Abstract
This work determined the feasibility of producing two highly demanded products (cornmeal (CM) snacks and gelatin power) with antioxidant properties and using dry-salted brown cannonball jellyfish umbrella (UM) and oral arms (OAs). Desalted and rehydrated UM and OAs were subjected to drying and milling processes to produce jellyfish flours (UMF and OAF). Five cornmeal snacks were made: 100% CM and ones containing 20% UMF; 50% UMF; 20% OAF; and 50% OAF. Meanwhile, gelatin was obtained from UM and OAs through 0.5 M HCl hydrolysis and thermal and cold maturation treatment. Jellyfish flours increased the protein content of cornmeal snacks. Only OAF improved its antioxidant activity. The addition of 20% OAF did not affect the sensory characteristics of cornmeal snacks. Gelatin from UM had a lower crude protein level, and a gelatin β-component was not observed. Higher hydroxyproline content by HPLC and proton peaks at higher 1H–NMR fields were observed in OA gelatin. OA gelatin exhibited higher viscosity, foam, and in vitro antioxidant properties.
1. Introduction
The proliferation of jellyfish in many countries’ coastal waters and the growth of sustainable and novel foods present the possibility of considering these organisms as alternative raw materials for novel foods [1]. Moreover, these organisms are characterized by their nutraceutical attributes [2,3,4,5,6,7,8,9,10,11,12]. Furthermore, no allergic reaction has been detected in jellyfish chemical compounds [13].
Among the commercially exploited jellyfish species, brown cannonball jellyfish (Stomolophus meleagris) are emerging in fisheries in the Pacific Ocean, ranging from California to Ecuador. They thrive during the summer and fall months when they approach shallow waters to reproduce, representing 16% of the biomass of the region’s coastal zone [14]. Given their intrinsic physical and chemical properties such as high moisture content and high perishability, jellyfish are usually exported dry-salted to Asian countries, where they are considered a delicacy with therapeutical properties [14]. It is noteworthy that during the dry-salting process, umbrella and oral arms are separated, and the price of oral arms is generally lower than the umbrella region. Despite jellyfish fisheries being profitable in the Americas [10], jellyfish is not a common food or food ingredient in Western countries [10,14]. Because of this situation, the development of processing technologies that include umbrella and oral arms in the food industry will likely lead to their consumption by Western consumers.
Recently, it was stated that jellyfish have the potential to be a food of the future due to their high population density during seasonal blooms and bioactive properties [15]. Since jellyfish may improve consumers’ health and nutrition, they are of the particular interest to those involved in their capture and commercialization who seek to devise new jellyfish production and acquisition processes [10]. However, these processes must be designed to maintain the jellyfish´s nutritional properties and have sensory attributes suitable for consumers [1]. Within the context that most consumers’ products are cereal-based snacks, which are low in overall nutritional value [16], fried cornmeal snacks could be an attractive option.
Concerning fried cornmeal snacks, numerous studies have been conducted on the use of different types of seafood in the fortification of cereal snack products [16,17,18,19]. This is due to seafood being an excellent source of bioactive compounds that can be incorporated into cereal snacks to improve their bioactive properties [20]. However, they can affect the sensory attributes of products, so for success in the market, fried cornmeal snacks must retain their normal sensory attributes [21]. In this context, the application of dry-salted jellyfish has not yet been considered.
Based on the market opportunities for novel marine collagens, another alternative for jellyfish is to produce gelatin powder [11]. Several studies have reported that commercial gelatins obtained from pork and bovine by-products are limited mainly due to health-, social-, and culture-related concerns [22]. Marine gelatins have been studied as suitable replacements for terrestrial animal collagen [22]. However, few studies have reported the production of edible jellyfish gelatin [5,6,11,23,24,25]. Moreover, although the antioxidant activity of protein extracts, protein hydrolysates and peptides from jellyfish has been reported [3,5,7,8,10,12], information about the antioxidant capacity of gelatin obtained from commercial dry-salted cannonball jellyfish umbrella and oral arms is still scarce.
In order to achieve high added value and comprehensive utilization of brown cannonball jellyfish (Stomolophus meleagris), this study focused on determining the feasibility of producing two highly demanded products from commercial dry-slated S. meleagris: (1) fried cornmeal snacks from umbrella flour and oral arm flour, and (2) gelatin from rehydrated umbrella and oral arms. Analyses were conducted to determine the chemical composition and antioxidant activity in the dry-salted S. meleagris anatomical regions and flour, along with the evaluation of the physicochemical and antioxidant properties of S. meleagris gelatin.
2. Materials and Methods
2.1. Raw Material
Dry-salted umbrella and oral arms from brown cannonball jellyfish (Stomolophus meleagris) were obtained from Pesquera Asia, Rocky Point, Sonora, Mexico. The samples were transported to the laboratory in sealed cuvettes. Approximately 40 salted umbrellas and 40 oral arms were rinsed and soaked in tap water for seven days at 4 °C, with water refills every 48 h to remove the salt and rehydration.
2.2. Preliminary Characterization of Dry-Salted Umbrella and Oral Arms
Dry-salted jellyfish umbrella and oral arms were ground using a Hamilton Beach coffee grinder, and then analyzed for moisture, protein, total lipid, and ash using AOAC methods #925.45, #2001.1, #920.39, and #942.05, respectively [26]. The in vitro antioxidant activity was evaluated using three spectrophotometric assays: the quenching of DPPH• radical (1,1-Diphenyl-2-picrylhydrazyl) method [27], the quenching of ABTS·+ 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical [28], and ferric reducing/antioxidant power (FRAP) [29].
2.3. Preparation of Rehydrated Umbrella Flour and Oral Arms Flour
Rehydrated umbrella and oral arms were dried at 60–70 °C in a tunnel dryer for 7 h. Dried samples were ground using a Hamilton Beach coffee grinder and later with a Wiley 1 mm mesh grinder. Finally, flours were passed through a 30 cm sieve and packed in high-density polyethylene bags for later use during the creation of fried snacks.
2.4. Preparation of Cornmeal Snacks and Analysis
Umbrella flour (UMF) and oral arm flour (OAF) were used in the creation of snacks. Commercial cornmeal (CM) was used as a base flour, and the treatments were: (T1) 100% CM (control); (T2) 80% CM–20% UMF; (T3) 50% CM–50% UMF; (T4) 80% CM–20% OAF; and (T5) 50% CM–50% OAF.
Different ratios of the flours were mixed, and water was added until an appropriate consistency was reached for the doughs to be shaped. Once the doughs were ready, a tortilla was shaped, cut into 4 triangular sections, and fried in corn oil at 160 °C for 5–6 min. Tortilla chips were cooled down at room temperature (25 °C) and vacuum-packed for later analysis.
2.4.1. Chemical Analysis of Flour and Snacks
Moisture, protein, lipids, and ash of the cornmeal, jellyfish flour, and fried snacks were determined using the AOAC methods [26]. The carbohydrate-free nitrogen content of fried snacks was calculated, along with the differences in 100 g of all the analyzed components, using the method #986.25 [26].
2.4.2. Antioxidant Activity of Flour and Snacks
The antioxidant activities of cornmeal, jellyfish flour, and cornmeal fried snacks were evaluated using the DPPH•, ABTS·+, and FRAP assays.
The DPPH assay was carried out according to the method described previously [27]. Samples of 1 mg were dissolved in 1 mL of methanol. Afterward, an aliquot of 20 μL of each extract was combined with 200 μL of DPPH solution (0.004% w/v). The samples were left in the dark for 30 min at 25 °C, and their absorbance was read at 517 nm using a microplate reader (Thermo Fisher Scientific Inc. Multiskan GO, NY, USA). The results were expressed as the scavenging activity percentage of Abs517 (RSA%).
The ABTS radical assay was performed using the method based on the ABTS·+ radical discoloration due to interactions with hydrogen and electron donor species [28]. For this purpose, 19.3 mg of ABTS·+ was dissolved in 5 mL of water. The solution was kept at 25 °C for 16 h in the dark before use; then, 20 μL of the samples (0.75 mg/mL) was added to 270 μL of the cationic radical solution diluted with ethanol, and after 30 min, the absorbance was read at 734 nm. Water was used as a control. A Trolox curve was made, and the results were expressed as μM TE/g ds.
The FRAP assay using the method based on the reduction of a complex formed by 2,4,6-tripyridyl-s-triazine (TPTZ) and ferric iron (Fe+3), a colorless complex, as opposed to Fe+2, exhibited a greenish-blue color when antioxidants were present in an acid medium [29]. For this purpose, 20 μL of the sample was mixed with 280 μL of a FRAP solution (acetic acid–sodium acetate (pH 3.4), TPTZ and FeCl3, 10:1:1) and placed in a microplate reader. After 30 min, the absorbance was read at 638 nm. The results were expressed as μM TE/g ds.
2.4.3. Sensory Analysis of Snacks
To evaluate the consumer acceptance, sensory analysis was carried out by employing quantitative descriptive analysis [30] and following the methods described in the Codex Alimentarius (CXS 222-2001) [31]. Twenty-five consumers, students and professors at the University of Sonora (14 females and 11 males aged between 20 and 50 years old), evaluated the five created types of snacks. The session of sensory analysis was about 45 min in duration. Before the sensory analysis, each consumer was informed about the sensory attributes in this study: odor (1 = dislike extremely, 5 = like extremely), color (1 = dark, 5 = yellow), crunchy (1 = very soft, 5 = very crunchy), and flavor (1 = dislike extremely, 5 = like extremely). All samples were served simultaneously to each consumer and coded with a three-digit number. The different treatments were served on a white plate under normal white light simultaneously to carry out the evaluation. Each panelist received a total of five samples plus an evaluation sheet and a glass of water for mouth washing. Samples receiving a rate below 2.5 were considered as unacceptable.
2.5. Preparation and Analysis of Gelatin Powders
The process for producing gelatin powder was the same as a previously described method but with some modifications [6]. Rehydrated umbrella and oral arms were chopped, soaked in HCl 0.5 M in the ratio 1:15 (m/v), and homogenized with UltraTurrax equipment (IKA-UltraTurrax T–25, Germany) at 500 rpm for 15 min. Then, the mixtures were stirred continuously at 4 °C for 24 h using a stirring plate (Thermo Scientific, SP88857100, Marietta, OH, USA). After that, the samples were filtered using a double layer cheesecloth. The pellet samples were sequentially treated with tap water until a neutral pH was achieved and heated at 60 °C for 12 h, with constant stirring, in a water bath (Model 2870 Thermo Scientific, Marietta, OH, USA). The gelatin solutions were cooled down at 4 °C for 12 h and then freeze-dried. The extraction yield was calculated gravimetrically, using the weight of the dry-salted jellyfish sample as a reference.
2.5.1. Chemical Analysis
Moisture content, crude protein, content lipid content, and ash content of gelatin powder was determined using the AOAC methods [26]. A factor of 5.55 was used to convert nitrogen values to gelatin protein [32].
The proline and hydroxyproline content of gelatin were analyzed with a reverse-phase high-performance liquid chromatography (HPLC) system (Agilent Technologies Inc., Palo Alto, CA, USA). Proline and hydroxyproline were quantified from standard curves measured with amino acid standards. Prior to the analysis, the samples were hydrolyzed in 6 M HCl containing sodium thioglycolate (1:1 v/v), at 150 °C for 6 h. The hydrolyzed samples were diluted with 0.4 M sodium borate buffer and derivatized at 60 °C for 5 min with 9-fluromethyl chloroformate. Amino acid separation was performed with a C18 column (4.6 mm ID × 100 mm; Agilent Technologies, Inc., Palo Alto, CA, USA). Chromatograms were analyzed using ChemStation software [33]. The results were expressed as mg/100 g protein.
2.5.2. Electrophoretic Profile (SDS-PAGE)
The protein pattern of jellyfish gelatin was established by using sodium dodecyl sulphate (SDS)-polyacrylamide (PAGE) gels with 8% and 4% concentration, respectively, and separation systems were prepared. In total, 20 µL of the sample was injected. The gels were stained with Coomassie R–250 blue and destained with methanol: water (1:1, v/v) solution [34].
2.5.3. Nuclear Magnetic Resonance of Proton (1H-NMR)
1H–NMR spectra were obtained with a nuclear magnetic spectrometer Bruker Advance 400 (Billerica, MA, EE. UU.) at 400 MHz. The dried gelatin samples (1 mg) were dissolved in 0.5 mL of deuterated KaOH 1% (v/v) in 40% deuterated water. Dimethylsilapentane-5-sulfonic acid (DSS) was used as a reference. The spectral frame was 20 ppm and we carried out the readings at 24 ± 1 °C [35].
2.5.4. Viscosity
Gelatin solutions (6.7% w/v) were prepared in distilled water following heating to 60 °C [36]. The viscosity (cP) of 200 mL of gelatin solution was evaluated in a rheometer (Anton Paar MCR 102) [37]. The gradient in velocity was a fixed constant at 100/s. The viscosity was registered as the average of 30 data points over 240 s [37].
2.5.5. Foaming Properties
The foaming capacity (FC) and foaming stability (FS) of umbrella and oral arm gelatins were determined following a method described elsewhere [38], and 50 mL of gelatin solution (1%) was mixed for 2 min using UltraTurrax equipment. The solution was carefully transferred to a graduated cylinder. FC (Equation (1)) was calculated by measuring the foam volume within 10 s after the foam was set in the cylinder (zero time). FS (Equation (2)) was calculated as the percentage of foam remaining after 60 min at 25 °C.
where Va is the volume after homogenization, Vb is the volume before homogenization, V60 is the volume after 60 min, and V0 is the volume at time zero.
2.5.6. Antioxidant Properties
The antioxidant activity of the gelatin was determined by the DPPH and ABTS methods described previously. The DPPH and ABTS results were expressed in terms of the sample concentration required to inhibit 50% radical DPPH or ABTS (IC50). The IC50 value was determined using a standard curve with the % inhibition against different gelatin concentrations.
2.6. Statistical Analyses
The design of the experiments applied in the present work was wholly randomized, and each of the trials was repeated at least three times. The data obtained were analyzed through a one-way analysis of variance. A comparison of means was carried out using the Tukey–Kramer method. The non-parametric Kruskal–Wallis trial was used during the sensory analysis. A significance level of p < 0.05 was used for every case. All the data were analyzed using the INFOSTAT statistical software. Descriptive statistics was applied for the electrophoretic analysis and RMN data.
3. Results and Discussion
3.1. Preliminary Characterization of Jellyfish Umbrella and Oral Arms
Knowledge of the original composition of any sample is considered critical in the development of any particular food, especially one with functional attributes [11]. As expected, dry-salted umbrella and oral arms had a high content of ash (Table 1). The ash value was significantly higher (p < 0.05) in the umbrella compared with oral arms. The umbrella of jellyfish is responsible for the floating capacity of the organisms; hence, its high content of ash can be due to more minerals present in this anatomical region, which are remnants of the buffering activities of the organism to maintain osmotic balance [5]. Although soft-bodied invertebrates commonly contain a high content of water, the moisture of the jellyfish was very low due to the salt-drying process applied [39]. Similar findings were reported for the same jellyfish species [11]. Remarkably, it was found that jellyfish oral arms have approximately 22% more protein content than the umbrella region. The oral arms of jellyfish are mainly responsible for the movements of the organism, resulting in relative higher density of muscle cells and proteins [5]. Comparable results for other edible jellyfish species have been reported [5].

Table 1.
Chemical composition (%) and antioxidant activity measured in terms of DPPH (%), ABTS-radical scavenging activity (μM TE/g dm) and FRAP activities (μM TE/g dm) of dry-salted jellyfish umbrella (SUM), oral arms (SOA) and umbrella (UMF), oral arm (OAF) flour and cornmeal (CM) 1.
The antioxidant results (Table 1) suggested that both umbrella and oral arms possess free-radical-scavenging capacity and reducing power. The highest free-radical-scavenging capacity, measured by DPPH and ABTS assays, was found in oral arms. No differences (p > 0.05) were observed between FRAP capacity of umbrella and oral arms. The antioxidant activity of jellyfish [2,3,4,7,8,9,10,12] was linked to the capacity of marine organisms to produce many chemical compounds with different functional and biological properties. This capacity may be attributed to the fact that they are exposed to various environmental changes and have developed multiple mechanisms to adapt and defend themselves [10].
3.2. Snacks
3.2.1. Chemical Analysis of Flours and Snacks
The content of moisture, protein, and lipids of the jellyfish umbrella and oral arms flours did not differ (p > 0.05) from dry-salted jellyfish (Table 1). As previously noted, oral arm flour had the lowest ash and highest protein content. When comparing jellyfish flours with cornmeal, those from jellyfish flours showed the highest (p < 0.05) moisture, protein, and ash content, but lower lipid concentration (Table 2). The inclusion of umbrella and oral arm flours in cornmeal snacks resulted in a slightly lower effect on the moisture content, with values ranging from 4.36 to 4.69 (Table 2). This was probably due to the lower initial moisture content in the jellyfish flours, as well as its lower hydration properties, hence retaining less moisture after frying. However, there was a significant increase in the levels of protein and ash, and a significant decrease in the lipid and carbohydrate-free nitrogen content (p < 0.05) regarding the concentration of jellyfish increment. The protein content increased because jellyfish flour had about 18 to 22% protein compared with cornmeal being 12%. The high ash content in jellyfish snack samples is due to the high amount of salt, which might be due to the low carbohydrate levels in the snacks. Higher protein and ash and lower carbohydrate levels were also found with red fish meat in fried snacks [40].

Table 2.
Chemical composition 1 of corn snacks: 100% CM (T1), 80% CM–20% UMF (T2), 50% CM–50% UMF (T3), 80% CM–20% OAF (T4), and 50% CM–50% UMF (T5) 2.
3.2.2. In Vitro Antioxidant Activity of Flour and Snacks
The antioxidant activity was measured by DPPH, ABTS, and FRAP assays to evaluate the potential antioxidant power of jellyfish flour and snacks. Overall, antioxidant activity was detectable in jellyfish flour (Table 1) and snacks (Table 3). The DPPH, ABTS, and FRAP values of the jellyfish umbrella and oral arm flours did not significantly differ (p > 0.05) from dry-salted jellyfish (Table 1). The highest DPPH and ABTS radical scavenging ability was found in oral arm flour and its related snack products. There were no differences among the flours in terms of FRAP values, and the inclusion of jellyfish in snacks did not affect the snack’s capability to reduce ferric ions (Table 3). Although jellyfish showed a similar capacity to reduce ferric ions to cornmeal flour (since there are several antioxidant mechanisms), the obtained results suggest that dry-salted oral arm flour has a better capacity than umbrella flour to increase the antioxidant activity of the snacks. This behaviour could be attributed to the high protein observed in oral arms [5,10].

Table 3.
Antioxidant activity of corn snacks 1: 100% CM (T1), 80% CM–20% UMF (T2), 50% CM–50% UMF (T3), 80% CM–20% OAF (T4), and 50% CM–50% UMF (T5), as measured in terms of DPPH, ABTS-radical scavenging activity, and FRAP-activities 2.
3.2.3. Sensory Analysis of Snacks
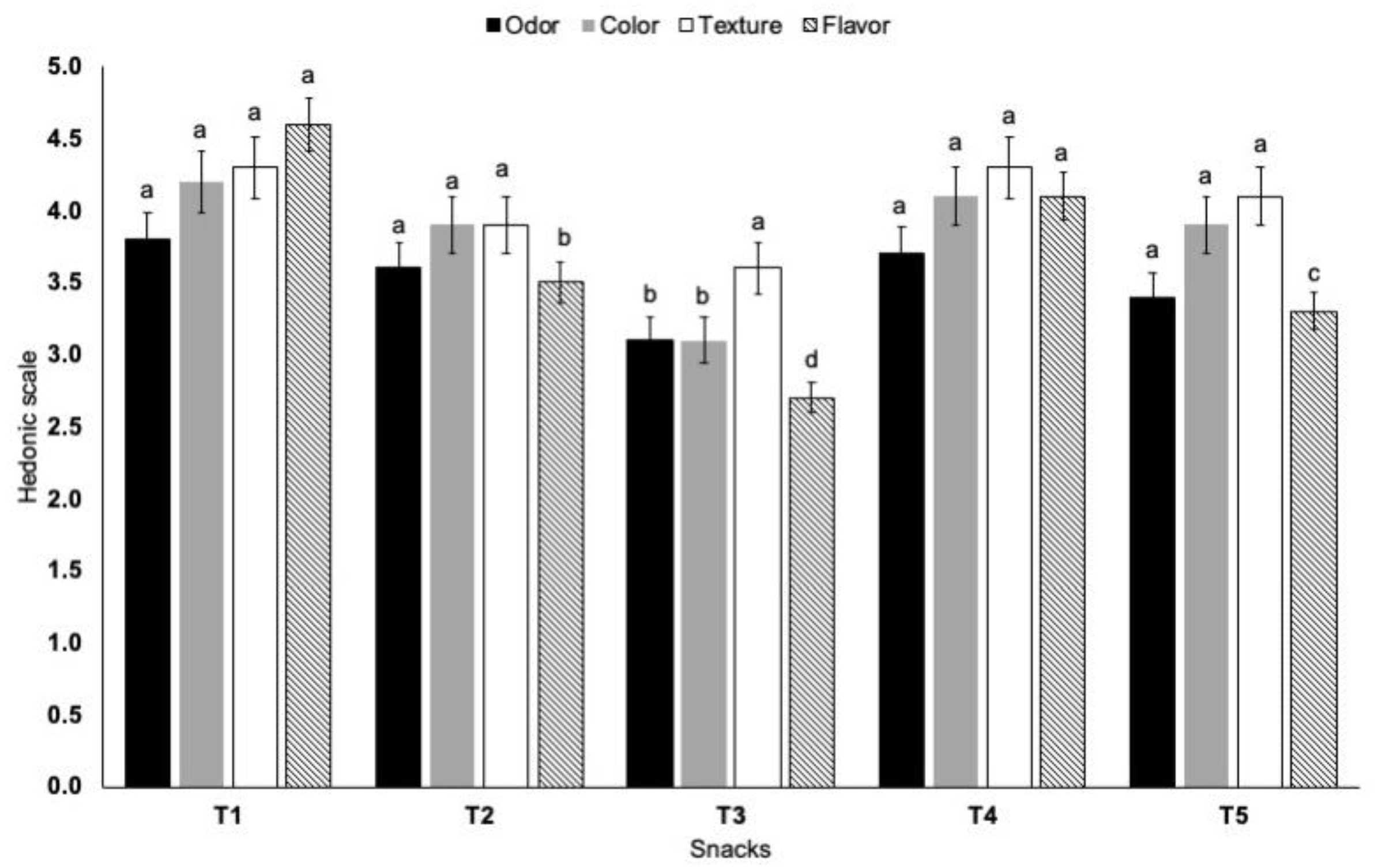
The objective of this section was to establish if the addition of umbrella flour or oral arms flour would affect the attributes of odor, color, texture, and flavor of corn snacks (Figure 1). Concerning odor, the lowest score was given to corn snacks prepared with 50% jellyfish flour, while a high rating was given to corn snacks and samples with 20% jellyfish oral arm flour. The dominance of odor in 50% jellyfish snacks was due to the characteristic jellyfish smell compared with cornmeal. The yellow color score of snacks was high in 100% corn and 20% jellyfish snacks, while further addition of 50% jellyfish umbrella decreased the color ratings. It is noteworthy that the incorporation of jellyfish flour did not affect the crunchiness attribute of the corn snacks. For the evaluation of flavor, the highest score was given to snacks prepared with 100% cornmeal and 20% oral arm flour. Although more studies are required, fried snacks with 20 g oral arm flour per 100 g may be formulated on a commercial scale.
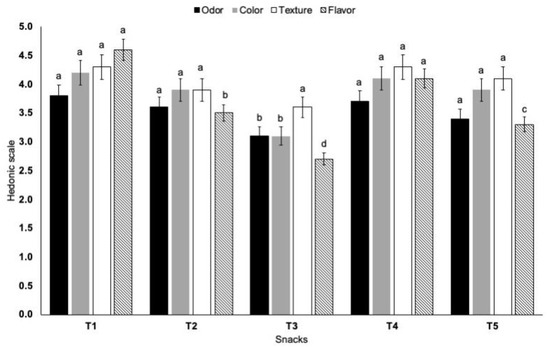
Figure 1.
Sensory evaluation of 100% cornmeal snacks (T1), 80% cornmeal–20% umbrella snacks (T2), 50% cornmeal–50% umbrella snacks (T3), 80% cornmeal–20% oral arms snacks (T4), and 50% cornmeal–50% oral arms snacks (T5). Different letters among snacks for each sensory attribute are statistically different (p < 0.05).
3.3. Gelatin
3.3.1. Yield
To obtain gelatin, collagen is subjected to a thermal process, using methods that break the inter- and intramolecular collagen crosslinks, avoiding peptide cleavage, for the polypeptide chain to remain intact [41]. In this study, chemical hydrolysis was performed, resulting in an acid-solubilized gelatin solution followed by a thermal treatment at 60 °C. The higher extraction yield was obtained in jellyfish oral arms (Table 4). One of the factors that may have influenced the gelatin yield extraction is the protein content in the tissue analyzed. The lower yield in umbrella can be due to the low protein content in this anatomical region.

Table 4.
Yield and chemical composition of umbrella and oral arm gelatins from dry-salted brown cannonball jellyfish 1.
Yield values were lower for those reported for jellyfish Lobonema smithii (26.4%) obtained under similar conditions [6]. The lower degree of conversion of collagen to gelatin detected in this work was likely due to the loss of extracted collagen through leaching during the washing process [41] and properties of the jellyfish species that were employed [22].
3.3.2. Chemical Composition
The chemical composition of the jellyfish umbrella gelatin and oral arm gelatin indicated significant differences (p < 0.05) among samples in protein and ash (Table 3). The protein content in both gelatins were comparable to those of gelatin extracted from jellyfish Rhopilema hispidum (75.3%) [23] and higher in the same jellyfish species (29.54%) [11], indicating the efficiency of the used extraction method.
The moisture values of both gelatins were similar to the ones reported for Rhopilema hispidum jellyfish gelatin (12.2%) [23] but higher than those reported for the same jellyfish species (4.8%) [11]. The lower ash found in the umbrella and oral arm gelatins compared to the same jellyfish species (56.6%) [11] could explain the higher moisture level of our gelatin. However, according to US standards of food [42], the maximum ash content of gelatin is 3%. Thus, the obtained gelatin from dry-salted jellyfish contained higher ash than the regulation level. Therefore, future studies should establish better methods to eliminate the salt.
The analysis of the total imino acids (Proline, Pro + hydroxyproline, Hyp) revealed that gelatin from oral arms had a higher (p < 0.05) Hyp content than umbrella (Table 3). Unlike Hyp content, no difference (p > 0.05) was observed between the Pro content of the umbrella and oral arms. It is known that glycine, proline and hydroxyproline are the most abundant amino acids found in gelatin, but their values depend on the source, as imino acids participated in hydrogen bond formation [43]. The total amounts of imino acids (proline and hydroxyproline) of both anatomical regions are comparable to the umbrella of desalted jellyfish (Lobonema smithii) gelatin (30 mg/100 g protein) and the content of oral arms was 40 mg/100 g protein [44]. Under the conditions of this study, this result indicated that the collagen tissue derived from oral arms possessed a higher degree of crosslinks than umbrella. This was confirmed by SDS-PAGE.
3.3.3. Electrophoretic Profile
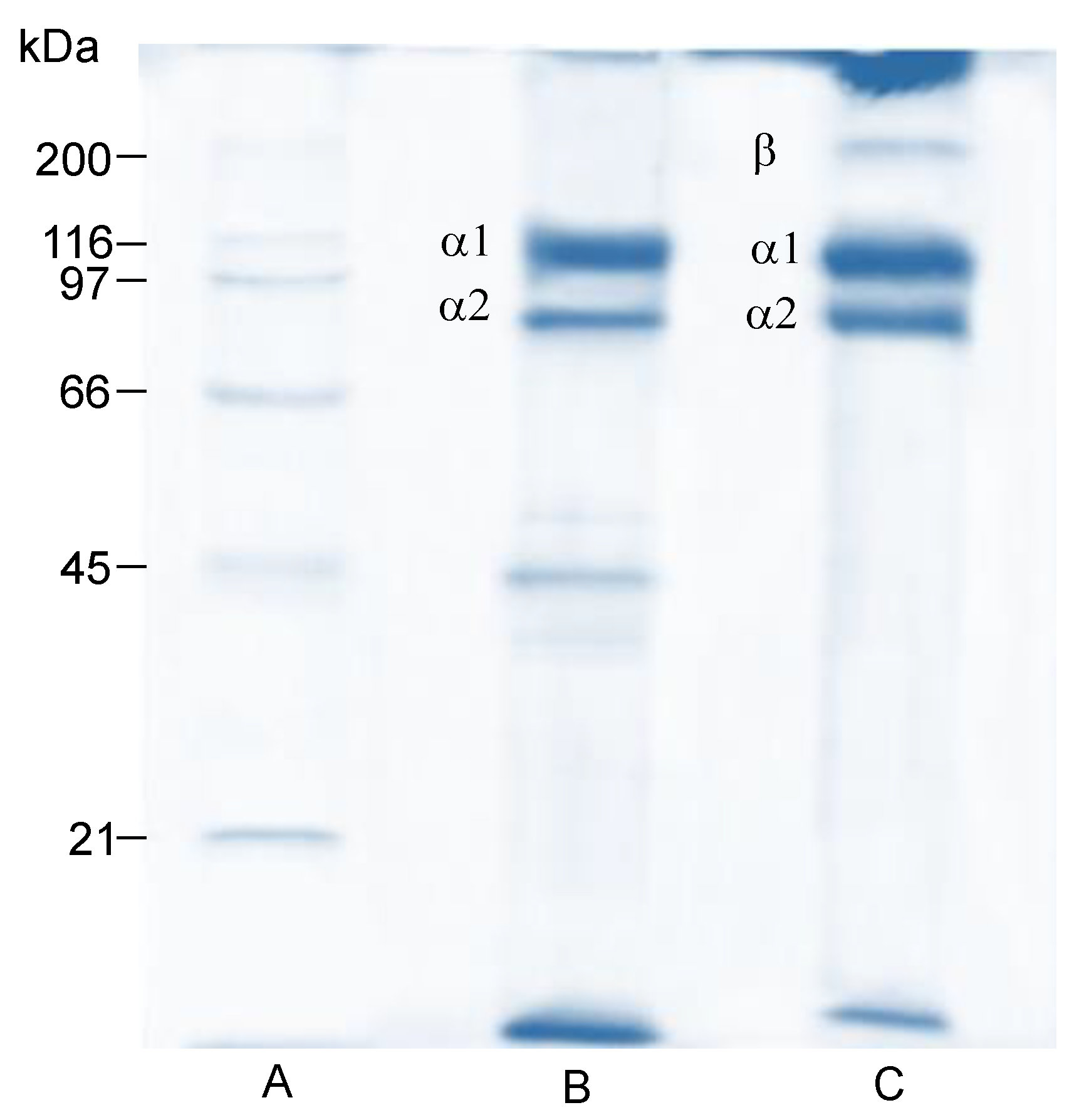
The electrophoretic pattern of the obtained jellyfish umbrella gelatin exhibited two different bands in a continuous position, with a molecular weight of approximately 116 kDa and 97 kDa (Figure 2, Lane B). This suggests that the umbrella gelatin molecules comprise at least two different α collagen-chains, α1 and α2 [45]. Meanwhile, the electrophoretic pattern of oral arm gelatin comprises the α chains and the crosslinked component β (band about 190 kDa) (Figure 2, Lane C); this component is considered a dimer of α-chains [46]. Moreover, bands with a lower molecular weight of the α2 chain were detected in umbrella gelatin, which might affect its functional gelatin properties [47].
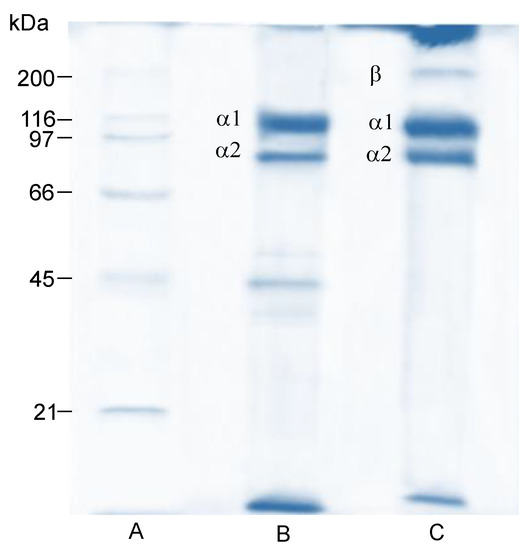
Figure 2.
SDS-polyacrylamide protein pattern of jellyfish (Stomolophus meleagris) gelatin. Lane A: molecular weight marker; Lane B: umbrella; and Lane C: oral arms.
3.3.4. Nuclear Magnetic Resonance of Proton (1H-NMR)
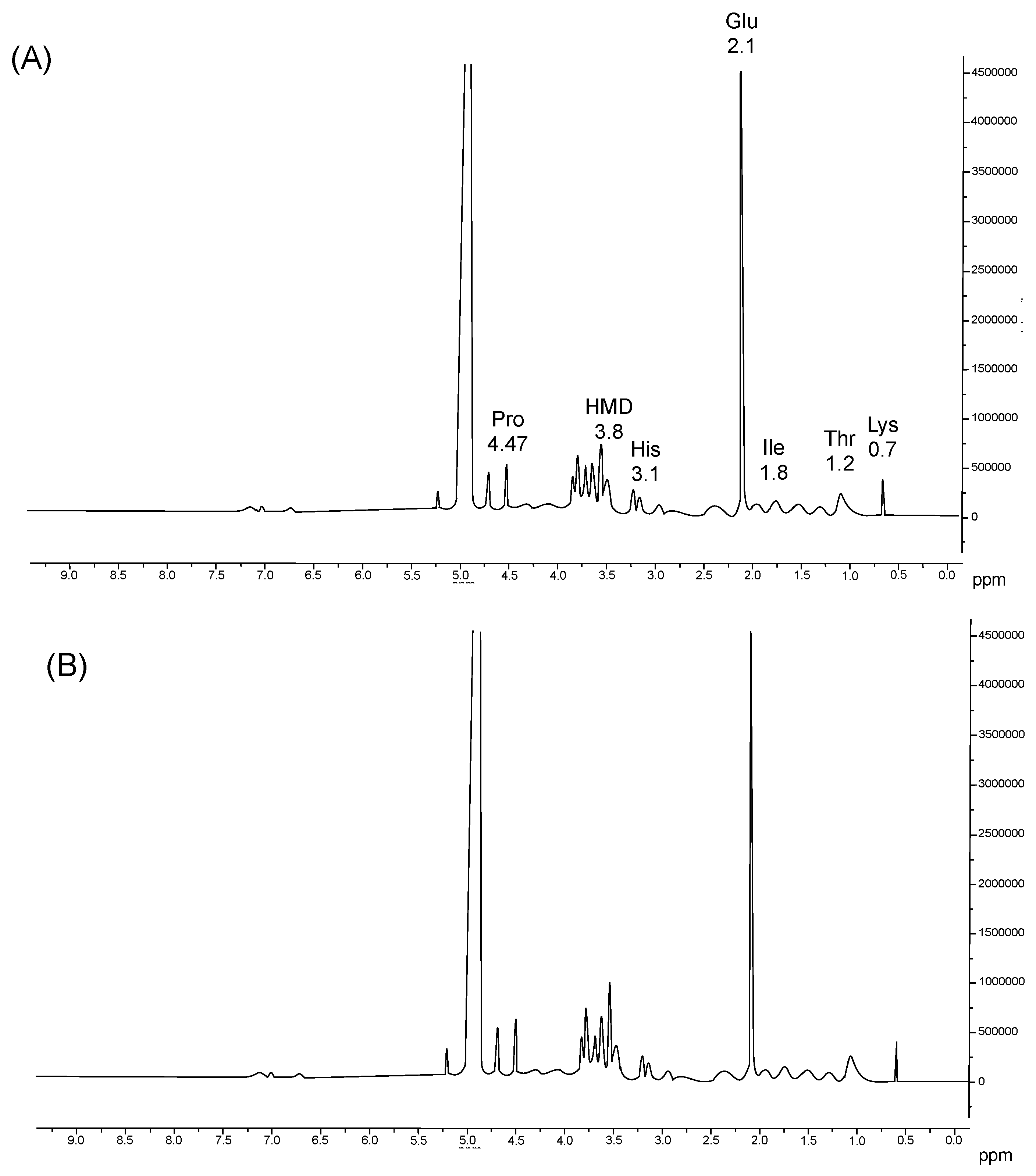
The 1H-NMR analysis of umbrella and oral arm gelatin (Figure 3) indicated the presence of water molecules (4.8 ppm), proline (4.47 ppm), histidine (3.1), imino amino acid groups of hydroxymerodesmosine (HMD) (3.8 ppm), glutamate (2.1 ppm), isoleucine (1.8 ppm), threonine (1.2 ppm), and lysine (0.7 ppm) [46]. Proton alpha-Pro and protons present in HMD chemical displacements were similar in both samples. However, the intensity of this peak varied. More intense peaks were observed in gelatin from oral arms than in umbrellas. These observations are consistent with the above-discussed Pro and Hyp content. In addition, the NMR results further help with the partial characterization of the proteins present in the jellyfish samples.
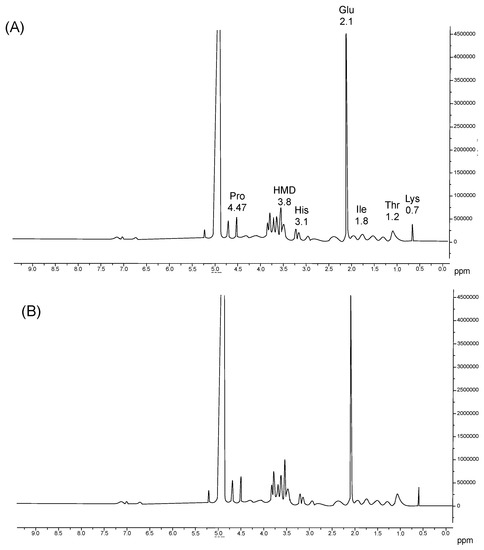
Figure 3.
1H-NMR spectra of jellyfish umbrella (A) and oral arm (B) gelatins. Amino acids are indicated by their corresponding peaks: Lysine (Lys), threonine (Thr), isoleucine (Ile), glutamate (Glu), proline (Pro), histidine (His) and imino amino acid group of hydroxymerodesmosine (HMD).
3.3.5. Viscosity
The viscosity of the obtained gelatin solution (10%) from the cold maturation process varied significantly (p < 0.05) among the evaluated jellyfish anatomical regions. The greatest value obtained from oral arm gelatins (Table 5) could be due to differences in the proportion of hydroxyproline and the proline content in each region. Moreover, the low viscosity detected in the umbrella gelatin could be associated more with the bands with a lower molecular weight than the α2 observed in this gelatin [45]. Although only the value from the oral arm gelatin is comparable to those observed for jellyfish Lobonema smithii (7.7 cP) [25], the viscosity of both anatomical regions could be considered acceptable due to the results agreeing with commercial gelatin [37].

Table 5.
Viscosity (VIS), foaming capacity (FC), and antioxidant activity as measured in terms of DPPH and ABTS-radical scavenging activity, of umbrella and oral arms gelatins from dry-salted brown cannonball jellyfish 1.
3.3.6. Foaming Properties
Foam analysis determines the ability of protein molecules to penetrate the air–water interface and to form a strong, flexible, and cohesive film, inhibiting the bubble coalescence [48]. Oral arm gelatin demonstrates better foam capacity than umbrella gelatin (Table 5); both gelatins were maintained at 100% foam formation for 60 min after the gelatin solution was homogenized. Moreover, the foam formed with jellyfish gelatin had excellent stability, and oral arm gelatin showed a similar FC to that reported for giant grouper skin gelatin (81.6%) [47], but lower than the umbrella gelatin of dried desalted jellyfish Lobonema smithii gelatin (250%) [6]. The lower FC detected in the umbrella gelatin suggests that the obtained gelatin might contain a lower ratio of nonpolar/polar side chains [49].
3.3.7. Antioxidant Properties
The ability to trap DPPH and ABTS radicals of both jellyfish umbrella gelatin and oral arm gelatin (Table 5) confirms previous reports on the antioxidant activity of jellyfish [3,4,7,8,10,12]. The highest antioxidant activity produced was with oral arm gelatin (Table 4). This result agreed with previous work, where the umbrella showed lower antioxidant activity than oral arms separated from Aurelia coerulea, Cotylorhiza tuberculate, and Rhizostoma pulmo [10]. Finally, the antioxidant activity of the obtained gelatin was considered effective since their IC50 value (Table 5) was similar to those for some fractions from white jellyfish (Lobonema smithii) protein hydrolysates [12]. The antioxidant activity of jellyfish collagen is linked to its high content of certain amino acids, such as glycine, proline, glutamic, aspartic, valine and arginine [50].
4. Conclusions
This work demonstrated that dry-salted brown cannonball jellyfish (Stomolophus meleagris) have the potential to be used as a source of protein supplement in cornmeal snacks and for the creation of gelatin products, mainly using their oral arms. When 20% or 50% (w/w) oral arms of jellyfish was used, the antioxidant activity of cornmeal snacks increased. The sensory attributes indicated that the samples with 20% oral arms flour were not affected. Moreover, oral arm gelatin showed better viscosity, foaming and antioxidant properties. This information could form a base of knowledge for further investigations to find new applications for dry-salted cannonball jellyfish as a functional ingredient in the food industry.
Author Contributions
Conceptualization, B.d.S.V.-U., L.P.V.-V., S.J.B.-P. and J.M.E.-B.; data curation, B.d.S.V.-U. and C.L.D.T.-S.; formal analysis, B.d.S.V.-U., L.P.V.-V., S.J.B.-P. and J.E.C.-H.; funding acquisition, J.M.E.-B.; investigation, B.d.S.V.-U. and J.M.E.-B.; methodology, C.L.D.T.-S. and J.E.C.-H.; project administration, J.M.E.-B.; resources, J.M.E.-B.; supervision, C.L.D.T.-S., J.E.C.-H. and J.M.E.-B.; writing—original draft preparation, B.d.S.V.-U., L.P.V.-V. and S.J.B.-P.; writing—review and editing, C.L.D.T.-S., J.E.C.-H. and J.M.E.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by a grant from the Aquaculture Institute of the State of Sonora, Mexico (grant CV-IAS-006-2018). Villalba-Urquidy received a scholarship number 862134 from the National Research and Technology Council (CONACyT) via the Mexico Government.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article. Further information is available upon request from the corresponding author.
Acknowledgments
The authors would like to thank Ricardo Iván González-Vega for his technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bleve, G.L.; Ramires, F.A.; Gallo, A.; Leone, A. Identification of safety and quality parameters for preparation of jellyfish based novel food products. Foods 2019, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.F.; Li, Y.-Y.; Xu, J.-J.; Su, X.R.; Gao, X.; Yue, F.-P. Study on effect of jellyfish collagen hydrolysate on anti-fatigue and anti-oxidant. Food Hydrocoll. 2011, 25, 1350–1353. [Google Scholar] [CrossRef]
- Barzideh, Z.; Latiff, A.A.; Gan, C.-Y.; Abedin, M.Z.; Alias, A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.). Food Technol. Biotechnol. 2014, 52, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Lecci, R.M.; Durante, M.; Meli, F.; Piraino, S. The bright side of gelatinous blooms: Nutraceutical value and antioxidant properties of three Mediterranean jellyfish (Scyphozoa). Mar. Drugs 2015, 13, 4654–4681. [Google Scholar] [CrossRef]
- Khong, N.M.H.; Yusoff, F.M.; Jamilah, B.; Basri, M.; Maznah, I.; Chan, K.W.; Nishikawa, J. Nutritional composition and total collagen content of three commercially important edible jellyfish. Food Chem. 2016, 196, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Rodsuwan, U.; Thumthanaruk, B.; Kerdchoechuen, O.; Laohakunjit, N. Functional properties of type A gelatin from jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 507–514. Available online: http://www.ifrj.upm.edu.my/23%20(02)%202016/(8).pdf (accessed on 28 March 2022).
- Prieto, L.; Enrique-Navarro, A.; Volsi, R.L.; Ortega, M.J. The large jellyfish Rhizostoma luteum as sustainable a resource for antioxidant properties, nutraceutical value and biomedical applications. Mar. Drugs 2018, 16, 396. [Google Scholar] [CrossRef]
- Zhang, Q.; Song, C.; Zhao, J.; Shi, X.; Sun, J.; Liu, J.; Fu, Y.; Jin, W.; Zhu, B. Separation and characterization of antioxidative and angiotensin converting enzyme inhibitory peptide from jellyfish gonad hydrolysate. Molecules 2018, 23, 94. [Google Scholar] [CrossRef]
- De Domenico, S.; De Rinaldis, G.; Paulmery, M.; Piraino, S.; Leone, A. Barrel jellyfish (Rhizostoma pulmo) as source of antioxidant peptides. Mar. Drugs 2019, 17, 134. [Google Scholar] [CrossRef]
- Leone, A.; Lecci, R.M.; Milisenda, G.; Piraino, S. Mediterranean jellyfish as novel food: Effects of thermal processing on antioxidant, phenolic, and protein contents. Eur. Food Res. Technol. 2019, 245, 1611–1627. [Google Scholar] [CrossRef]
- Chiarelli, P.G.; Pegg, R.B.; Kumar, D.; Sloval, K.M. Exploring the feasibility of developing novel gelatine powders from salted, dried cannonball jellyfish (Stomolophus meleagris). Food Biosci. 2021, 44, 101397. [Google Scholar] [CrossRef]
- Upata, M.; Siriwoharn, T.; Makkun, S.; Yarnpakdee, S.; Regenstein, J.M.; Wangtueai, S. Tyrosinase inhibitory and antioxidant activity of enzymatic protein hydrolysate from jellyfish (Lobonema smithii). Foods 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed]
- Amaral, L.; Raposo, A.; Morais, Z.; Coimbra, A. Jellyfish ingestion was safe for patients with crustaceans, cephalopods, and fish allergy. Asia Pac. Allergy 2018, 8, e3. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, J.; Álvarez-Tello, J. The jellyfish fishery in Mexico. Agric. Sci. 2013, 4, 57. [Google Scholar] [CrossRef]
- Future of Food. Available online: https://www.about.sainsburys.co.uk/~/media/Files/S/Sainsburys/pdf-downloads/futureoffood-10c.pdf (accessed on 2 September 2022).
- Jozinović, A.; Šubarić, D.; Ačkar, Đ.; Babić, J.; Orkić, V.; Guberac, S.; Miličević, B. Food industry by-products as raw materials in the production of value-added corn snack products. Foods 2021, 10, 946. [Google Scholar] [CrossRef]
- Murphy, M.; Skonberg, D.; Camire, M.; Dougherty, M.; Bayer, R.; Briggs, J. Chemical composition and physical properties of extruded snacks containing crab-processing by-product. J. Sci. Food Agric. 2003, 83, 1163–1167. [Google Scholar] [CrossRef]
- Choudhury, G.; Gautam, A. Hydrolyzed fish muscle as a modifier of rice flour extrudate characteristics. J. Food Sci. 2003, 68, 1713–1721. [Google Scholar] [CrossRef]
- Cortez-Netto, J.; Campagnoli-de Oliveira, P.; Lapa-Guimarães, J.; Villegas, M. Physicochemical and sensory characteristics of snack made with minced Nile tilapia. Food Sci. Technol. 2014, 34, 591–596. [Google Scholar] [CrossRef]
- Shaviklo, A.R. Fish snacks from value addition of low value fish and processing by-products. Infofish Inter. 2012, 5, 42–44. Available online: www.infofish.org (accessed on 30 September 2022).
- Shaviklo, A.R.; Azaribeh, M.; Moradi, Y.; Zangeneh, P. Formula optimization and storage stability of extruded puffed corn-shrimp snacks. LWT-Food Sci. Technol. 2015, 63, 307–314. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatin. Food Hydrocoll. 2009, 23, 563–567. [Google Scholar] [CrossRef]
- Cho, S.; Ahn, J.R.; Koo, J.S.; Kim, S.B. Physicochemical properties of gelatin from jellyfish Rhopilema hispidum. Fish. Aquatic Sci. 2014, 17, 299–304. [Google Scholar] [CrossRef]
- Chancharern, P.; Laohakunjit, N.; Kerdchoechuen, O.; Thumthanaru, B. Extraction of type A and type B gelatin from jellyfish (Lobonema smithii). Int. Food Res. J. 2016, 23, 419–424. Available online: www.ifrj.upm.edu.my/23%20(01)%202016/(63).pdf (accessed on 15 July 2022).
- Lueyot, A.; Rungsardthong, V.; Vatanyoopaisarn, S.; Hutangura, P.; Wonganu, B.; Wongsa-Ngsari, P.; Charoenlappanit, S.; Roytrakul, S.; Thumthanaruk, B. Influence of collagen and some proteins on gel properties of jellyfish gelatin. PLoS ONE 2021, 16, e0253254. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, W. Official Methods of Analysis of AOAC International, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Protoggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Meilgaard, M.C.; Civille, G.V.; Caar, B.T. Sensory Evaluation Techniques, 4th ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007; pp. 264–270. [Google Scholar]
- Codex Alimentarius, Normas Internacionales de los Alimentos. Norma del Codex para galletas de pescado marino y de agua dulce y de mariscos, crustáceos y moluscos. Organización de las Naciones Unidas, Organización Mundial de la Salud, 2001, CXS 222-2001. Available online: https://www.fao.org/fao-who-codexalimentarius.org (accessed on 23 August 2022).
- Sarbon, N.M.; Badii, F.; Howell, N.K. Preparation and characterisation of chicken skin gelatin as an alternative to mammalian gelatin. Food Hydrocoll. 2013, 30, 143–151. [Google Scholar] [CrossRef]
- Vázquez-Ortíz, F.A.; Morón-Fuenmayor, O.E.; González-Méndez, N.F. Hydroxyproline Measurement by HPLC: Improved Method of Total Collagen Determination in Meat Samples. J. Liq. Chromatogr. Relat. 2004, 27, 2771–2780. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Sarabia-Sainz, H.M.; Torres-Arreola, W.; Márquez-Ríos, E.; Santacruz-Ortega, H.C.; Rouzaud-Sández, O.; Valenzuela-Soto, E.M.; Burgara-Estrella, A.J.; Ezquerra-Brauer, J.M. Interrelation of collagen chemical structure and nanostructure with firmness of three body regions of jumbo squid (Dosidicus gigas). Food Biophys. 2017, 12, 491–499. [Google Scholar] [CrossRef]
- Mirzapour-Kouhdasht, A.; Sabzipour, F.; Taghizadeh, M.S.; Moosavi-Nasab, M. Physicochemical, rheological, and molecular characterization of colloidal gelatin produced from common carp by-products using microwave and ultrasound-assisted extraction. J. Texture Stud. 2019, 50, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Uriarte-Montoya, M.H.; Santacruz-Ortega, H.; Cinco-Moroyoqui, F.J.; Rouzaud-Sández, O.; Plascencia-Jatomea, M.; Ezquerra-Brauer, J.M. Giant squid skin gelatin: Chemical composition and biophysical characterization. Food Res. Int. 2011, 44, 3243–3249. [Google Scholar] [CrossRef]
- Coffman, C.W.; Garcia, V.V. Functional properties and amino acid content of protein isolate from mung bean flour. J. Food Technol. 1977, 12, 473–484. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, C.; He, C.; Wang, H. Mechanical properties, anisotropic swelling behaviours and structures of jellyfish mesoglea. J. Mech. Behav. Biomed. Mater. 2012, 6, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, A.; Xiong, Z.; Li, Q.; Xiong, H.; Irshad, S.; Chen, L.; Wang, P.; Zhang, M.; Hina, S.; Regenstein, J.M. Evaluation of physicochemical, textural and sensory quality characteristics of red fish meat-based fried snacks. J. Sci. Food Agric. 2019, 99, 5771–5777. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Mohd Pu’ad, N.A.S.; Lee, T.C.; Nayan, N.H.M.; Sahari, N.; Basri, H.; Idris, M.I.; Abdullah, H.Z. A review of gelatin: Properties, sources, process, applications, and commercialization. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- FAO. Compendium of Food Additive Specifications; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006; ISSN 1817-7077. [Google Scholar]
- Ledward, D.A. Gelation of gelatin. In Functional Properties of Food Macromolecules; Mitchell, J.R., Ledward, D.A., Eds.; Elsevier: London, UK, 1986; pp. 171–201. [Google Scholar]
- Klaiwong, T.; Hutangura, P.; Rutatip, S.; Wongsa-ngasri, P.; Thumthanaruk, B. Comparative properties of pepsin hydrolyzed jellyfish protein from salted jellyfish. J. Agric. Sci. Technol. B 2014, 4, 555–564. [Google Scholar]
- Kimura, S.; Miyauchi, Y.; Uchida, N. Scale and bone type I collagens of carp (Cyprinus carpio). Comp. Biochem. Physiol. B 1991, 99, 473–476. [Google Scholar] [CrossRef]
- Masuda, M.; Karube, S.; Hayashi, Y.; Shindo, H.; Igarashi, M. Direct measurement of collagen crosslinks with automatic amino acid analyzer—Identification of peaks due to crosslinks. FEBS Lett. 1976, 63, 245–249. [Google Scholar] [CrossRef]
- Chen Lin, C.; Kuei Chiou, T.; Chieh Sung, W. Characteristics of gelatin from giant grouper (Epinephelus lanceolatus) skin. Int. J. Food Prop. 2015, 18, 2339–2348. [Google Scholar] [CrossRef]
- Dickinson, E. Properties of emulsion stabilized with milk proteins: Overview of some recent development. J. Dairy Sci. 1997, 80, 2607–2619. [Google Scholar] [CrossRef]
- Johnson, T.M.; Zabik, M.E. Ultrastructural examination of egg albumen protein foams. J. Food Sci. 1981, 46, 1237–1240. [Google Scholar] [CrossRef]
- Zhuang, Y.-L.; Zhoa, X.; Li, B.F. Optimization of antioxidant activity by response surface methodology in hydrolysates of jellyfish (Rhopilema esculentum) umbrella collagen. J. Zhejiang Univ. Sci. B 2009, 10, 572–579. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).