| BMI (kg/m2) | 24.8 ± 0.6 |
| VO2peak (mL/kg/min) | 55.0 ± 18.3 |
| HPM | LPM | |
|---|---|---|
| Outdoor PM2.5 concentration (μg/m3) | 150.9 ± 27.1 (71, 280) | 17.6 ± 4.7 (5, 40) * |
| Indoor PM2.5 concentration (μg/m3) | 59.0 ± 2.1 (50, 73) | 7.8 ± 1.0 (5, 13) * |
| HPM | LPM | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
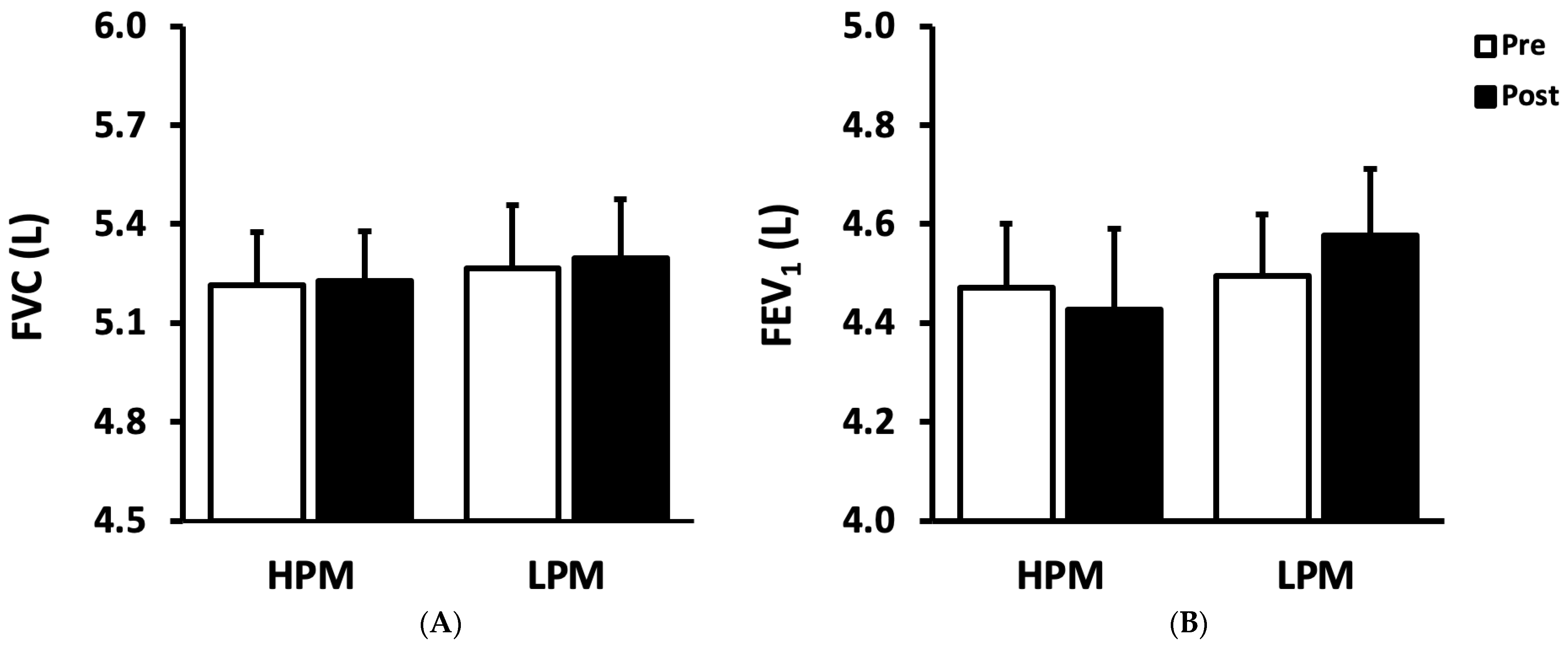
| FVC (L) | 5.22 ± 0.16 | 5.23 ± 0.15 | 5.27 ± 0.19 | 5.30 ± 0.18 |
| (% predicted) | 106.67 ± 2.84 | 106.78 ± 2.84 | 107.44 ± 3.22 | 108.22 ± 3.20 |
| FEV1 (L) | 4.47 ± 0.13 | 4.43 ± 0.16 | 4.49 ± 0.13 | 4.58 ± 0.13 |
| (% predicted) | 106.22 ± 3.08 | 105.33 ± 3.33 | 106.56 ± 3.12 | 108.67 ± 3.30 |
| FEV1/FVC (%) | 86.01 ± 2.49 | 85.16 ± 3.68 | 85.76 ± 2.44 | 86.76 ± 2.39 |
| (% predicted) | 99.33 ± 2.81 | 98.44 ± 4.14 | 98.89 ± 2.61 | 100.00 ± 2.60 |
| FEF25–75 (%) | 5.01 ± 0.43 | 4.94 ± 0.47 | 4.96 ± 0.38 | 5.21 ± 0.42 |
| (% predicted) | 105.33 ± 9.28 | 101.56 ± 9.85 | 104.00 ± 8.30 | 109.00 ± 9.19 |
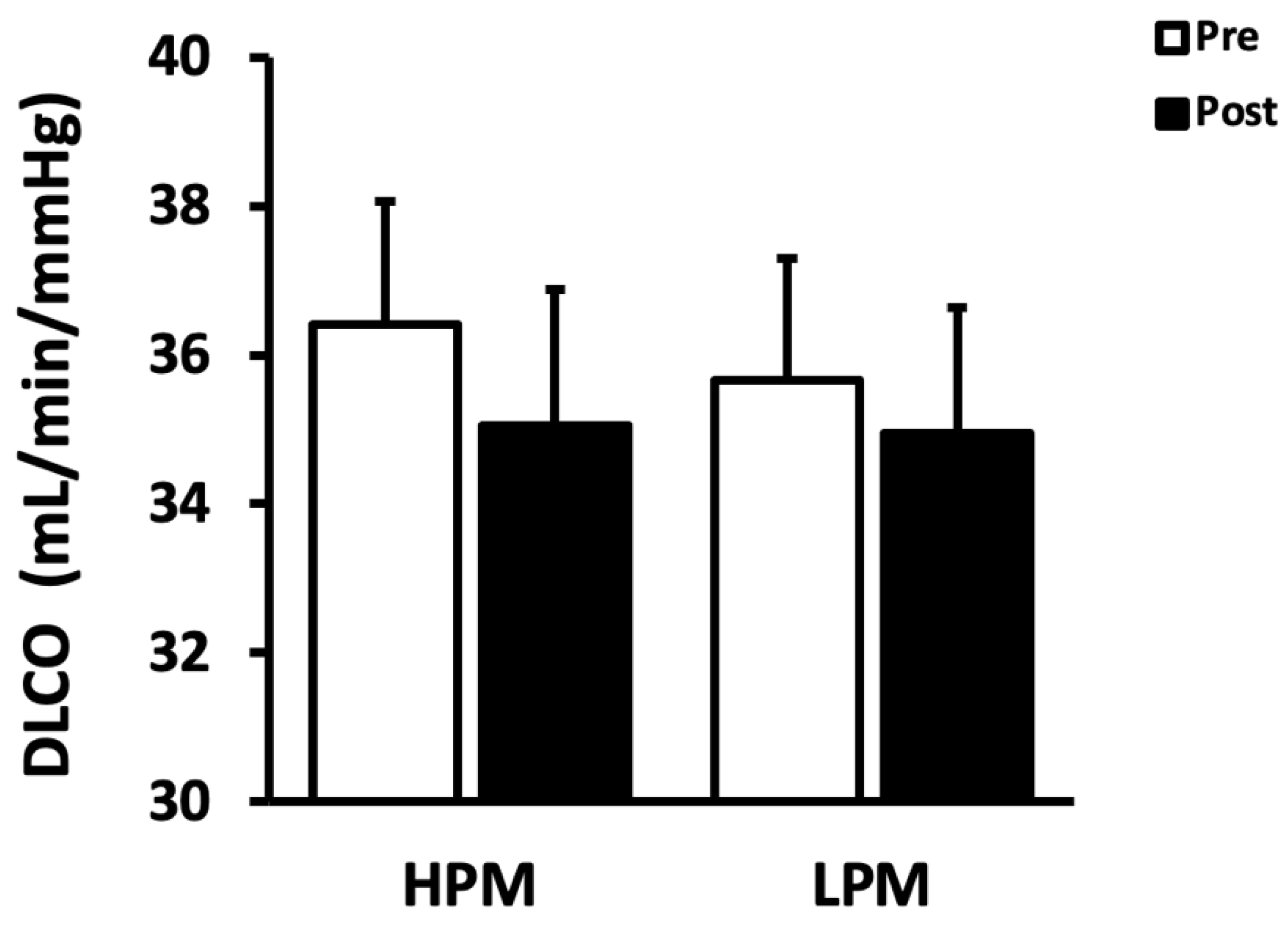
| DLCO (mL/min/mmHg) | 36.41 ± 1.66 | 35.05 ± 1.83 | 35.66 ± 1.64 | 34.94 ± 1.70 |
| (% predicted) | 101.44 ± 4.11 | 97.56 ± 4.50 | 99.33 ± 4.53 | 97.33 ± 4.46 |
| DLCO/VA (mL/min/mmHg/L) | 5.40 ± 0.12 | 5.24 ± 0.13 | 5.25 ± 0.13 | 5.02 ± 0.11 |
| (% predicted) | 104.56 ± 2.27 | 101.44 ± 2.56 | 101.44 ± 2.29 | 97.33 ± 2.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-S.; Lee, D.G.; Wang, L.; Kang, H.; Hwang, M.-H. Acute Moderate-Intensity Aerobic Exercise under High PM2.5 Levels Does Not Influence the Pulmonary Function and Lung Diffusion Capacity in Healthy Young Men. Appl. Sci. 2022, 12, 10080. https://doi.org/10.3390/app121910080
Kim J-S, Lee DG, Wang L, Kang H, Hwang M-H. Acute Moderate-Intensity Aerobic Exercise under High PM2.5 Levels Does Not Influence the Pulmonary Function and Lung Diffusion Capacity in Healthy Young Men. Applied Sciences. 2022; 12(19):10080. https://doi.org/10.3390/app121910080
Chicago/Turabian StyleKim, Jin-Su, Do Gyun Lee, Lin Wang, Heechan Kang, and Moon-Hyon Hwang. 2022. "Acute Moderate-Intensity Aerobic Exercise under High PM2.5 Levels Does Not Influence the Pulmonary Function and Lung Diffusion Capacity in Healthy Young Men" Applied Sciences 12, no. 19: 10080. https://doi.org/10.3390/app121910080
APA StyleKim, J.-S., Lee, D. G., Wang, L., Kang, H., & Hwang, M.-H. (2022). Acute Moderate-Intensity Aerobic Exercise under High PM2.5 Levels Does Not Influence the Pulmonary Function and Lung Diffusion Capacity in Healthy Young Men. Applied Sciences, 12(19), 10080. https://doi.org/10.3390/app121910080







