Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
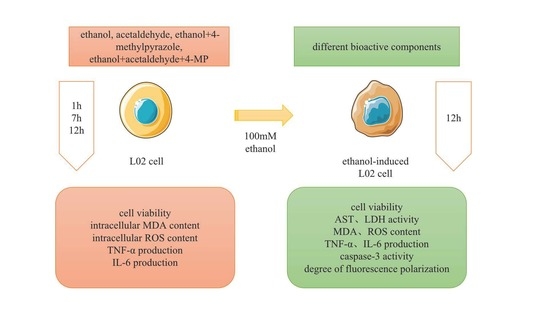
2.2. Cell Culture and Treatment
2.3. Cell Viability Assay
2.4. Status of Intracellular Oxidative Stress
2.5. Activity of AST, ALT, and LDH Levels
2.6. Intracellular Levels of MDA, TNF-α, and IL-6
2.7. Caspase 3 Activity Assay
2.8. Membrane Potential
2.9. Statistical Analysis
3. Results
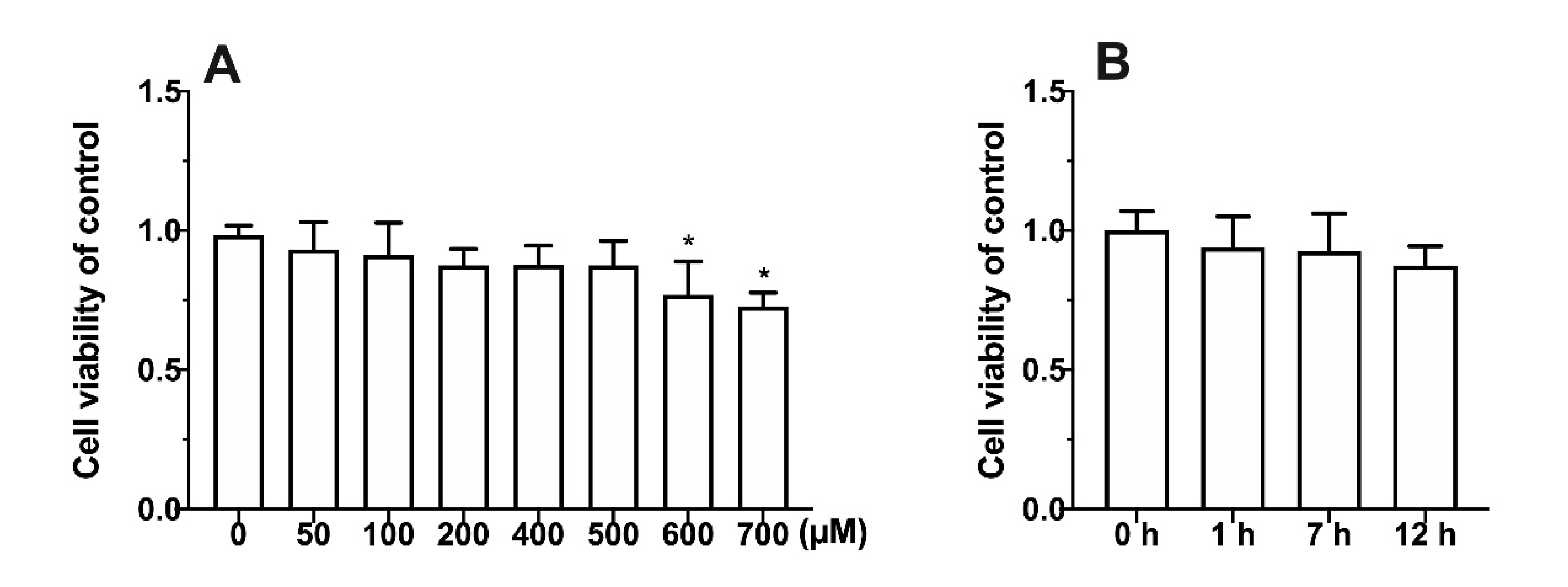
3.1. Effect of Metabolic Inhibitors on Cell Relative Survival Rate
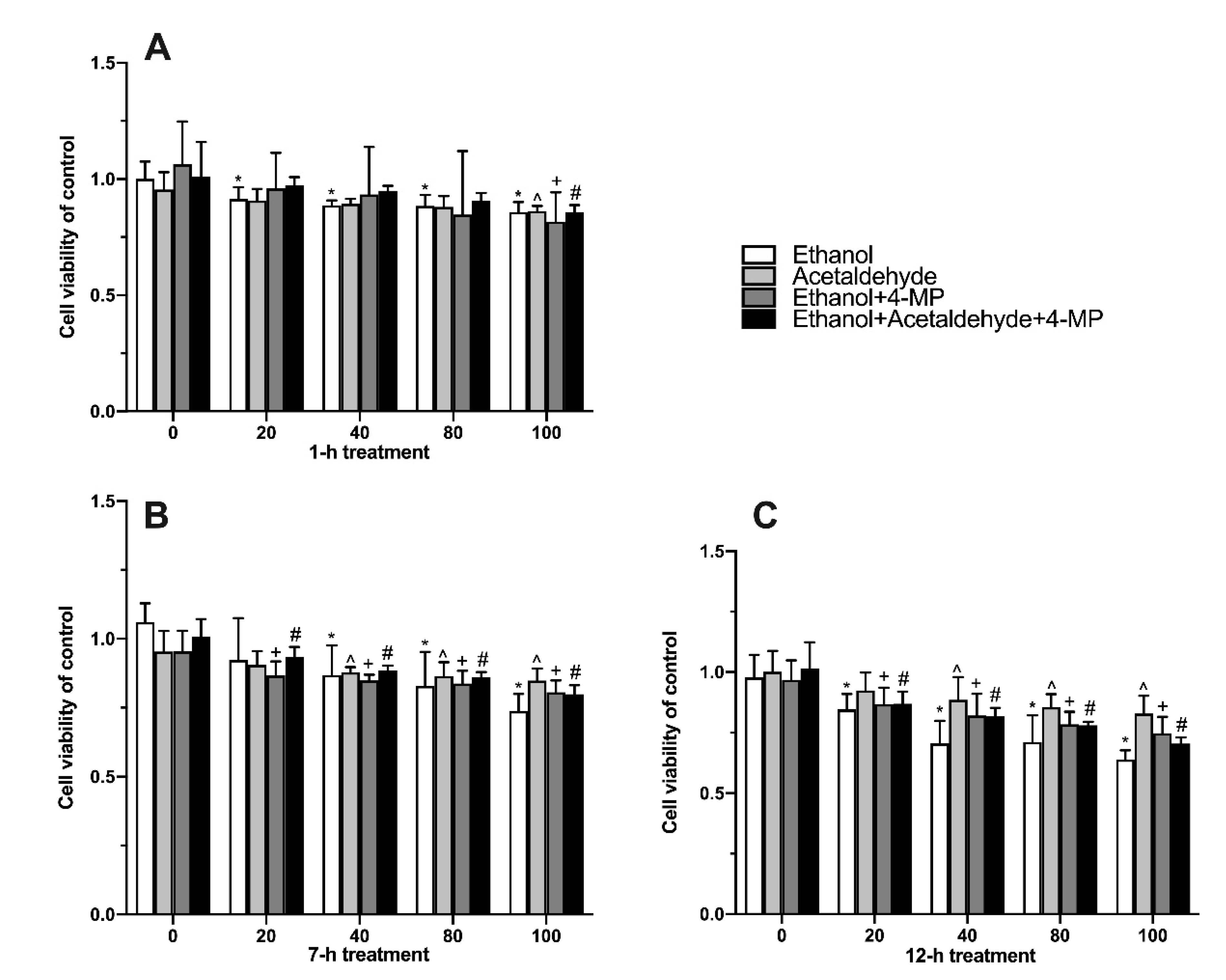
3.2. Effects of Four Different Treatments on Cell Proliferation
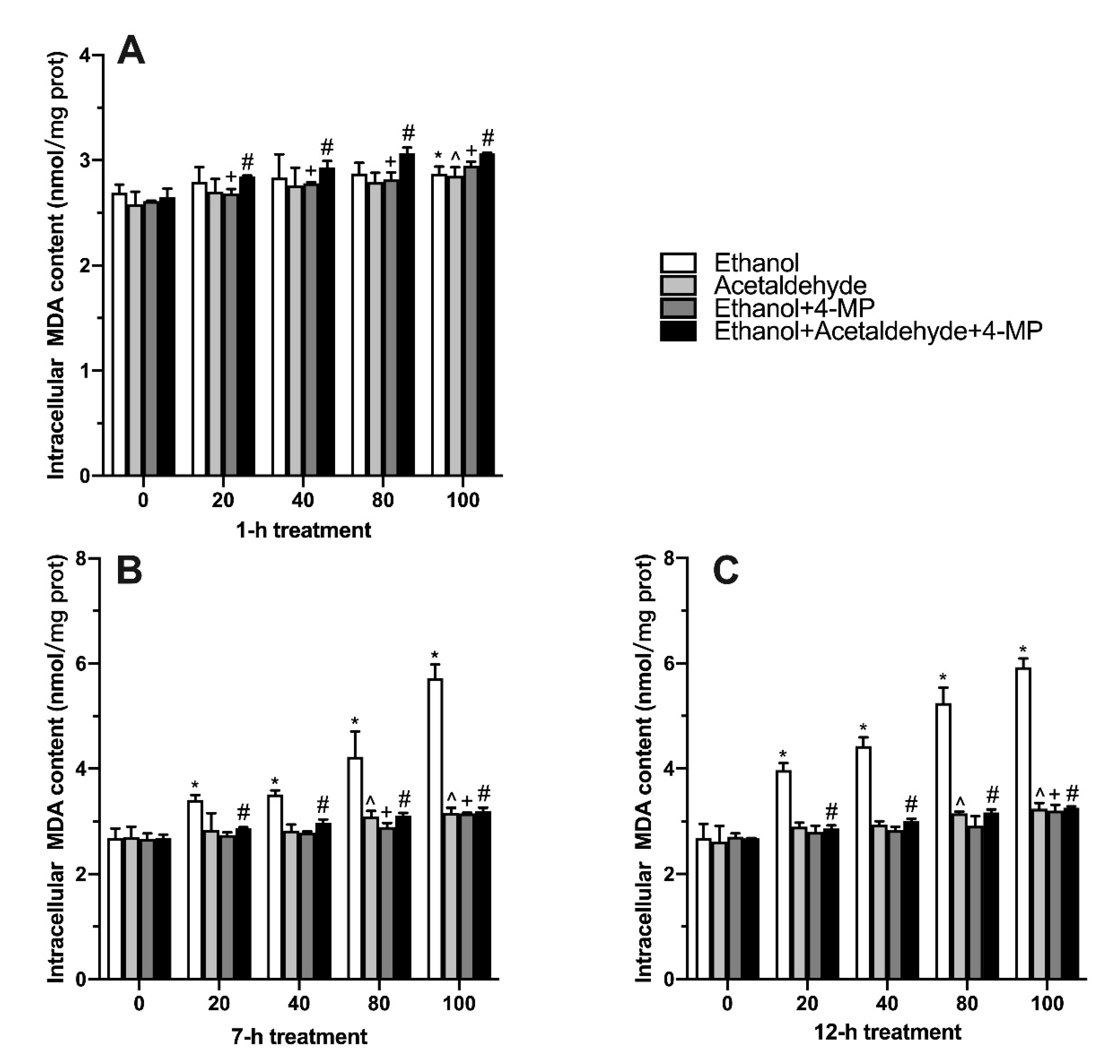
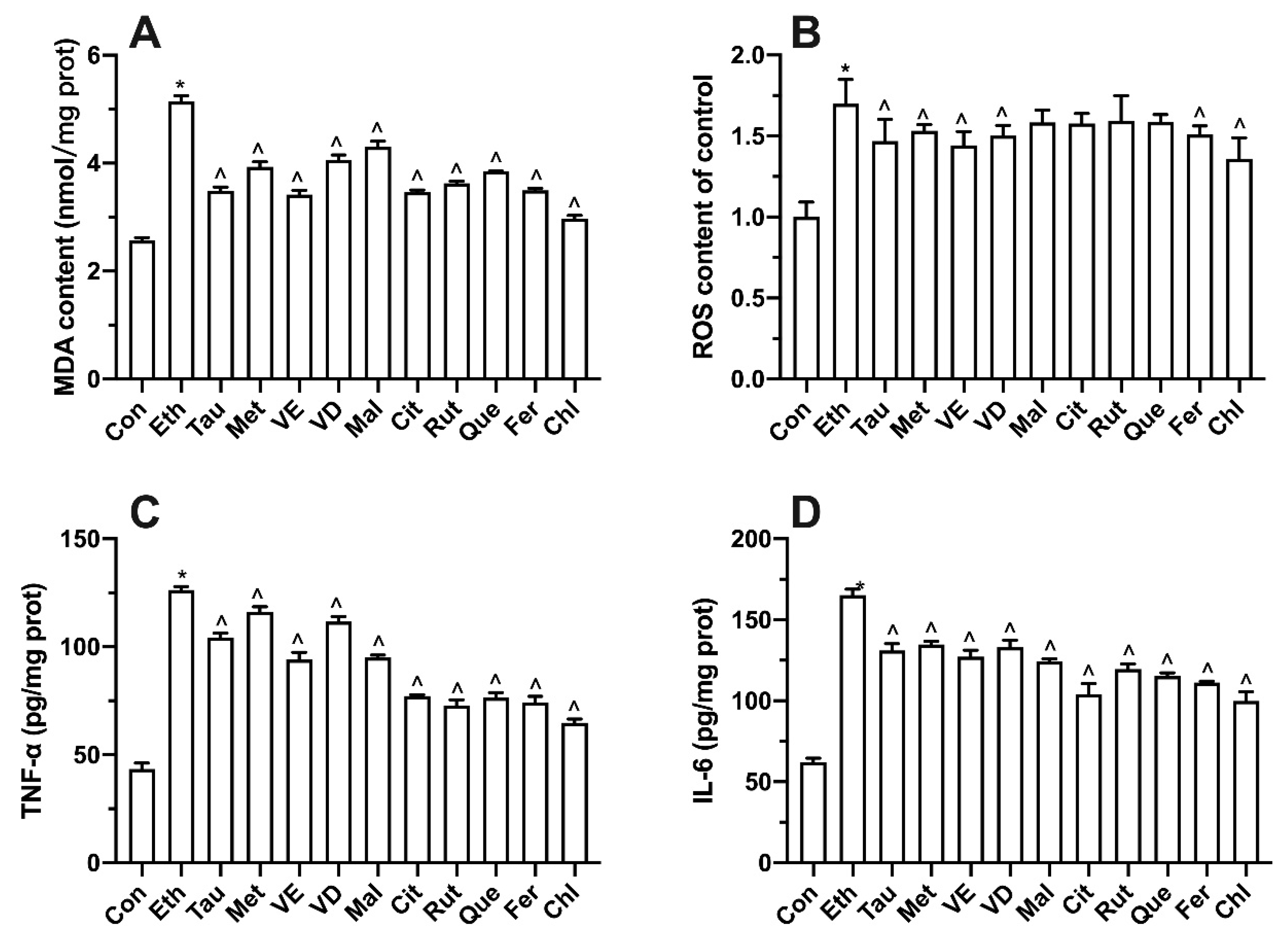
3.3. Effects of Four Different Treatments on Intracellular MDA Production
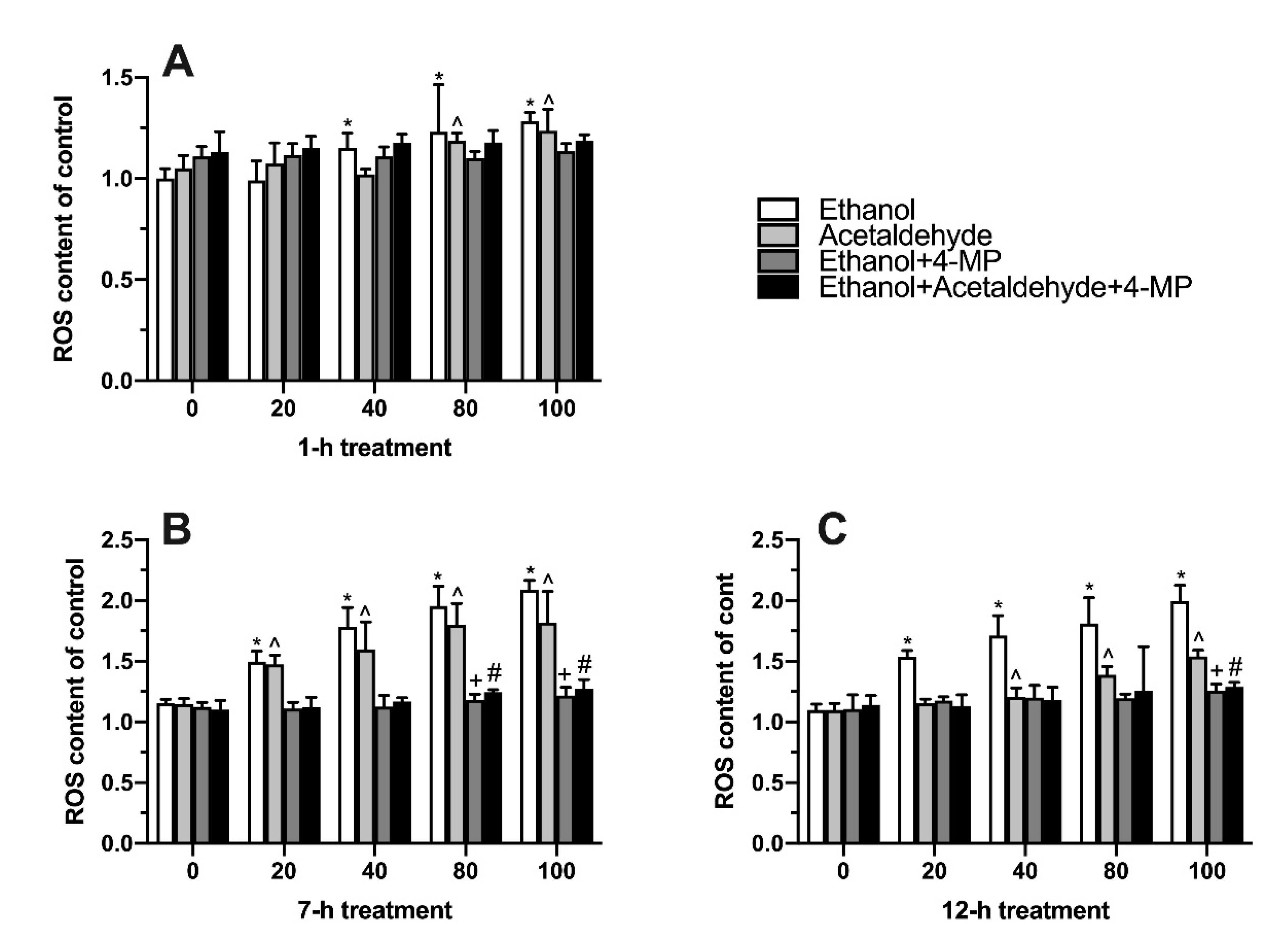
3.4. Effects of Four Different Treatments on Intracellular Oxidative Stress
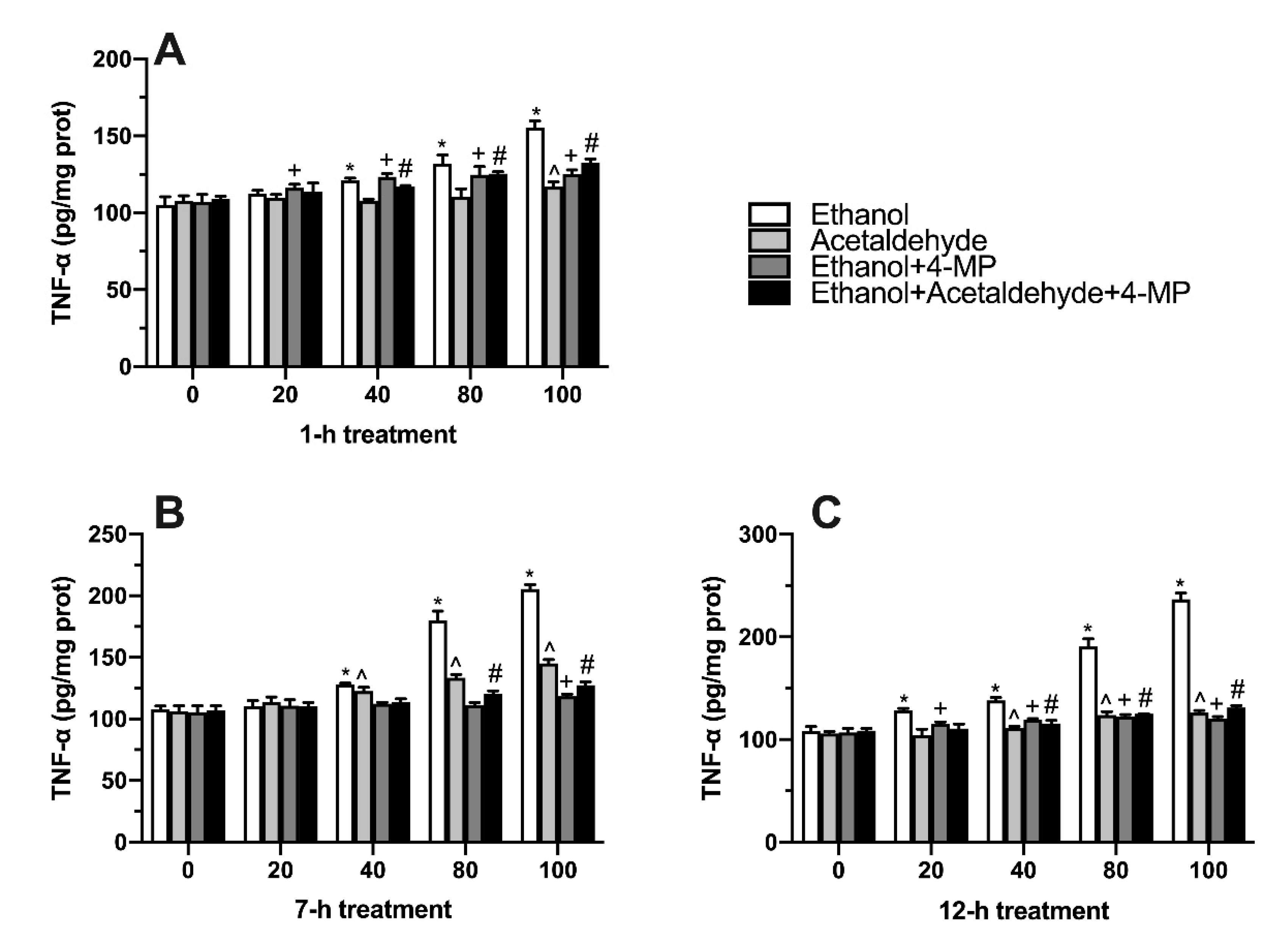
3.5. Effects of Four Different Treatments on TNF-α Production
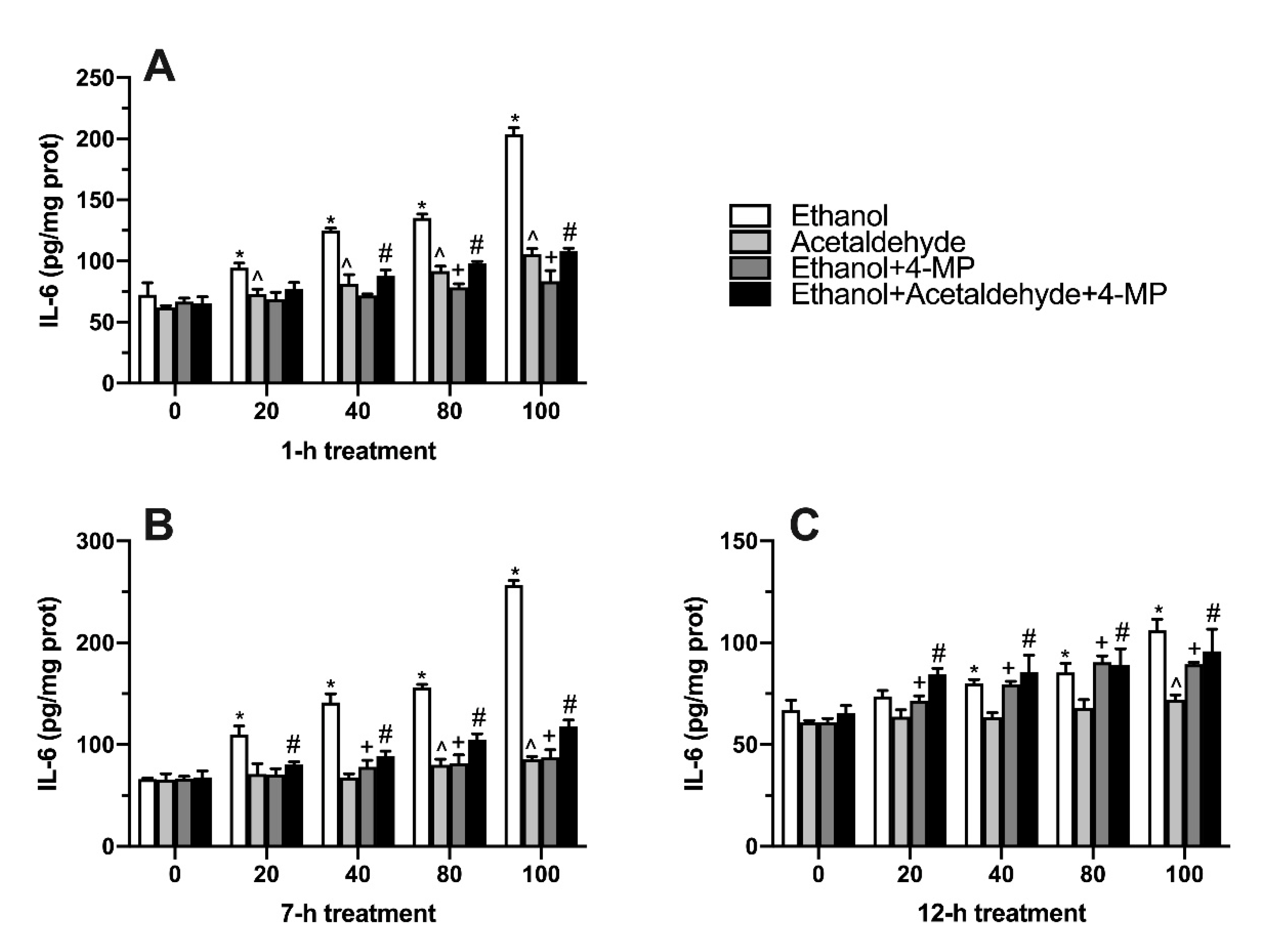
3.6. Effects of Four Different Treatments on IL-6 Production
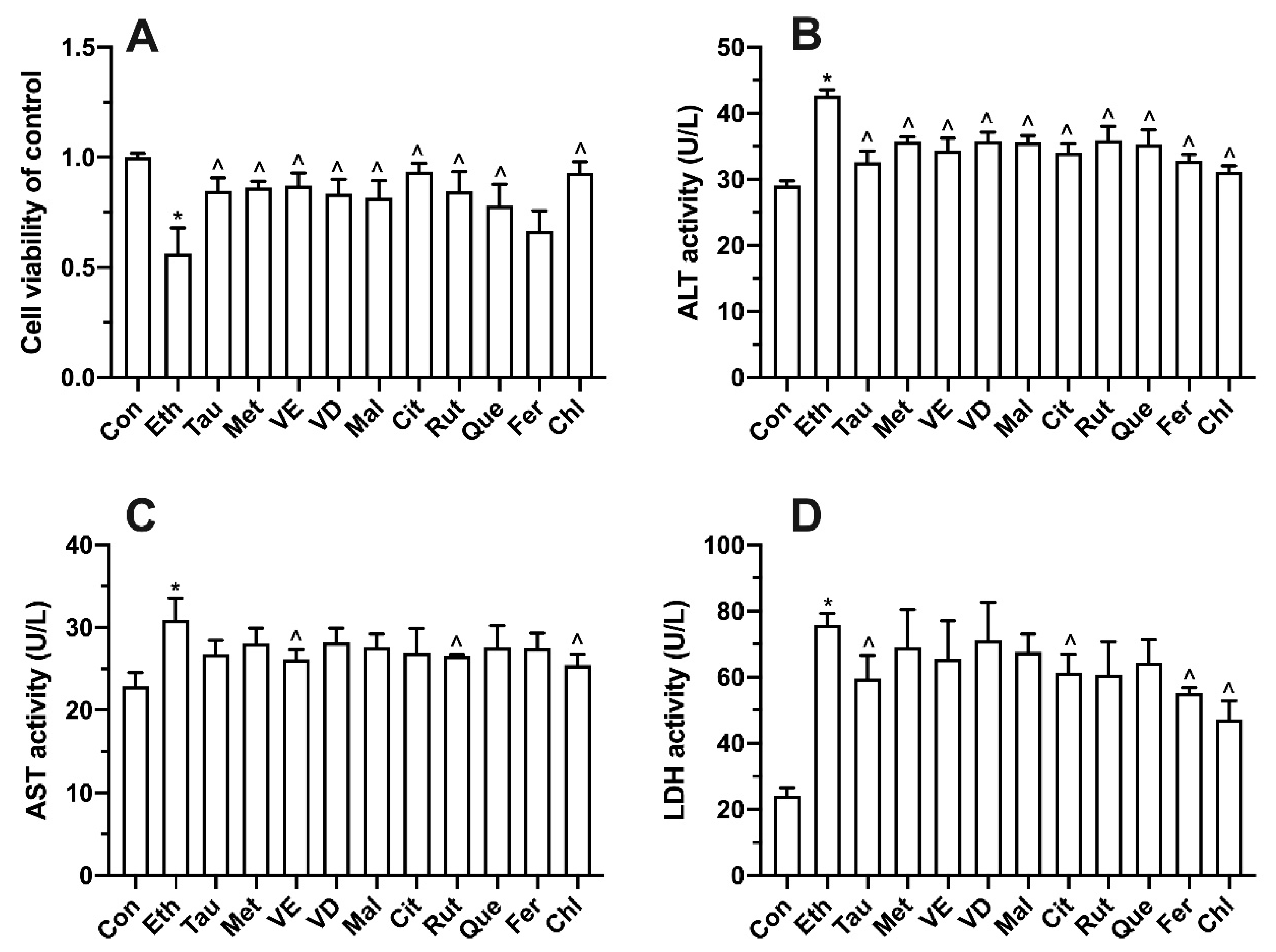
3.7. Effects of Bioactive Components on Ethanol-Induced Cell Proliferation, Transaminase and LDH
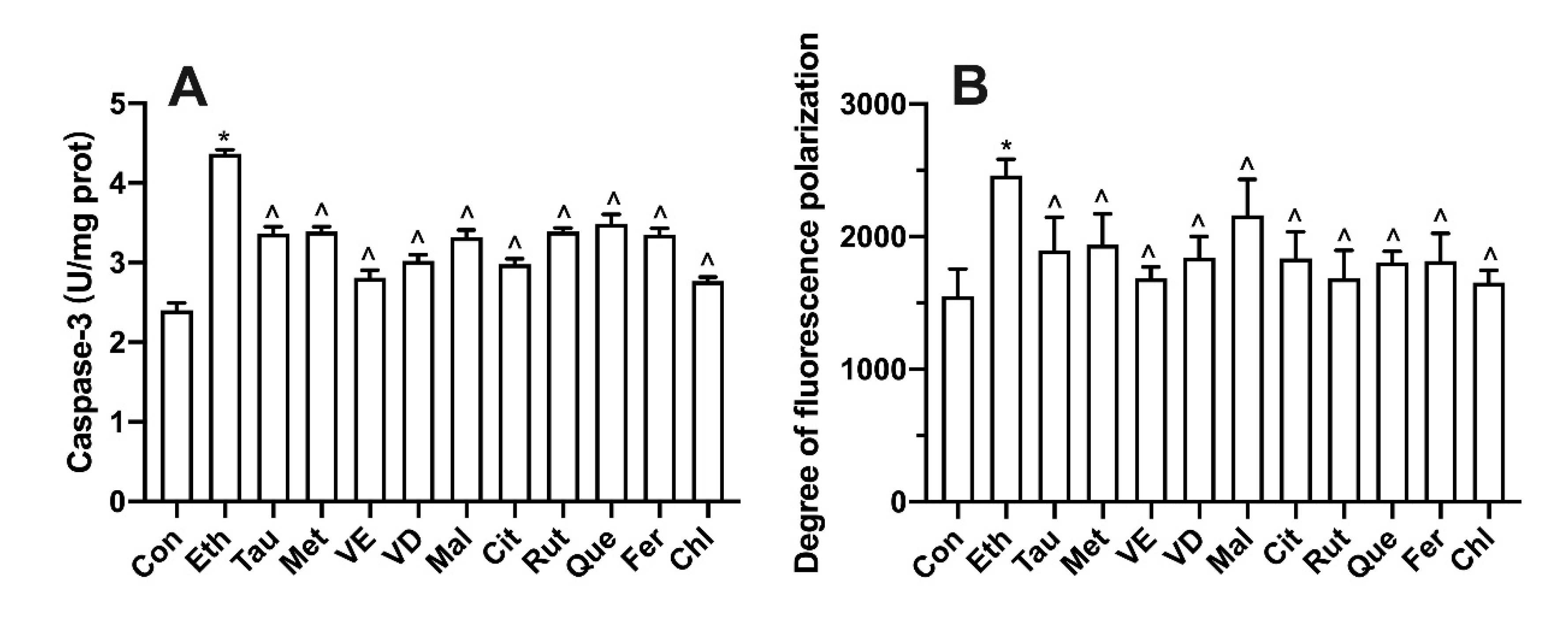
3.8. Effect of Bioactive Components on Intracellular Oxidative Stress and Inflammatory Factors
3.9. Effect of Bioactive Components on Membrane Potential
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gereben, O. Ring structure analysis of ethanol-water mixtures. J. Mol. Liq. 2015, 211, 812–820. [Google Scholar] [CrossRef]
- Chacko, K.R.; Reinus, J. Spectrum of Alcoholic Liver Disease. Clin. Liver Dis. 2016, 20, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ching, R.C.T.; Mak, S.P.; Wong, M.C.S.; Lam, M.; Chan, W.M.; Wong, M.F.Y.; Liang, R.; Lam, T.H.; Hong Kong Alliance, A. Response to the World Health Organization’s working document for the development of a global action plan to reduce alcohol-related harm: Position Statement of the Hong Kong Alliance for Advocacy Against Alcohol. Hong Kong Med. J. 2021, 27, 4–6. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Status Report on Alcohol and Health 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Seyedsadjadi, N.; Acevedo, M.B.; Alfaro, R.; Ramchandani, V.A.; Plawecki, M.H.; Rowitz, B.; Pepino, M.Y. Site of Alcohol First-Pass Metabolism Among Women. JAMA Netw. Open 2022, 5, e223711. [Google Scholar] [CrossRef]
- Boyd, S.J.; Corbin, W.R. Faster Alcohol Metabolism Is Associated with Increased Stimulation and Within Session Consumption. Exp. Clin. Psychopharmacol. 2018, 26, 168–176. [Google Scholar] [CrossRef]
- Wunder, C.; Pogoda, W.; Paulke, A.; Toennes, S.W. Assay of ethanol and congener alcohols in serum and beverages by headspace gas chromatography/mass spectrometry. MethodsX 2021, 8, 101563. [Google Scholar] [CrossRef]
- Gawlikowski, T.; Piekoszewski, W.; Gomolka, E.; Krol, A. Acetaldehyde concentration in acute ethanol-intoxicated patients addicted to alcohol. Prz. Lek. 2004, 61, 310–313. [Google Scholar]
- Zhu, L.; Pei, W.; Thiele, I.; Mahadevan, R. Integration of a physiologically-based pharmacokinetic model with a whole-body, organ-resolved genome-scale model for characterization of ethanol and acetaldehyde metabolism. PLoS Comput. Biol. 2021, 17, e1009110. [Google Scholar] [CrossRef]
- Simic, M.; Tasic, M. The relationship between alcohol elimination rate and increasing blood alcohol concentration-calculated from two consecutive blood specimens. Forensic Sci. Int. 2007, 172, 28–32. [Google Scholar] [CrossRef]
- Seitz, H.K.; Suter, P.M. Ethanol Toxicity and Nutritional Status. In Nutritional Toxicology; Taylor & Francis: London, UK, 2002; pp. 122–154. [Google Scholar]
- Hyun, J.; Han, J.; Lee, C.; Yoon, M.; Jung, Y. Pathophysiological Aspects of Alcohol Metabolism in the Liver. Int. J. Mol. Sci. 2021, 22, 5717. [Google Scholar] [CrossRef]
- Jeon, S.; Carr, R. Alcohol effects on hepatic lipid metabolism. J. Lipid Res. 2020, 61, 470–479. [Google Scholar] [CrossRef]
- Lieber, C.S. Alcohol and the liver: Metabolism of alcohol and its role in hepatic and extrahepatic diseases. Mt. Sinai J. Med. 2000, 67, 84–94. [Google Scholar]
- Contreras-Zentella, M.L.; Villalobos-García, D.; Hernández-Muñoz, R. Ethanol Metabolism in the Liver, the Induction of Oxidant Stress, and the Antioxidant Defense System. Antioxidants 2022, 11, 1258. [Google Scholar] [CrossRef]
- Gerasimova, O.N.; Dankovtseva, E.N.; Nakonechnikov, S.N.; Zateyshchikov, D.A. Inflammation and chronic heart failure: The role of tumor necrosis factor. Kardiol. Vestn. 2014, IX, 86–93. [Google Scholar]
- Shijun, W.; Zou, Y.; Aijun, S.U.N.; Danling, X.U.; Keqiang, W.; Junbo, G.E. Oxidative stress plays an important role in acetaldehyde -induced cardio- myocytes apoptosis. Chin. J. Pathophysiol. 2008, 24, 1464–1468. [Google Scholar]
- Grishko, V.I.; Rachek, L.I.; Spitz, D.R.; Wilson, G.L.; LeDoux, S.P. Contribution of mitochondrial DNA repair to cell resistance from oxidative stress. J. Biol. Chem. 2005, 280, 8901–8905. [Google Scholar] [CrossRef]
- Radosavljevic, T.; Mladenovic, D.; Vucevic, D. The role of oxidative stress in alcoholic liver injury. Med. Pregl. 2009, 62, 547–553. [Google Scholar] [CrossRef]
- Jia, C.; Huang, F.; Wang, Y.; Liu, X.; Chen, Y.; Guo, L.; Shen, G.; Zhu, H.; Jiang, S. Effect of LPS on the expressions of YAP/p-YAP in hepatocytes. J. Trop. Med. 2016, 16, 557–560. [Google Scholar]
- Park, J.H.; Kim, K.H.; Kim, S.J.; Lee, W.R.; Lee, K.G.; Han, S.M.; Park, K.K. Bee venom protects against TNF-α-induced hepatic cell damage via inhibition of pro-inflammatory cytokine production and NF-κB pathway. J. Apic. 2010, 25, 173–180. [Google Scholar]
- Shabangu, C.S.; Huang, J.F.; Hsiao, H.H.; Yu, M.L.; Chuang, W.L.; Wang, S.C. Liquid Biopsy for the Diagnosis of Viral Hepatitis, Fatty Liver Steatosis, and Alcoholic Liver Diseases. Int. J. Mol. Sci. 2020, 21, 3732. [Google Scholar] [CrossRef]
- Thomes, P.G.; Rasineni, K.; Saraswathi, V.; Kharbanda, K.K.; Clemens, D.L.; Sweeney, S.A.; Kubik, J.L.; Donohue, T.M., Jr.; Casey, C.A. Natural recovery by the liver and other organs after chronic alcohol use. Alcohol Res.-Curr. Rev. 2021, 41, 5. [Google Scholar] [CrossRef]
- Garcia-Tirado, J.; Rieger-Reyes, C.; Saz-Peiro, P. Effect of flavonoids in the prevention of lung cancer: Systematic review. Med. Clin. 2012, 139, 358–363. [Google Scholar] [CrossRef]
- Ide, N.; Lau, B.H.S. Garlic compounds protect vascular endothelial cells from oxidized low density lipoprotein-induced injury. J. Pharm. Pharmacol. 1997, 49, 908–911. [Google Scholar] [CrossRef]
- Zea, L.; Medina, M. Vitamin E and vitamin C linked with the natural lipid antioxidant system. Aliment. Equipos Tecnol. 2006, 25, 40–43. [Google Scholar]
- Kharbanda, K.K. Methionine metabolic pathway in alcoholic liver injury. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 89–95. [Google Scholar] [CrossRef]
- Devi, A.K.P.; Martin, A. Recent insights into the molecular regulators and mechanisms of taurine to modulate lipid metabolism: A review. Crit. Rev. Food Sci. Nutr. 2022, 18, 1–13. [Google Scholar] [CrossRef]
- Ma, L.; Huo, C.-Y.; Zhang, X.-Y.; Qin, C.-Q.; Ren, D.-F.; Lu, J. Protective effect of Letinous edodes foot peptides against ethanol-induced liver injury in L02 cells. Mol. Med. Rep. 2018, 18, 1858–1866. [Google Scholar] [CrossRef]
- Neuman, M.G.; Maor, Y.; Nanau, R.M.; Melzer, E.; Mell, H.; Opris, M.; Cohen, L.; Malnick, S. Alcoholic Liver Disease: Role of Cytokines. Biomolecules 2015, 5, 2023–2034. [Google Scholar] [CrossRef]
- Wang, A.L.; Wang, J.P.; Wang, H.; Chen, Y.H.; Zhao, L.; Wang, L.S.; Wei, W.; Xu, D.X. A dual effect of N-acetylcysteine on acute ethanol-induced liver damage in mice. Hepatol. Res. 2006, 34, 199–206. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C. Protective Effects of Ellagic Acid Against Alcoholic Liver Disease in Mice. Front. Nutr. 2021, 8, 744520. [Google Scholar] [CrossRef]
- Gawel, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Wang, J.; Yang, X.; Zhang, J. Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic beta cells. Cell. Signal. 2016, 28, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Zulli, A.; Lau, E.; Wijaya, B.; Jin, X.; Sutarga, K.; Schwartz, G.; Learmont, J.; Wookey, P.; Zinello, A.; Carru, C.; et al. High Dietary Taurine Reduces Apoptosis and Atherosclerosis in the Left Main Coronary Artery: Association with Reduced CHOP, Homocysteine, and HOCL-LDL. In Proceedings of the 10th Annual Conference on Arteriosclerosis, Thrombosis and Vascular, Washington, DC, USA, 29 April–1 May 2009. [Google Scholar]
- Wang, L.; Xie, Y.; Zhang, G. Application of phytogenic antioxidants and its mechanisms. Chin. J. Anim. Nutr. 2017, 29, 1481–1488. [Google Scholar]
- Qiao, L.; Fu, Y.; Ye, X.; Sun, Y. Recent Advances of Phenolic Acid Bioactivities. J. Chin. Inst. Food Sci. Technol. 2013, 13, 144–152. [Google Scholar]
- Sha’fie, M.S.A.; Rathakrishnan, S.; Hazanol, I.N.; Dali, M.H.I.; Khayat, M.E.; Ahmad, S.; Hussin, Y.; Alitheen, N.B.; Jiang, L.H.; Syed Mortadza, S.A. Ethanol Induces Microglial Cell Death via the NOX/ROS/PARP/TRPM2 Signalling Pathway. Antioxidants 2020, 9, 1253. [Google Scholar] [CrossRef]
- Mira, M.L.; Manso, C.F. Alcohol and free oxygen radicals. Acta Med. Port. 1993, 6, 193–198. [Google Scholar]
- Li, L.; Ji, H.; Duan, J. The inhibition study of chlorogenic acid on the oxidation and N-nitrosodiethylamine(NDEA) formation in pork myofibrillar protein. Sci. Technol. Food Ind. 2018, 39, 29–33. [Google Scholar]
- Wang, X.; Xi, Y.; Fan, X.; Cao, J.; Jiang, W. Research Progress on Bioavailability and Antioxidant Activity of Chlorogenic Acid. J. Chin. Inst. Food Sci. Technol. 2019, 19, 271–279. [Google Scholar]
- Niki, E. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: In vitro and in vivo evidence. Free Radic. Biol. Med. 2014, 66, 3–12. [Google Scholar] [CrossRef]
- Saqib, M.; Iqbal, S.; Mahmood, A.; Akram, R. Theoretical Investigation for Exploring the Antioxidant Potential of Chlorogenic Acid: A Density Functional Theory Study. Int. J. Food Prop. 2016, 19, 745–751. [Google Scholar] [CrossRef]
- Volynets, V.; Kueper, M.A.; Strahl, S.; Maier, I.B.; Spruss, A.; Wagnerberger, S.; Koenigsrainer, A.; Bischoff, S.C.; Bergheim, I. Nutrition, Intestinal Permeability, and Blood Ethanol Levels Are Altered in Patients with Nonalcoholic Fatty Liver Disease (NAFLD). Dig. Dis. Sci. 2012, 57, 1932–1941. [Google Scholar] [CrossRef]
- Giraldo, L.F.; Passino, K.M.; Clapp, J.D.; Ruderman, D. Dynamics of Metabolism and Decision Making during Alcohol Consumption: Modeling and Analysis. IEEE Trans. Cybern. 2017, 47, 3955–3966. [Google Scholar] [CrossRef]
- Chen, J.J.; Yu, B.P. Alterations in mitochondrial-membrane fluidity by lipid-peroxidation products. Free Radic. Biol. Med. 1994, 17, 411–418. [Google Scholar] [CrossRef]
- Ying, L.I.U.; Zhi-sheng, W.; An-guo, Z.; Jing-yi, C.A.I. In vivo and in vitro Antioxidant Effects of Hesperidin and Chlorogenic Acid. Food Sci. 2009, 30, 196–199. [Google Scholar]
- Chen, C.; Wang, S.; Liu, P. Deferoxamine Enhanced Mitochondrial Iron Accumulation and Promoted Cell Migration in Triple-Negative MDA-MB-231 Breast Cancer Cells Via a ROS-Dependent Mechanism. Int. J. Mol. Sci. 2019, 20, 4952. [Google Scholar] [CrossRef]
- Zhao, L.; Mehmood, A.; Yuan, D.; Usman, M.; Murtaza, M.A.; Yaqoob, S.; Wang, C. Protective Mechanism of Edible Food Plants against Alcoholic Liver Disease with Special Mention to Polyphenolic Compounds. Nutrients 2021, 13, 1612. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Ong, C.-N.; Yang, X.-F.; Shen, H.-M. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett. 2010, 293, 109–116. [Google Scholar] [CrossRef]
- Haghgoo, S.M.; Sharafi, H.; Alavian, S.M. Serum cytokines, adipokines and ferritin for non-invasive assessment of liver fibrosis in chronic liver disease: A systematic review. Clin. Chem. Lab. Med. 2019, 57, 577–610. [Google Scholar] [CrossRef]
- Wang, P.; Guo, L.; Jiang, Z.; Guo, Y.; Ma, X.; Li, Y. Synergistic antioxidation of polysaccharides and flavonoid from Auricularia auricula by oxygen-resistant extraction. Sci. Technol. Food Ind. 2018, 39, 54–58, 63. [Google Scholar]
- Winkler, J.; Cochran, F. Apoptosis: Insight into its role in inflammation. Inflamm. Res. 1997, 46, 3. [Google Scholar]
- Mancini, M.; Nicholson, D.W.; Roy, S.; Thornberry, N.A.; Peterson, E.P.; Casciola-Rosen, L.A.; Rosen, A. The caspase-3 precursor has a cytosolic and mitochondrial distribution: Implications for apoptotic signaling. J. Cell Biol. 1998, 140, 1485–1495. [Google Scholar] [CrossRef]
| Category | Compound | Structure | Concentration (µM) |
|---|---|---|---|
| amino acids | Taurine | 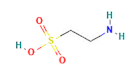 | 400 |
| methionine |  | 200 | |
| vitamins | vitamin E | 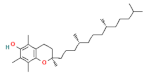 | 100 |
| vitamin D | 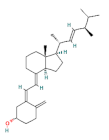 | 100 | |
| organic acids | malic acid | 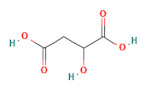 | 200 |
| citric acid | 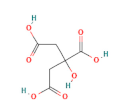 | 200 | |
| flavonoids | rutin | 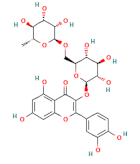 | 100 |
| quercetin | 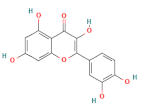 | 100 | |
| phenolic acids | ferulic acid | 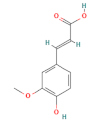 | 100 |
| chlorogenic acid | 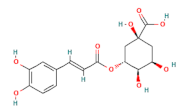 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, N.; Zhou, J.; Sun, P.; Zhao, L.; Zhou, F. Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol. Foods 2022, 11, 3014. https://doi.org/10.3390/foods11193014
Wang Y, Zhang N, Zhou J, Sun P, Zhao L, Zhou F. Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol. Foods. 2022; 11(19):3014. https://doi.org/10.3390/foods11193014
Chicago/Turabian StyleWang, Yashen, Nanhai Zhang, Jingxuan Zhou, Peng Sun, Liang Zhao, and Feng Zhou. 2022. "Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol" Foods 11, no. 19: 3014. https://doi.org/10.3390/foods11193014
APA StyleWang, Y., Zhang, N., Zhou, J., Sun, P., Zhao, L., & Zhou, F. (2022). Protective Effects of Several Common Amino Acids, Vitamins, Organic Acids, Flavonoids and Phenolic Acids against Hepatocyte Damage Caused by Alcohol. Foods, 11(19), 3014. https://doi.org/10.3390/foods11193014