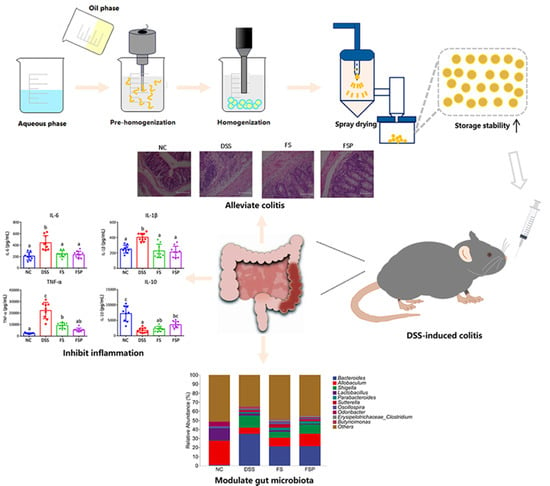
Encapsulation of Functional Plant Oil by Spray Drying: Physicochemical Characterization and Enhanced Anti-Colitis Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsion
2.3. Particle Size Distribution of Emulsion
2.4. Preparation of Oil Powders
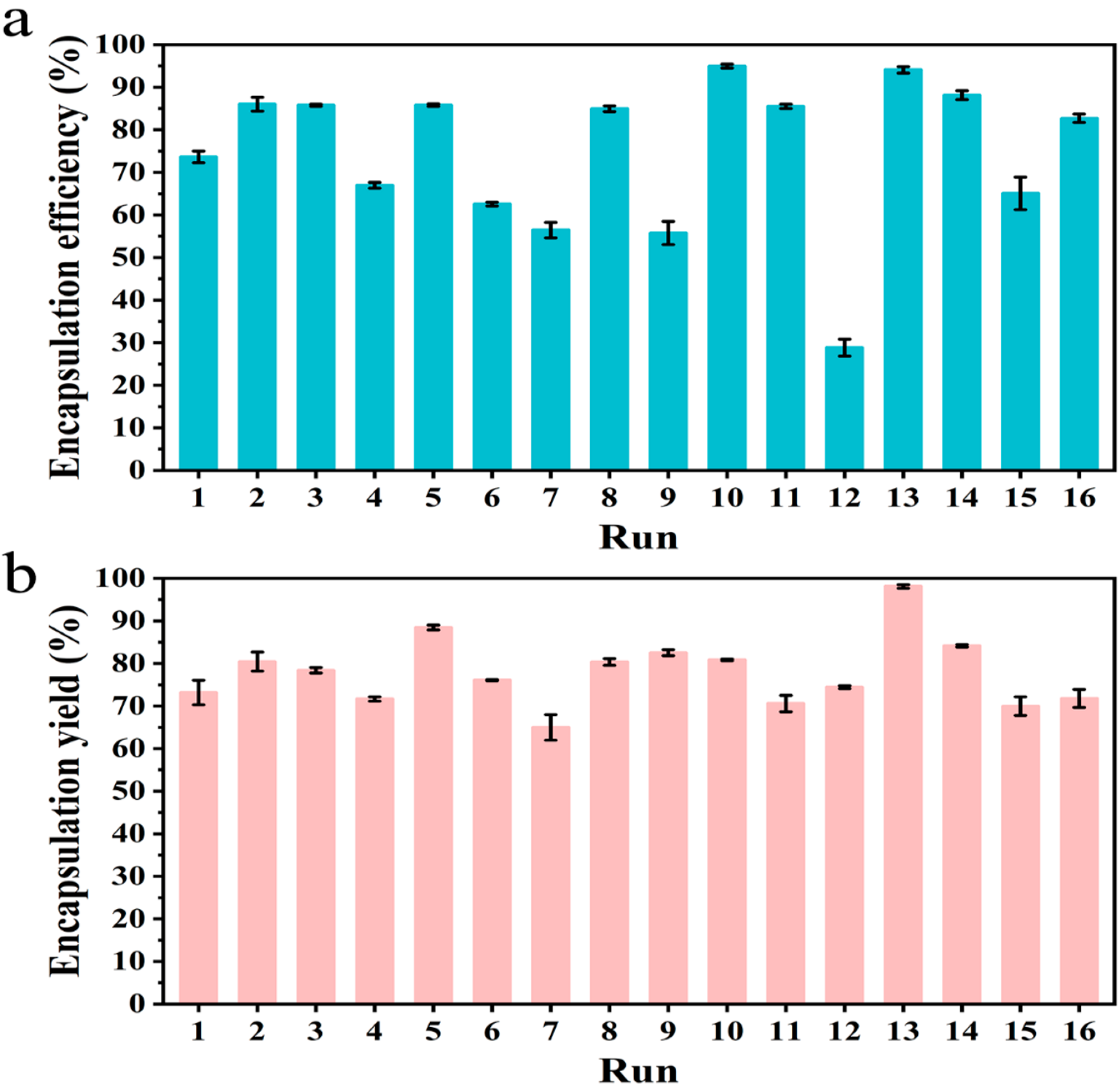
2.5. Orthogonal Experiment Design
2.6. Characterization of the Oil Powders
2.6.1. Total Oil Content
2.6.2. Surface Oil Content
2.6.3. Encapsulation Efficiency
2.6.4. Moisture Content
2.6.5. Acidity Value Analysis
2.6.6. Peroxide Value Analysis
2.6.7. Powder Morphology
2.7. Storage Stability of the Spray-Dried Oil Powders
2.8. Animal Experiment
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Raw Material Compositions on Spray-Dried Flaxseed Oil Powders
3.2. Effect of Emulsification and Spray Drying Conditions on Spray-Dried Flaxseed Oil Powders
3.3. Physicochemical Properties of Oil Emulsions and Powders
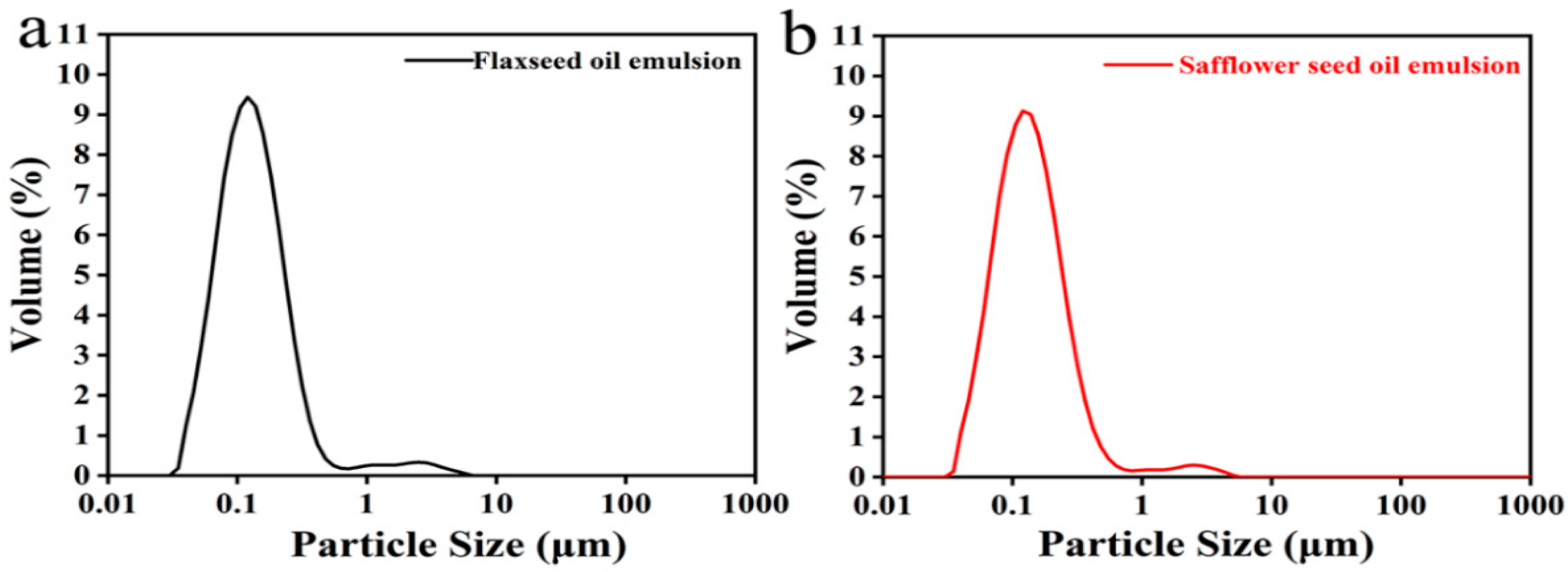
3.3.1. Emulsion Droplet Size
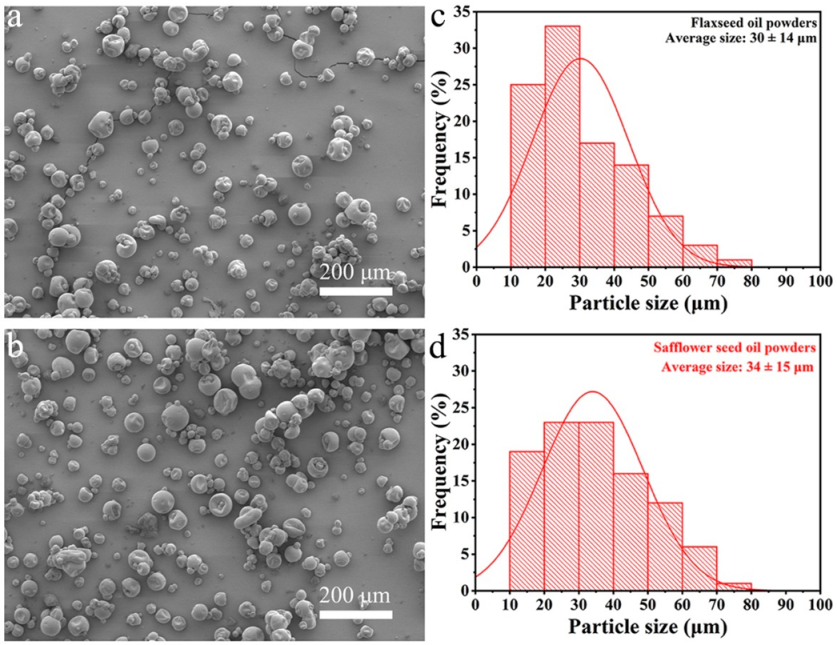
3.3.2. Powder Morphology
3.3.3. Physicochemical Properties of the Oil Powders
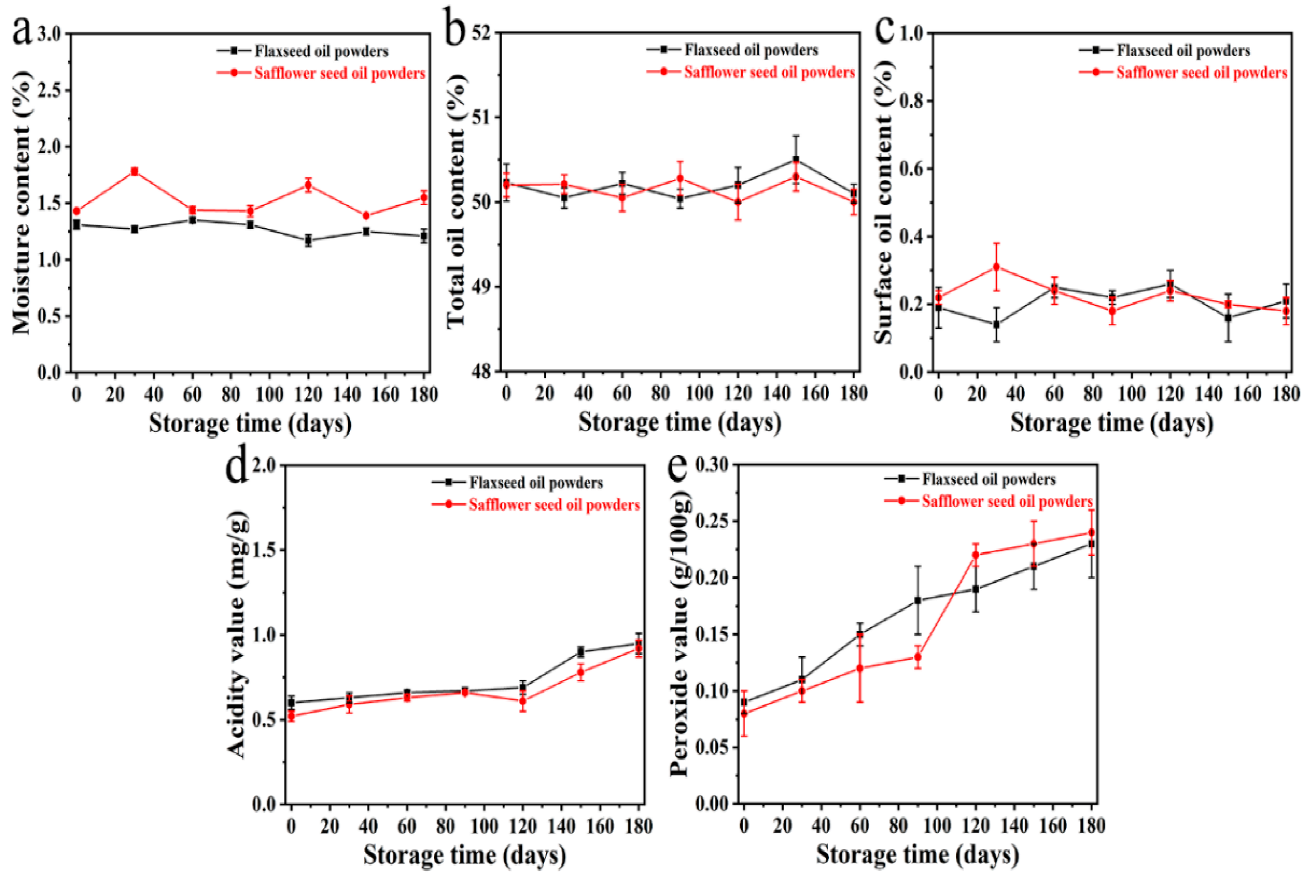
3.4. Storage Stability
3.5. Alleviation of DSS-Induced Colitis
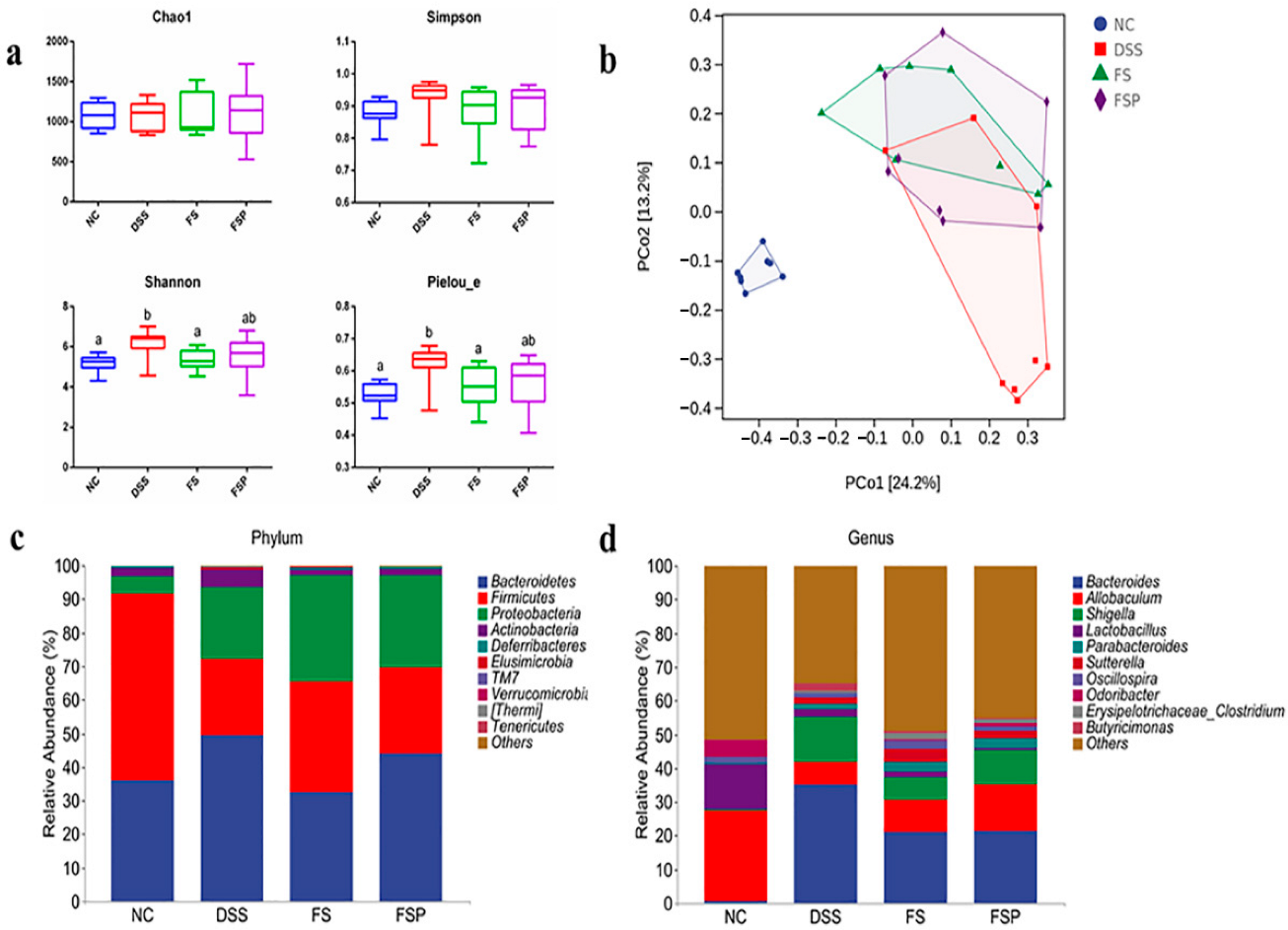
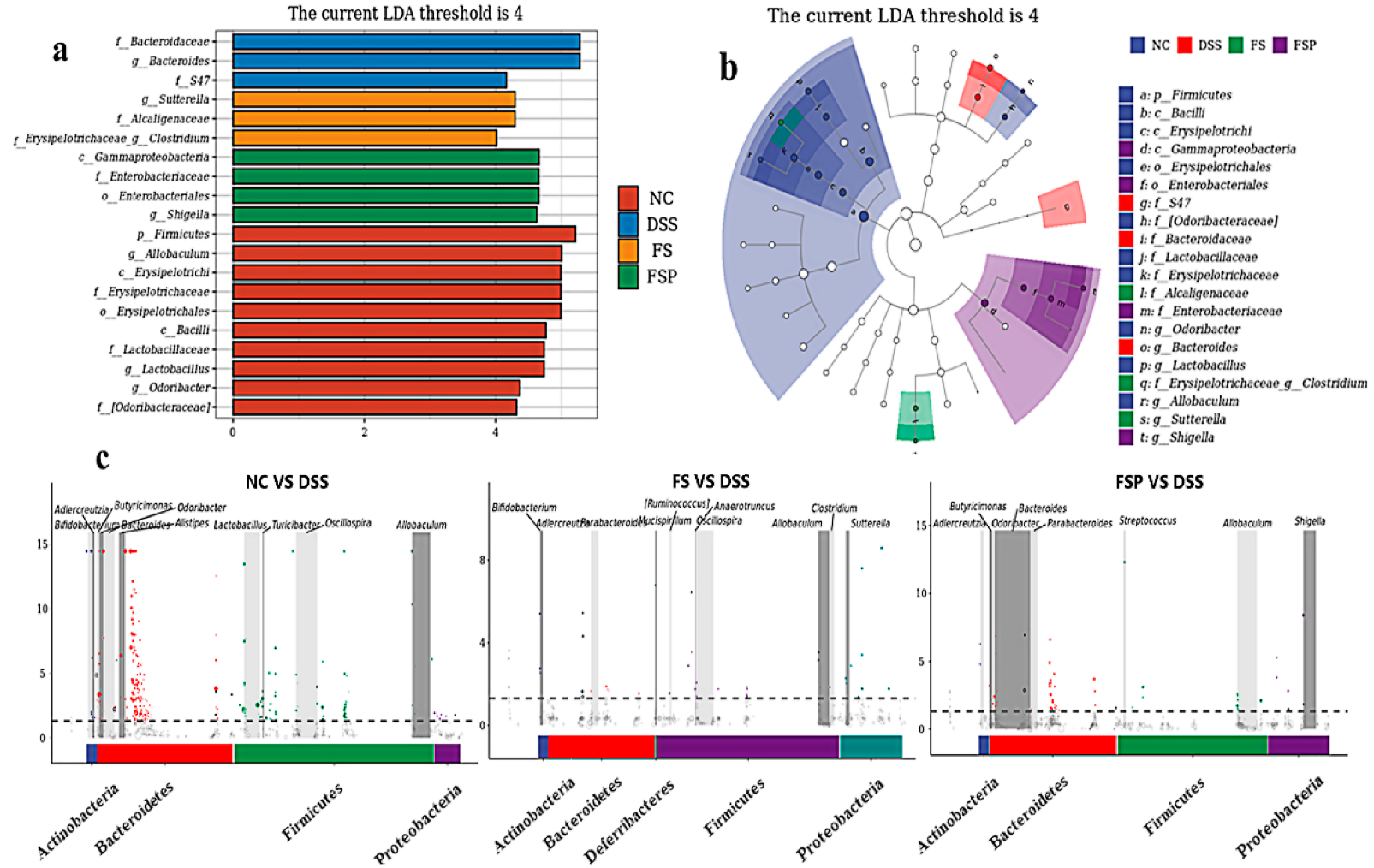
3.6. Regulation of Gut Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simopoulos, A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Bakry, A.M.; Fang, Z.; Ni, Y.Z.; Cheng, H.; Chen, Y.Q.; Liang, L. Stability of tuna oil and tuna oil/peppermint oil blend microencapsulated using whey protein isolate in combination with carboxymethyl cellulose or pullulan. Food Hydrocolloid 2016, 60, 559–571. [Google Scholar] [CrossRef]
- Fioramonti, S.A.; Stepanic, E.M.; Tibaldo, A.M.; Pavon, Y.L.; Santiago, L.G. Spray dried flaxseed oil powdered microcapsules obtained using milk whey proteins-alginate double layer emulsions. Food Res. Int. 2019, 119, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Polavarapu, S.; Oliver, C.M.; Ajlouni, S.; Augustin, M.A. Physicochemical characterisation and oxidative stability of fish oil and fish oil–extra virgin olive oil microencapsulated by sugar beet pectin. Food Chem. 2011, 127, 1694–1705. [Google Scholar] [CrossRef]
- Carneiro, H.C.F.; Tonon, R.V.; Grosso, C.R.F.; Hubinger, M.D. Encapsulation efficiency and oxidative stability of flaxseed oil microencapsulated by spray drying using different combinations of wall materials. J. Food Eng. 2013, 115, 443–451. [Google Scholar] [CrossRef]
- Ahn, J.H.; Kim, Y.P.; Lee, Y.M.; Seo, E.M.; Lee, K.W.; Kim, H.S. Optimization of microencapsulation of seed oil by response surface methodology. Food Chem. 2008, 107, 98–105. [Google Scholar] [CrossRef]
- Bastioglu, A.Z.; Koc, M.; Yalcin, B.; Ertekin, F.K.; Otles, S. Storage characteristics of microencapsulated extra virgin olive oil powder: Physical and chemical properties. J. Food Meas. Charact. 2017, 11, 1210–1226. [Google Scholar] [CrossRef]
- Venugopalan, V.K.; Gopakumar, L.R.; Kumaran, A.K.; Chatterjee, N.S.; Soman, V.; Peeralil, S.; Mathew, S.; McClements, D.J.; Nagarajarao, R.C. Encapsulation and Protection of Omega-3-Rich Fish Oils Using Food-Grade Delivery Systems. Foods 2021, 10, 1566. [Google Scholar] [CrossRef]
- Kouame, K.J.E.P.; Bora, A.F.M.; Li, X.D.; Sun, Y.; Liu, L. Novel trends and opportunities for microencapsulation of flaxseed oil in foods: A review. J. Funct. Foods 2021, 87, 104812. [Google Scholar] [CrossRef]
- Perez, E.M.D.; De Alencar, N.M.N.; De Figueiredo, I.S.T.; Aragao, K.S.; Gaban, S.V.F. Effect of safflower oil (Carthamus tinctorius L.) supplementation in the abdominal adipose tissues and body weight of male Wistar rats undergoing exercise training. Food Chem. Mol. Sci. 2022, 4, 100083. [Google Scholar] [CrossRef]
- Stefani, F.D.; de Campo, C.; Paese, K.; Guterres, S.S.; Costa, T.M.H.; Flores, S.H. Nanoencapsulation of linseed oil with chia mucilage as structuring material: Characterization, stability and enrichment of orange juice. Food Res. Int. 2019, 120, 872–879. [Google Scholar] [CrossRef]
- National Standard GB/T 5009.6–2016; Determination of Fat in Food. China Standard Press: Beijing, China, 2016.
- Chinese Aquaculture Industry Standard SC/T 3505-2006; Microcapsules of Fish Oil. Ministry of Agriculture of PRC: Beijing, China, 2006.
- National Standard GB/T 5009.3–2016; Determination of Moisture Content in Food. China Standard Press: Beijing, China, 2016.
- National Standard GB/T 5009.229–2016; Determination of Acid Value in Food. China Standard Press: Beijing, China, 2016.
- National Standard GB/T 5009.227–2016; Determination of Peroxide Value in Food. China Standard Press: Beijing, China, 2016.
- Xu, Z.X.; Tang, H.; Huang, F.H.; Qiao, Z.X.; Wang, X.; Yang, C.; Deng, Q.C. Algal Oil Rich in n-3 PUFA Alleviates DSS-Induced Colitis via Regulation of Gut Microbiota and Restoration of Intestinal Barrier. Front. Microbiol. 2020, 11, 615404. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Qiao, Z.X.; Xu, Z.X.; Wang, X.; Deng, Q.C.; Chen, W.C.; Huang, F.H. Algal Oil Rich in Docosahexaenoic Acid Alleviates Intestinal Inflammation Induced by Antibiotics Associated with the Modulation of the Gut Microbiome and Metabolome. J. Agr. Food Chem. 2021, 69, 9124–9136. [Google Scholar] [CrossRef]
- Mohseni, F.; Goli, S. Encapsulation of flaxseed oil in the tertiary conjugate of oxidized tannic acid-gelatin and flaxseed (Linum usitatissimum) mucilage. Int. J. Biol. Macromol. 2019, 140, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, P.; Dowling, K.; Mcknight, S.; Barrow, C.J.; Adhikari, B. Microencapsulation of flaxseed oil in flaxseed protein and flaxseed gum complex coacervates. Food Res. Int. 2016, 86, 1–8. [Google Scholar] [CrossRef]
- Lai, Q.D.; Doan, N.T.T.; Nguyen, T.T.T. Influence of Wall Materials and Homogenization Pressure on Microencapsulation of Rice Bran Oil. Food Bioprocess. Tech. 2021, 14, 1885–1896. [Google Scholar] [CrossRef]
- Pangeni, R.; Sharma, S.; Mustafa, G.; Ali, J.; Baboota, S. Vitamin E loaded resveratrol nanoemulsion for brain targeting for the treatment of Parkinson’s disease by reducing oxidative stress. Nanotechnology 2014, 25, 485102. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Mobli, H.; Madadlou, A.; Rafiee, S. Influence of Wall Material and Inlet Drying Air Temperature on the Microencapsulation of Fish Oil by Spray Drying. Food Bioprocess. Tech. 2013, 6, 1561–1569. [Google Scholar] [CrossRef]
- Nguyen, P.T.N.; Nguyen, H.T.A.; Hoang, Q.B.; Nguyen, T.D.P.; Nguyen, T.V.; Mai, H.C. Influence of spray drying parameters on the physicochemical characteristics of microencapsulated orange (Citrus sinensis L.) essential oil. Mater. Today Proc. 2022, 60, 2026–2033. [Google Scholar] [CrossRef]
- Delfanian, M.; Razavi, S.; Haddad Khodaparast, M.; Esmaeilzadeh Kenari, R.; Golmohammadzadeh, S. Influence of main emulsion components on the physicochemical and functional properties of W/O/W nano-emulsion: Effect of polyphenols, Hi-Cap, basil seed gum, soy and whey protein isolates. Food Res. Int. 2018, 108, 136–143. [Google Scholar] [CrossRef]
- Carvalho, A.G.S.; Silva, V.M.; Hubinger, M.D. Microencapsulation by spray drying of emulsified green coffee oil with two-layered membranes. Food Res. Int. 2014, 61, 236–245. [Google Scholar] [CrossRef]
- Edris, A.; Kalemba, D.; Adamiec, J.; Piątkowski, M. Microencapsulation of Nigella sativa oleoresin by spray drying for food and nutraceutical applications. Food Chem. 2016, 204, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, M.; Xiao, M.; Lu, S.; Wang, Z.; Yao, J.; Yang, L. Microencapsulated β-carotene preparation using different drying treatments. J. Zhejiang Univ Sc. B 2019, 20, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Chew, S.C.; Tan, C.P.; Nyam, K.L. Microencapsulation of refined kenaf (Hibiscus cannabinus L.) seed oil by spray drying using β-cyclodextrin/gum arabic/sodium caseinate. J. Food Eng. 2018, 237, 78–85. [Google Scholar] [CrossRef]
- Patel, S.S.; Pushpadass, H.A.; Franklin, M.E.E.; Battula, S.N.; Vellingiri, P. Microencapsulation of curcumin by spray drying: Characterization and fortification of milk. J. Food Sci. Tech. Mys. 2022, 59, 1326–1340. [Google Scholar] [CrossRef]
- Polekkad, A.; Franklin, M.E.E.; Pushpadass, H.A.; Battula, S.N.; Rao, S.B.N.; Pal, D.T. Microencapsulation of zinc by spray-drying: Characterisation and fortification. Powder Technol. 2021, 381, 1–16. [Google Scholar] [CrossRef]
- Wang, B.; Adhikari, B.; Barrow, C.J. Optimisation of the microencapsulation of tuna oil in gelatin-sodium hexametaphosphate using complex coacervation. Food Chem. 2014, 158, 358–365. [Google Scholar] [CrossRef]
- Elik, A.; Yanik, D.K.; Gogus, F. A comparative study of encapsulation of carotenoid enriched-flaxseed oil and flaxseed oil by spray freeze-drying and spray drying techniques. Lwt Food Sci. Technol. 2021, 143, 111153. [Google Scholar] [CrossRef]
- Avramenko, N.A.; Chang, C.; Low, N.H.; Nickerson, M.T. Encapsulation of flaxseed oil within native and modified lentil protein-based microcapsules. Food Res. Int. 2016, 81, 17–24. [Google Scholar] [CrossRef]
- Zhou, Q.; Ma, L.; Zhao, W.Y.; Zhao, W.; Han, X.; Niu, J.H.; Li, R.B.; Zhao, C.H. Flaxseed oil alleviates dextran sulphate sodium-induced ulcerative colitis in rats. J. Funct. Foods 2020, 64, 103602. [Google Scholar] [CrossRef]
- Jangale, N.M.; Devarshi, P.P.; Bansode, S.B.; Kulkarni, M.J.; Harsulkar, A.M. Dietary flaxseed oil and fish oil ameliorates renal oxidative stress, protein glycation, and inflammation in streptozotocin-nicotinamide-induced diabetic rats. J. Physiol. Biochem. 2016, 72, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, X.J.; Yan, C.; He, C.; Li, P.; Chen, M.; Su, H.; Wan, J.B. Preventive effect of α-linolenic acid-rich flaxseed oil against ethanol-induced liver injury is associated with ameliorating gut-derived endotoxin-mediated inflammation in mice. J. Funct. Foods 2016, 23, 532–541. [Google Scholar] [CrossRef]
- Petrey, A.C.; de la Motte, C.A. The extracellular matrix in IBD: A dynamic mediator of inflammation. Curr. Opin. Gastroen. 2017, 33, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Loddo, I.; Romano, C. Inflammatory bowel disease: Genetics, epigenetics, and pathogenesis. Front. Immunol. 2015, 6, 551. [Google Scholar] [CrossRef]
- Calder, P.C.; Ahluwalia, N.; Albers, R.; Bosco, N.; Bourdet-Sicard, R.; Haller, D.; Holgate, S.T.; Jonsson, L.S.; Latulippe, M.E.; Marcos, A.; et al. A Consideration of Biomarkers to Be Used for Evaluation of Inflammation in Human Nutritional Studies. Ann. Nutr. Metab. 2013, 63, 15. [Google Scholar] [CrossRef]
- Nordlander, S.; Pott, J.; Maloy, K.J. NLRC4 expression in intestinal epithelial cells mediates protection against an enteric pathogen. Mucosal. Immunol. 2014, 7, 775–785. [Google Scholar] [CrossRef]
- Weingarden, A.R.; Vaughn, B.P. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut Microbes 2017, 8, 238–252. [Google Scholar] [CrossRef]
- Rashidi, A.; Ebadi, M.; Rehman, T.U.; Elhusseini, H.; Nalluri, H.; Kaiser, T.; Holtan, S.G.; Khoruts, A.; Weisdorf, D.J.; Staley, C. Gut microbiota response to antibiotics is personalized and depends on baseline microbiota. Microbiome 2021, 9, 211. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Gut microbiota as a regulator of energy homeostasis and ectopic fat deposition: Mechanisms and implications for metabolic disorders. Curr. Opin. Lipidol. 2010, 21, 76–83. [Google Scholar] [CrossRef]
- Xu, Z.X.; Chen, W.C.; Deng, Q.C.; Huang, Q.D.; Wang, X.; Yang, C.; Huang, F.H. Flaxseed oligosaccharides alleviate DSS-induced colitis through modulation of gut microbiota and repair of the intestinal barrier in mice. Food Funct. 2020, 11, 8077–8088. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, L.X.; Li, C.; Wu, H.; Ran, D.; Zhang, Z.Y. Sulforaphane alter the microbiota and mitigate colitis severity on mice ulcerative colitis induced by DSS. Amb. Express 2020, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Yamanishi, S.; Sohn, J.; Alekseyenko, A.V.; Leung, J.M.; Cho, I.; Kim, S.G.; Li, H.; Gao, Z.; Mahana, D.; et al. Altering the Intestinal Microbiota during a Critical Developmental Window Has Lasting Metabolic Consequences. Cell 2014, 158, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.T.; Li, Y.Q.; Wang, J.K.; Li, P.F.; Duan, Y.H.; Dai, H.Y.; An, Y.C.; Cheng, L.; Wang, T.S.; Wang, C.G.; et al. Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomed. Pharm. 2020, 124, 109873. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.L.; Mikkelsen, D.; Hoedt, E.C.; Williams, B.A.; Flanagan, B.M.; Morrison, M.; Gidley, M.J. In vitro fermentation outcomes of arabinoxylan and galactoxyloglucan depend on fecal inoculum more than substrate chemistry. Food Funct. 2020, 11, 7892–7904. [Google Scholar] [CrossRef]
- Fry, L. Microbiome of chronic plaque psoriasis. In The Human Microbiota and Chronic Disease Dysbiosis as a Cause of Human Pathology, 1st ed.; Nibali, L., Henderson, B., Eds.; Wiley: New York, NY, USA, 2016; pp. 327–337. [Google Scholar]
- van Muijlwijk, G.H.; van Mierlo, G.; Jansen, P.W.T.C.; Vermeulen, M.; Bleumink-Pluym, N.M.C.; Palm, N.W.; van Putten, J.P.M.; de Zoete, M.R. Identification of Allobaculum mucolyticum as a novel human intestinal mucin degrader. Gut Microbes 2021, 13, 1966278. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tian, T.; Mao, Q.; Zou, T.; Zhou, C.J.; Xie, J.; Chen, J.J. Associations between disordered gut microbiota and changes of neurotransmitters and short-chain fatty acids in depressed mice. Transl. Psychiat. 2020, 10, 350. [Google Scholar] [CrossRef] [PubMed]

| Level | Factors | |||
|---|---|---|---|---|
| Wall Ratio | Core/Wall Ratio | Solid Concentration | Emulsifier Content | |
| 1 | 0.6:1 | 1:1 | 26% | 0.5% |
| 2 | 1:1 | 1:0.9 | 34% | 1% |
| 3 | 1:0.6 | 1:0.8 | 42% | 2% |
| 4 | 1:0.2 | 1:0.7 | 50% | 5% |
| Run | Factors | |||
|---|---|---|---|---|
| Wall Ratio | Core/Wall Ratio | Solid Concentration | Emulsifier Content | |
| 1 | 0.6:1 | 1:1 | 26% | 0.5% |
| 2 | 0.6:1 | 1:0.9 | 34% | 1% |
| 3 | 0.6:1 | 1:0.8 | 42% | 2% |
| 4 | 0.6:1 | 1:0.7 | 50% | 5% |
| 5 | 1:1 | 1:1 | 34% | 2% |
| 6 | 1:1 | 1:0.9 | 26% | 5% |
| 7 | 1:1 | 1:0.8 | 50% | 0.5% |
| 8 | 1:1 | 1:0.7 | 42% | 1% |
| 9 | 1:0.6 | 1:1 | 42% | 5% |
| 10 | 1:0.6 | 1:0.9 | 50% | 2% |
| 11 | 1:0.6 | 1:0.8 | 26% | 1% |
| 12 | 1:0.6 | 1:0.7 | 34% | 0.5% |
| 13 | 1:0.2 | 1:1 | 50% | 1% |
| 14 | 1:0.2 | 1:0.9 | 42% | 0.5% |
| 15 | 1:0.2 | 1:0.8 | 34% | 5% |
| 16 | 1:0.2 | 1:0.7 | 26% | 2% |
| Factors | Level | Surface Oil (%) |
|---|---|---|
| Homogenization pressure | 20 MPa | 3.70 ± 0.12 |
| 40 MPa | 2.94 ± 0.14 | |
| 60 MPa | 2.88 ± 0.09 | |
| 80 MPa | 2.90 ± 0.11 | |
| Homogenization cycles | 1 time | 3.12 ± 0.13 |
| 2 times | 2.90 ± 0.09 | |
| 3 times | 2.22 ± 0.10 | |
| 4 times | 2.18 ± 0.04 | |
| Inlet air temperature | 150 °C | 2.78 ± 0.22 |
| 160 °C | 2.22 ± 0.15 | |
| 170 °C | 1.02 ± 0.07 | |
| 180 °C | 1.07 ± 0.13 | |
| Emulsion feeding rate | 4 L/h | 0.22 ± 0.03 |
| 6 L/h | 0.23 ± 0.01 | |
| 8 L/h | 0.19 ± 0.06 | |
| 10 L/h | 1.02 ± 0.02 |
| Flaxseed Oil Emulsion | Safflower Seed Oil Emulsion | |
|---|---|---|
| D [0.1] | 0.067 μm | 0.069 μm |
| D [0.5] | 0.135 μm | 0.140 μm |
| D [0.9] | 0.301 μm | 0.321 μm |
| D [3,2] | 0.116 μm | 0.120 μm |
| D [4,3] | 0.234 μm | 0.226 μm |
| Span | 1.732 | 1.797 |
| Parameters | Flaxseed Oil Powders | Safflower Seed Oil Powders |
|---|---|---|
| Moisture content (%) | 1.31 ± 0.04 | 1.43 ± 0.01 |
| Total oil (%) | 50.23 ± 0.22 | 50.20 ± 0.14 |
| Surface oil (%) | 0.19 ± 0.06 | 0.22 ± 0.02 |
| Encapsulation efficiency (%) | 99.6 | 99.6 |
| Acidity value (mg/g) | 0.60 ± 0.04 | 0.52 ± 0.03 |
| Peroxide value (g/100 g) | 0.09 ± 0.01 | 0.08 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Xu, Z.; Qiao, Z.; Wang, X.; Tang, H.; Yang, C.; Huang, F. Encapsulation of Functional Plant Oil by Spray Drying: Physicochemical Characterization and Enhanced Anti-Colitis Activity. Foods 2022, 11, 2993. https://doi.org/10.3390/foods11192993
Zhang H, Xu Z, Qiao Z, Wang X, Tang H, Yang C, Huang F. Encapsulation of Functional Plant Oil by Spray Drying: Physicochemical Characterization and Enhanced Anti-Colitis Activity. Foods. 2022; 11(19):2993. https://doi.org/10.3390/foods11192993
Chicago/Turabian StyleZhang, Hao, Zhenxia Xu, Zhixian Qiao, Xu Wang, Hu Tang, Chen Yang, and Fenghong Huang. 2022. "Encapsulation of Functional Plant Oil by Spray Drying: Physicochemical Characterization and Enhanced Anti-Colitis Activity" Foods 11, no. 19: 2993. https://doi.org/10.3390/foods11192993
APA StyleZhang, H., Xu, Z., Qiao, Z., Wang, X., Tang, H., Yang, C., & Huang, F. (2022). Encapsulation of Functional Plant Oil by Spray Drying: Physicochemical Characterization and Enhanced Anti-Colitis Activity. Foods, 11(19), 2993. https://doi.org/10.3390/foods11192993