Application of Pineapple Waste to the Removal of Toxic Contaminants: A Review
Abstract
1. Introduction
2. Pineapple Waste Application as an Economic-Development Means of Waste Management
2.1. Pineapple Waste as an Environmental Threat
2.2. Pineapple Adsorbent and Composites Used for the Elimination of Inorganic and Organic Pollutants
2.3. Pineapples Applied as an Adsorbent for the Removal of Heavy Metals
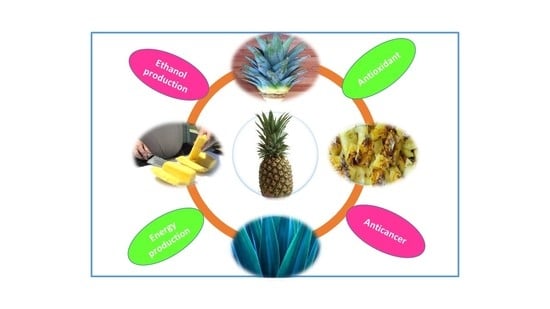
2.4. Application of Pineapple Wastes
2.4.1. Energy Production and as a Carbon Source
2.4.2. Antioxidant Activity
2.4.3. Anticancer and Antibacterial Activity
2.4.4. Pharmaceutical and Food Industry
2.4.5. Production of Ethanol
2.4.6. Production of Vinegar
2.5. Nutritional and Health Benefits
2.6. Challenge and Future Trends
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Worldbank Water in Agriculture. Available online: https://www.worldbank.org/en/topic/water-in-agriculture#1 (accessed on 31 May 2022).
- Mugagga, F.; Nabaasa, B.B. The Centrality of Water Resources to the Realization of Sustainable Development Goals (SDG). A Review of Potentials and Constraints on the African Continent. Int. Soil Water Conserv. Res. 2016, 4, 215–223. [Google Scholar] [CrossRef]
- Bavel, J. Van The World Population Explosion: Causes, Backgrounds and Projections for the Future. Facts Views Vis. ObGyn 2013, 5, 281. [Google Scholar] [PubMed]
- National Academy of Sciences. The Growth of World Population: Analysis of the Problems and Recommendations for Research and Training; The National Academies Press: Washington, DC, USA, 1963. [Google Scholar] [CrossRef]
- World Health Organization Drinking-Water. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 3 September 2022).
- Da̧browski, A.; Hubicki, Z.; Podkościelny, P.; Robens, E. Selective Removal of the Heavy Metal Ions from Waters and Industrial Wastewaters by Ion-Exchange Method. Chemosphere 2004, 56, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Masindi, V.; Muedi, K.L. Environmental Contamination by Heavy Metals. In Heavy Metals; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Murugesan, G.S.; Sathishkumar, M.; Swaminathan, K. Arsenic Removal from Groundwater by Pretreated Waste Tea Fungal Biomass. Bioresour. Technol. 2006, 97, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Adewoye, T.L.; Ogunleye, O.O.; Abdulkareem, A.S.; Salawudeen, T.O.; Tijani, J.O. Optimization of the Adsorption of Total Organic Carbon from Produced Water Using Functionalized Multi-Walled Carbon Nanotubes. Heliyon 2021, 7, e05866. [Google Scholar] [CrossRef]
- Moosavi, S.; Lai, C.W.; Gan, S.; Zamiri, G.; Akbarzadeh Pivehzhani, O.; Johan, M.R. Application of Efficient Magnetic Particles and Activated Carbon for Dye Removal from Wastewater. ACS Omega 2020, 5, 20684–20697. [Google Scholar] [CrossRef]
- Qasem, N.A.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 36. [Google Scholar] [CrossRef]
- Bankole, M.T.; Abdulkareem, A.S.; Tijani, J.O.; Ochigbo, S.S.; Afolabi, A.S.; Roos, W.D. Chemical Oxygen Demand Removal from Electroplating Wastewater by Purified and Polymer Functionalized Carbon Nanotubes Adsorbents. Water Resour. Ind. 2017, 18, 33–50. [Google Scholar] [CrossRef]
- Fathy, M.; El-Sayed, M.; Ramzi, M.; Abdelraheem, O.H. Adsorption Separation of Condensate Oil from Produced Water Using ACTF Prepared of Oil Palm Leaves by Batch and Fixed Bed Techniques. Egypt. J. Pet. 2018, 27, 319–326. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.H.; Jiang, J.J.; Wang, H.P.; Wei, Z.W.; Zhu, X.; Pan, M.; Su, C.Y. A Stable Metal Cluster-Metalloporphyrin MOF with High Capacity for Cationic Dye Removal. J. Mater. Chem. A 2018, 6, 17698–17705. [Google Scholar] [CrossRef]
- Ayad, M.; Zaghlol, S. Nanostructured Crosslinked Polyaniline with High Surface Area: Synthesis, Characterization and Adsorption for Organic Dye. Chem. Eng. J. 2012, 204–206, 79–86. [Google Scholar] [CrossRef]
- Dutta, S.; Srivastava, S.K.; Gupta, A.K. Polypyrrole–Polyaniline Copolymer Coated Green Rice Husk Ash as an Effective Adsorbent for the Removal of Hexavalent Chromium from Contaminated Water. Mater. Adv. 2021, 2, 2431–2443. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D Hierarchical Graphene Oxide-NiFe LDH Composite with Enhanced Adsorption Affinity to Congo Red, Methyl Orange and Cr(VI) Ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef]
- Sabzehmeidani, M.M.; Mahnaee, S.; Ghaedi, M.; Heidari, H.; Roy, V.A.L. Carbon Based Materials: A Review of Adsorbents for Inorganic and Organic Compounds. Mater. Adv. 2021, 2, 598–627. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent Advances for Dyes Removal Using Novel Adsorbents: A Review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Piriya, R.S.; Jayabalakrishnan, R.M.; Maheswari, M.; Boomiraj, K.; Oumabady, S. Comparative Adsorption Study of Malachite Green Dye on Acid-Activated Carbon. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Streit, A.F.M.; Collazzo, G.C.; Druzian, S.P.; Verdi, R.S.; Foletto, E.L.; Oliveira, L.F.S.; Dotto, G.L. Adsorption of Ibuprofen, Ketoprofen, and Paracetamol onto Activated Carbon Prepared from Effluent Treatment Plant Sludge of the Beverage Industry. Chemosphere 2021, 262, 128322. [Google Scholar] [CrossRef]
- Ahmad, F.; Zaidi, S. Potential Use of Agro/Food Wastes as Biosorbents in the Removal of Heavy Metals. In Emerging Contaminants; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Çelebi, H.; Gök, G.; Gök, O. Adsorption Capability of Brewed Tea Waste in Waters Containing Toxic Lead(II), Cadmium (II), Nickel (II), and Zinc(II) Heavy Metal Ions. Sci. Rep. 2020, 10, 17570. [Google Scholar] [CrossRef]
- Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; El-Azazy, M. Molecules Application of Pineapple Leaves as Adsorbents for Removal of Rose Bengal from Wastewater: Process Optimization Operating Face-Centered Central Composite Design (FCCCD). Molecules 2020, 25, 3752. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.R.; Hoque, M.E.; Deng, Y. A Review on Pineapple Leaves Fibre and Its Composites. Int. J. Polym. Sci. 2015, 2015, 950567. [Google Scholar] [CrossRef]
- Rabiu, Z.; Maigari, F.U.; Lawan, U.; Gidado Mukhtar, Z.; Rabiu, Z.; Maigari, F.U.; Lawan, U.; Mukhtar, Z.G. Pineapple Waste Utilization as a Sustainable Means of Waste Management. In Sustainable Technologies for the Management of Agricultural Wastes; Springer: Singapore, 2018; pp. 143–154. [Google Scholar] [CrossRef]
- Saifaddin Galal April 1 2021. South Africa: Production of Pineapples 2000–2019|Statista. Available online: https://www.statista.com/statistics/1155961/production-of-pineapples-in-south-africa/ (accessed on 1 September 2022).
- Stats sa Fact Sheets. 2020. CoCA 2017 Fact Sheets.pdf. Available online: http://www.statssa.gov.za/publications/Report-11-02-01/CoCA%202017%20Fact%20Sheets.pdf (accessed on 10 September 2022).
- United States Department of Agriculture Spike in Pineapple Consumption and Processing Amid Decline in Exports Due to COVID-19. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Spike%20in%20Pineapple%20Consumption%20and%20Processing%20Amid%20Decline%20in%20Exports%20due%20to%20COVID-19_Pretoria_South%20Africa%20-%20Republic%20of_08-14-2020 (accessed on 15 June 2022).
- DP Bartholomew The Pineapple, 2nd Edition: Botany, Production and Uses—Google Books. Available online: https://books.google.co.za/books?hl=en&lr=&id=mCKADwAAQBAJ&oi=fnd&pg=PA105&dq=+The+Pineapple:+Botany,+Production,+and+Uses,+&ots=xryh1ORUaS&sig=6VZa9PiMhKduqU598eRwrKZ2Ats&redir_esc=y#v=onepage&q=ThePineapple%3ABotany%2CProduction%2CandUses%2C&f=false (accessed on 16 April 2022).
- Cabrera, H.A.P.; Menezes, H.C.; Oliveira, J.V.; Batista, R.F.S. Evaluation of Residual Levels of Benomyl, Methyl Parathion, Diuron, and Vamidothion in Pineapple Pulp and Bagasse (Smooth Cayenne). J. Agric. Food Chem. 2000, 48, 5750–5753. [Google Scholar] [CrossRef]
- Kataki, M.S.; Sharma, D.; Kumar, S.; Yadav, R.S.; Rajkumari, A. Antibacterial activity, in vitro antioxidant activity and anthelmintic activity of methanolic extract of Plumbago zeylanica L. leaves. J. Pharm. Res. 2010, 3, 2908–2912. [Google Scholar]
- Xie, W.; Xing, D.; Sun, H.; Wang, W.; Ding, Y.; Du, L. The Effects of Ananas Comosus L. Leaves on Diabetic-Dyslipidemic Rats Induced by Alloxan and a High-Fat/High-Cholesterol Diet. Am. J. Chin. Med. 2012, 33, 95–105. [Google Scholar] [CrossRef]
- Zhang, X.; Aixinjueluo, Q.Y.; Li, S.Y.; Song, L.L.; Lau, C.-T.; Tan, R.; Bian, Z.X. Reporting quality of Cochrane systematic reviews with Chinese herbal medicines. Syst. Rev. 2019, 8, 302. [Google Scholar] [CrossRef]
- Kalpana, M.B.; Prasath, G.S.; Subramanian, S. Studies on the Antidiabetic Activity of Ananas Comosus Leaves in STZ Induced Diabetic Rats. Available online: https://www.scholarsresearchlibrary.com/abstract/studies-on-the-antidiabetic-activity-of-ananas-comosus-leaves-in-stz-induced--diabetic-rats-8186.html (accessed on 16 April 2022).
- Mishra, S.; Misra, M.; Tripathy, S.S.; Nayak, S.K.; Mohanty, A.K. Potentiality of Pineapple Leaf Fibre as Reinforcement in PALF-Polyester Composite: Surface Modification and Mechanical Performance. J. Reinf. Plast. Compos. 2016, 20, 321–334. [Google Scholar] [CrossRef]
- Lopattananon, N.; Panawarangkul, K.; Sahakaro, K.; Ellis, B. Performance of Pineapple Leaf Fiber–Natural Rubber Composites: The Effect of Fiber Surface Treatments. J. Appl. Polym. Sci. 2006, 102, 1974–1984. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Tripathy, P.C.; Misra, M.; Parija, S.; Sahoo, S. Chemical Modification of Pineapple Leaf Fiber: Graft Copolymerization of Acrylonitrile onto Defatted Pineapple Leaf Fibers. J. Appl. Polym. Sci. 2000, 77, 3035–3043. [Google Scholar] [CrossRef]
- Kengkhetkit, N.; Amornsakchai, T. A New Approach to “Greening” Plastic Composites Using Pineapple Leaf Waste for Performance and Cost Effectiveness. Mater. Des. 2014, 55, 292–299. [Google Scholar] [CrossRef]
- Musa, N.S.; Ahmad, W.A. Chemical Oxygen Demand Reduction in Industrial Wastewater Using Locally Isolated Bacteria. Malays. J. Fundam. Appl. Sci. 2014, 6, 88S92. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chakraborty, S.; Saha, P. Biosorption of Basic Green 4 from Aqueous Solution by Ananas Comosus (Pineapple) Leaf Powder. Colloids Surf. B Biointerfaces 2011, 84, 520–527. [Google Scholar] [CrossRef]
- Ponou, J.; Kim, J.; Wang, L.P.; Dodbiba, G.; Fujita, T. Sorption of Cr(VI) Anions in Aqueous Solution Using Carbonized or Dried Pineapple Leaves. Chem. Eng. J. 2011, 172, 906–913. [Google Scholar] [CrossRef]
- Astuti, W.; Sulistyaningsih, T.; Kusumastuti, E.; Thomas, G.Y.R.S.; Kusnadi, R.Y. Thermal Conversion of Pineapple Crown Leaf Waste to Magnetized Activated Carbon for Dye Removal. Bioresour. Technol. 2019, 287, 121426. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Ibrahim, A.; Shitu, A. Batch Removal of Hazardous Safranin-O in Wastewater Using Pineapple Peels as an Agricultural Waste Based Adsorbent. Int. J. Environ. Monit. Anal. 2014, 2, 128. [Google Scholar] [CrossRef]
- Veeramalai, S.; Ramlee, N.N.; Mahdi, H.I.; Manas, N.H.A.; Ramli, A.N.M.; Illias, R.M.; Azelee, N.I.W. Development of Organic Porous Material from Pineapple Waste as a Support for Enzyme and Dye Adsorption. Ind. Crops Prod. 2022, 181, 114823. [Google Scholar] [CrossRef]
- Yamuna, M.; Kamaraj, M. Pineapple Peel Waste Activated Carbon as an Adsorbent for the Effective Removal of Methylene Blue Dye from Aqueous Solution. Int. J. ChemTech Res. 2016, 9, 544–550. [Google Scholar]
- Nieva, A.D.; Avena, L.G.S.; Pascual, M.A.M.; Pamintuan, K.R.S. Characterization of Powdered Pineapple (Ananas comosus) Crown Leaves as Adsorbent for Crystal Violet in Aqueous Solutions. IOP Conf. Ser. Earth Environ. Sci. 2020, 563, 012010. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Zhang, Y.; Zhang, H.; Huang, H. Green and Facile Fabrication of Pineapple Peel Cellulose/Magnetic Diatomite Hydrogels in Ionic Liquid for Methylene Blue Adsorption. Cellulose 2019, 26, 3825–3844. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of Caffeine on Mesoporous Activated Carbon Fibers Prepared from Pineapple Plant Leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef]
- Fakhar, N.; Siddiqi, W.A.; Khan, T.A.; Siddiqui, M.F. Fabrication of Ananas comosus Leaf Extract Modified Titanium Dioxide Nano Bio Adsorbent for the Sequestration of Basic Dye from Aqueous Phase: Equilibrium and Kinetic Studies. Mater. Res. Express 2020, 7, 015077. [Google Scholar] [CrossRef]
- Jana, S.; Ray, J.; Mondal, B.; Samanta, S.K.; Tripathy, T. Equilibrium and Kinetics Study of Methyl Violet Adsorption by Pineapple Leaf Fibers-Cl-Poly(Acrylic Acid-Co-2-Dimethyl Amino Ethyl Acrylate) Hydrogel. J. Appl. Polym. Sci. 2021, 138, 50882. [Google Scholar] [CrossRef]
- Hameed, B.H.; Krishni, R.R.; Sata, S.A. A Novel Agricultural Waste Adsorbent for the Removal of Cationic Dye from Aqueous Solutions. J. Hazard. Mater. 2009, 162, 305–311. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.I.C.; Paranha, G.; Maia, L.S.; Mulinari, D.R. Development of Activated Carbon from Pineapple Crown Wastes and Its Potential Use for Removal of Methylene Blue. J. Nat. Fibers 2021, 1–16. [Google Scholar] [CrossRef]
- Rahmat, N.A.; Ali, A.A.; Salmiati; Hussain, N.; Muhamad, M.S.; Kristanti, R.A.; Hadibarata, T. Removal of Remazol Brilliant Blue R from Aqueous Solution by Adsorption Using Pineapple Leaf Powder and Lime Peel Powder. Water. Air. Soil Pollut. 2016, 227, 1–11. [Google Scholar] [CrossRef]
- Fegousse, A.; El Gaidoumi, A.; Miyah, Y.; El Mountassir, R.; Lahrichi, A. Pineapple Bark Performance in Dyes Adsorption: Optimization by the Central Composite Design. J. Chem. 2019, 2019, 3017163. [Google Scholar] [CrossRef]
- Laksono, E.W.; Marfuatun; Marwati, S. Adsorption Mechanism of Direct Red Dye on Cellulose Acetate from Ananas comosus Leaves. Orient. J. Chem. 2017, 33, 3144–3149. [Google Scholar] [CrossRef]
- Ugbe, F.A.; Anebi, P.O.; Ikudayisi, V.A. Biosorption of an Anionic Dye, Eosin Yellow onto Pineapple Peels: Isotherm and Thermodynamic Study. Int. Ann. Sci. 2018, 4, 14–19. [Google Scholar] [CrossRef]
- Kamaru, A.A.; Sani, N.S.; Malek, N.A.N.N. Raw and Surfactant-Modified Pineapple Leaf as Adsorbent for Removal of Methylene Blue and Methyl Orange from Aqueous Solution. Desalin. Water Treat. 2016, 57, 18836–18850. [Google Scholar] [CrossRef]
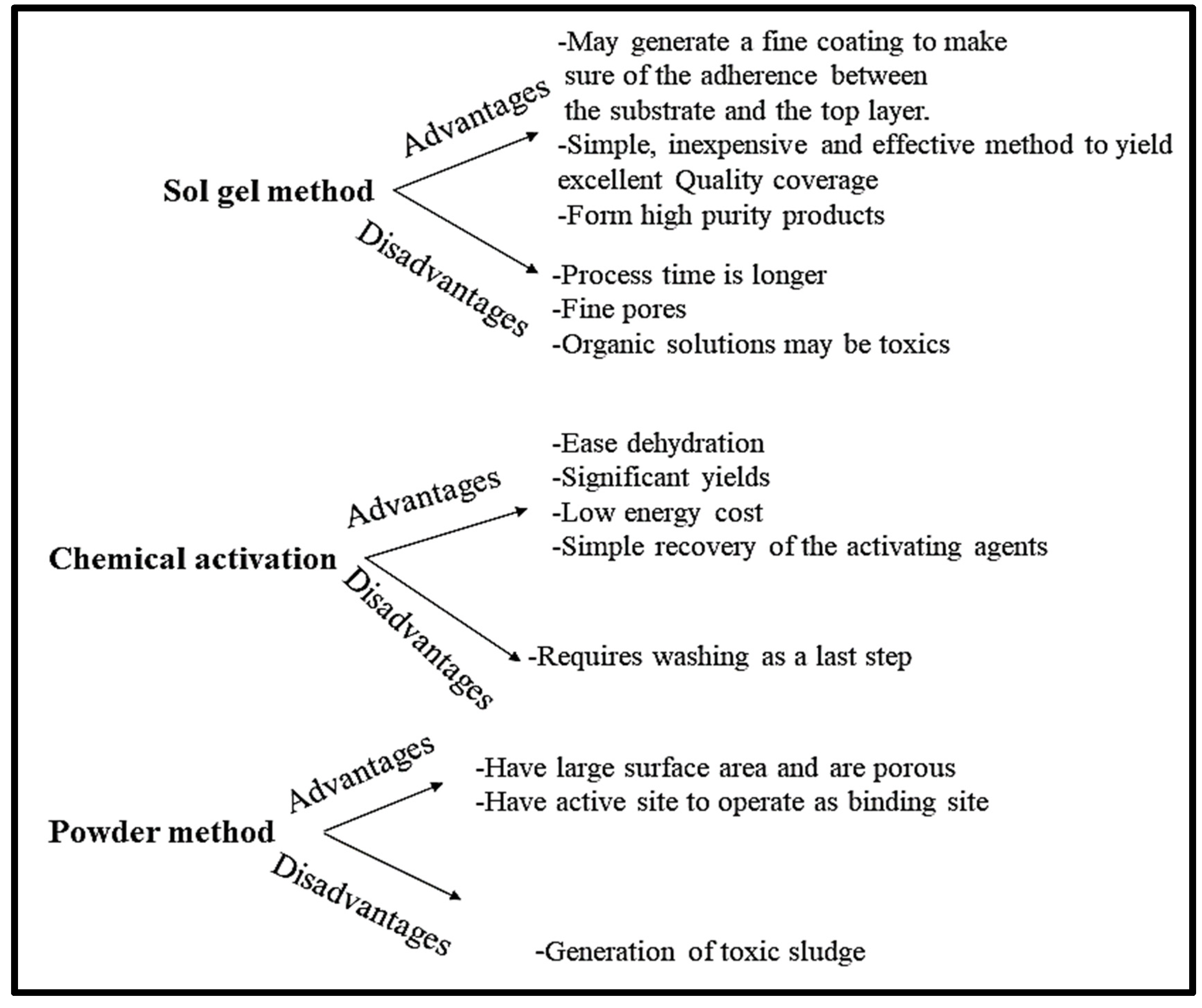
- Ho, S.M. A Review of Chemical Activating Agent on the Properties of Activated Carbon. Int. J. Chem. Res. 2022, 1, 1–13. [Google Scholar] [CrossRef]
- Modan, E.M.; Plăiașu, A.G. Advantages and Disadvantages of Chemical Methods in the Elaboration of Nanomaterials. Ann. Dunarea Jos Univ. Galati. Fascicle IX Metall. Mater. Sci. 2020, 43, 53–60. [Google Scholar] [CrossRef]
- Alcañiz-Monge, J.; Román-Martínez, M.D.C.; Lillo-Ródenas, M.Á. Chemical Activation of Lignocellulosic Precursors and Residues: What Else to Consider? Molecules 2022, 27, 1630. [Google Scholar] [CrossRef]
- Ayob, A.; Zamre, N.M.; Izzati, N.; Ariffin, M.; Hidayu, N.; Rani, A.; Mohamad, F. Pineapple Waste as an Adsorbent to Remove Lead from Synthetic Wastewater. Int. J. Latest Res. Eng. Manag. 2020, 4, 1–8. [Google Scholar]
- Mopoung, R.; Kengkhetkit, N. Lead and Cadmium Removal Efficiency from Aqueous Solution by NaOH Treated Pineapple Waste. Int. J. Appl. Chem. 2016, 12, 23–35. [Google Scholar]
- Mopoung, S.; Bunterm, T. KMnO4 Modified Carbon Prepared from Waste of Pineapple Leaf Fiber Production Processing for Removal of Ferric Ion from Aqueous Solution. Orig. Res. Pap. 2016, 13, 814–826. [Google Scholar] [CrossRef]
- Weng, C.-H.; Wu, Y.-C. Potential Low-Cost Biosorbent for Copper Removal: Pineapple Leaf Powder. J. Environ. Eng. 2011, 138, 286–292. [Google Scholar] [CrossRef]
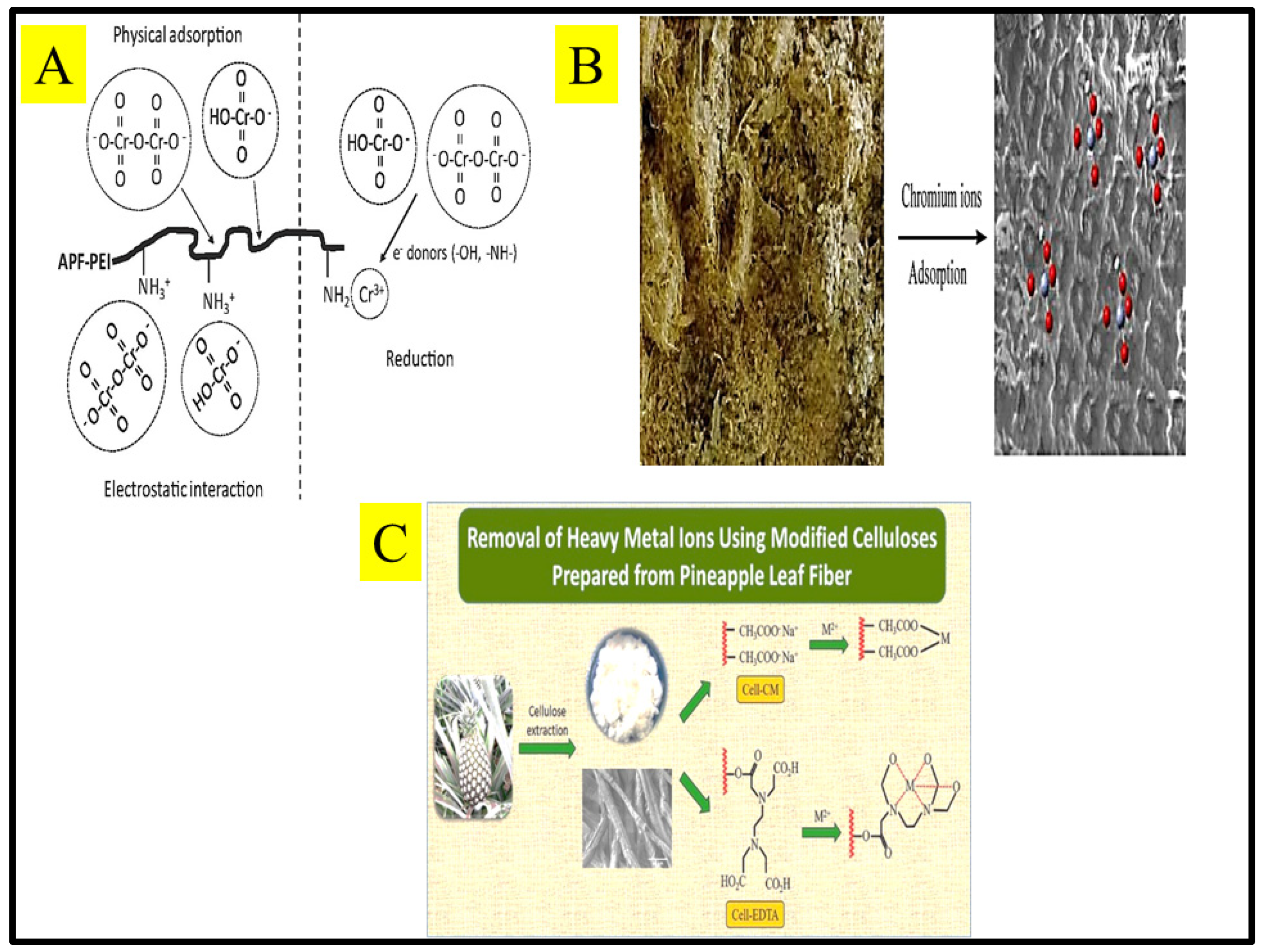
- Tangtubtim, S.; Saikrasun, S. Adsorption Behavior of Polyethyleneimine-Carbamate Linked Pineapple Leaf Fiber for Cr(VI) Removal. Appl. Surf. Sci. 2019, 467–468, 596–607. [Google Scholar] [CrossRef]
- Gogoi, S.; Chakraborty, S.; Saikia, M.D. Surface Modified Pineapple Crown Leaf for Adsorption of Cr(VI) and Cr(III) Ions from Aqueous Solution. J. Environ. Chem. Eng. 2018, 6, 2492–2501. [Google Scholar] [CrossRef]
- Tangtubtim, S.; Saikrasun, S. Effective Removals of Copper (II) and Lead (II) Cations from Aqueous Solutions by Polyethyleneimine-Immobilized Pineapple Fiber. Bioresour. Technol. Rep. 2019, 7, 100188. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, M.; Huang, H. Modification of Pineapple Peel Fiber as Metal Ion Adsorbent through Reaction with Succinic Anhydride in Pyridine and Dimethyl Sulfoxide Solvents on JSTOR. Water Environ. Res. 2010, 82, 733–741. [Google Scholar] [CrossRef]
- Daochalermwong, A.; Chanka, N.; Songsrirote, K.; Dittanet, P.; Niamnuy, C.; Seubsai, A. Removal of Heavy Metal Ions Using Modified Celluloses Prepared from Pineapple Leaf Fiber. ACS Omega 2020, 5, 5285–5296. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-setapar, S.; Khatoon, A.; Kumar, R.; Rafatullah, M. Chemically Oxidized Pineapple Fruit Peel for the Biosorption of Heavy Metals from Aqueous Solutions. Desalin. Water Treat. 2015, 57, 6432–6442. [Google Scholar] [CrossRef]
- Loh, V.; Ting, Z.; Ping, T.Y.; Abdullah, A.H. Removal of pb(ii) from aqueous solution by pineapple plant stem (Penyingkiran Pb(II) Dari Larutan Akueus Menggunakan Batang Tumbuhan Nanas). Malays. J. Anal. Sci. 2019, 23, 219–228. [Google Scholar] [CrossRef]
- Lim, Z.E.; Thai, Q.B.; Le, D.K.; Luu, T.P.; Nguyen, P.T.T.; Do, N.H.N.; Le, P.K.; Phan-Thien, N.; Goh, X.Y.; Duong, H.M. Functionalized Pineapple Aerogels for Ethylene Gas Adsorption and Nickel (II) Ion Removal Applications. J. Environ. Chem. Eng. 2020, 8, 104524. [Google Scholar] [CrossRef]
- Abdullah, A.; Mat, H. Characterisation of solid and liquid pineapple waste. Reaktor 2008, 12, 48. [Google Scholar] [CrossRef]
- Hikal, W.M.; Mahmoud, A.A.; Ahl, H.A.H.S.-A.; Bratovcic, A.; Tkachenko, K.G.; Kačániová, M.; Rodriguez, R.M. Pineapple (Ananas comosus L. Merr.), Waste Streams, Characterisation and Valorisation: An Overview. Open J. Ecol. 2021, 11, 610–634. [Google Scholar] [CrossRef]
- Olatunde, D.; Owolabi, J.B. Oranusi Solomon Biogas Generation from Water Melon Peels, Pine Apple Peels and Food Wastes|Request PDF. In Proceedings of the International Conference on African Development Issues, Igbara Odo, Nigeria, 11–13 May 2015. [Google Scholar]
- Mbuligwe, S.E.; Kassenga, G.R. Feasibility and Strategies for Anaerobic Digestion of Solid Waste for Energy Production in Dar Es Salaam City, Tanzania. Resour. Conserv. Recycl. 2004, 42, 183–203. [Google Scholar] [CrossRef]
- Prakash, E.V.; Singh, L.P. Biomethanation of Vegetable And Fruit Waste in Co-Digestion Process. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 493–495. [Google Scholar]
- Hammid, S.A.; Aini, N.; Selaman, R. Anaerobic Digestion of Fruit Wastes for Biogas Production. Int. J. Adv. Res. Innov. Ideas Educ. 2019, 5, 34–38. [Google Scholar]
- Kanakdande, A.; Agrwal, D.; Khobragade, C. Pineapple Waste and Wastewater: Route for Biodiesel Production from Candida Tropicalis (MF510172). Braz. Arch. Biol. Technol. 2020, 62, 19180499. [Google Scholar] [CrossRef]
- Wang, C.H.; Lin, P.J.; Chang, J.S. Fermentative Conversion of Sucrose and Pineapple Waste into Hydrogen Gas in Phosphate-Buffered Culture Seeded with Municipal Sewage Sludge. Process Biochem. 2006, 41, 1353–1358. [Google Scholar] [CrossRef]
- Kurosumi, A.; Sasaki, C.; Yamashita, Y.; Nakamura, Y. Utilization of Various Fruit Juices as Carbon Source for Production of Bacterial Cellulose by Acetobacter Xylinum NBRC 13693. Carbohydr. Polym. 2009, 76, 333–335. [Google Scholar] [CrossRef]
- Dibanda Romelle, F.; Rani, A.P.; Sai Manohar, R. Chemical Composition of Some Selected Fruit Peels a phenolic-rich herbal tea from Stathmostelma sp. (statroltea) inhibits in vitro pancreatic lipase activity view project chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Hajar, N.; Zainal, S.; Nadzirah, K.Z.; Roha, A.M.S.; Atikah, O.; Elida, T.Z.M.T. Physicochemical Properties Analysis of Three Indexes Pineapple (Ananas comosus) Peel Extract Variety N36. APCBEE Procedia 2012, 4, 115–121. [Google Scholar] [CrossRef]
- Hemalatha, R.; Anbuselvi, S. Physicohemical Constituents of Pineapple Pulp and Waste. J. Chem. Pharm. Res. 2013, 5, 240–242. [Google Scholar]
- Segovia Gómez, F.; Almajano Pablos, M.P. Pineapple Waste Extract for Preventing Oxidation in Model Food Systems. J. Food Sci. 2016, 81, C1622–C1628. [Google Scholar] [CrossRef]
- Saraswaty, V.; Risdian, C.; Primadona, I.; Andriyani, R.; Andayani, D.G.S.; Mozef, T. Pineapple Peel Wastes as a Potential Source of Antioxidant Compounds. IOP Conf. Ser. Earth Environ. Sci. 2017, 60, 012013. [Google Scholar] [CrossRef]
- Chompoo, J.; Upadhyay, A.; Kishimoto, W.; Makise, T.; Tawata, S. Advanced Glycation End Products Inhibitors from Alpinia Zerumbet Rhizomes. Food Chem. 2011, 129, 709–715. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chompoo, J.; Kishimoto, W.; Makise, T.; Tawata, S. HIV-1 Integrase and Neuraminidase Inhibitors from Alpinia Zerumbet. J. Agric. Food Chem. 2011, 59, 2857–2862. [Google Scholar] [CrossRef]
- Tawata, S.; Upadhyay, A. Applicability of Mimosine as Neuraminidase Inhibitors 2010.
- de Oliveira, A.C.; Valentim, I.B.; Silva, C.A.; Bechara, E.J.H.; de Barros, M.P.; Mano, C.M.; Goulart, M.O.F. Total Phenolic Content and Free Radical Scavenging Activities of Methanolic Extract Powders of Tropical Fruit Residues. Food Chem. 2009, 115, 469–475. [Google Scholar] [CrossRef]
- Yahya, N.A.; Wahab, R.A.; Xine, T.L.S.; Hamid, M.A. Ultrasound-Assisted Extraction of Polyphenols from Pineapple Skin. AIP Conf. Proc. 2019, 2155, 020002. [Google Scholar] [CrossRef]
- Li, T.; Shen, P.; Liu, W.; Liu, C.; Liang, R.; Yan, N.; Chen, J. Major Polyphenolics in Pineapple Peels and Their Antioxidant Interactions. Int. J. Food Prop. 2014, 17, 1805–1817. [Google Scholar] [CrossRef]
- Jayashree, J.; Priya, D.; Karthikeyan, S.; Ramesh Babu, N.G. Production and Characterization of Ferulic Acid from Pineapple Waste and Its Anticancer Activity Using HeLa Cell Line. Int. J. Innov. Res. Sci. Eng. Technol. 2017, 6, 1–6. [Google Scholar]
- Lubaina, A.S.; Renjith, P.R.; Kumar, P. Antibacterial Potential of Different Extracts of Pineapple Peel against Gram-Positive and Gram-Negative Bacterial Strains. Asian J. Pharm. Pharmacol. 2019, 5, 66–70. [Google Scholar] [CrossRef]
- Niramol, P.; Katemanee, S.; Wanwisa, S. Antimicrobial Activity of Pineapple Peel Extract. In Proceedings of the Innovation of Functional Foods in Asia Conference, Mae Ka, Thailand, 22–24 January 2018. [Google Scholar]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.S. Investigation of Antioxidant, Antibacterial, Antidiabetic, and Cytotoxicity Potential of Silver Nanoparticles Synthesized Using the Outer Peel Extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef] [PubMed]
- Nor, M.Z.M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Characteristic Properties of Crude Pineapple Waste Extract for Bromelain Purification by Membrane Processing. J. Food Sci. Technol. 2015, 52, 7103–7112. [Google Scholar] [CrossRef]
- De Freitas Coêlho, D.; Silveira, E.; Tambourgi, E.B. Purification Processes and Market Potential of Bromelain in Brazil. J. Chem. Chem. Eng. 2014, 8, 882–888. [Google Scholar] [CrossRef]
- Nor, M.Z.M.; Ramchandran, L.; Duke, M.; Vasiljevic, T. Separation of Bromelain from Crude Pineapple Waste Mixture by a Two-Stage Ceramic Ultrafiltration Process. Food Bioprod. Process. 2016, 98, 142–150. [Google Scholar] [CrossRef]
- Ketnawa, S.; Chaiwut, P.; Rawdkuen, S. Pineapple Wastes: A Potential Source for Bromelain Extraction. Food Bioprod. Process. 2012, 90, 385–391. [Google Scholar] [CrossRef]
- Amid, A.; Ismail, N.A.; Yusof, F.; Salleh, H.M. Expression, Purification, and Characterization of a Recombinant Stem Bromelain from Ananas comosus. Process Biochem. 2011, 46, 2232–2239. [Google Scholar] [CrossRef]
- Bernela, M.; Ahuja, M.; Thakur, R. Enhancement of Anti-Inflammatory Activity of Bromelain by Its Encapsulation in Katira Gum Nanoparticles. Carbohydr. Polym. 2016, 143, 18–24. [Google Scholar] [CrossRef]
- Bala, M.; Mel, M.; Jami, M.S.; Amid, A.; Salleh, H.M. Kinetic Studies on Recombinant Stem Bromelain. Adv. Enzym. Res. 2013, 1, 52–60. [Google Scholar] [CrossRef]
- Arshad, Z.I.M.; Amid, A.; Yusof, F.; Jaswir, I.; Ahmad, K.; Loke, S.P. Bromelain: An Overview of Industrial Application and Purification Strategies. Appl. Microbiol. Biotechnol. 2014, 98, 7283–7297. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, R.S.; Umesh Hebbar, H. Extraction of Bromelain from Pineapple Core and Purification by RME and Precipitation Methods. Sep. Purif. Technol. 2013, 111, 90–97. [Google Scholar] [CrossRef]
- Tap, F.M.; Majid, F.A.; Khaidurin, N.B. Structure Prediction of Stem Bromelain from Pineapples. Int. J. Appl. Eng. Res. 2016, 11, 6109–6111. [Google Scholar]
- Costa, H.B.; Fernandes, P.M.B.; Romão, W.; Ventura, J.A. A New Procedure Based on Column Chromatography to Purify Bromelain by Ion Exchange plus Gel Filtration Chromatographies. Ind. Crops Prod. 2014, 59, 163–168. [Google Scholar] [CrossRef]
- Hale, L.P.; Greer, P.K.; Trinh, C.T.; James, C.L. Proteinase Activity and Stability of Natural Bromelain Preparations. Int. Immunopharmacol. 2005, 5, 783–793. [Google Scholar] [CrossRef]
- Maynard, R.; Antonio, D.R.; Angelica, A.; Dela Cruz, C.; Quinto, A.S.; Cordero, P.R.; Natalia, M.; Dimaano, R. Bioethanol Production from Pineapple (Ananas comosus) Peelings Using Saccharomyces Cerevisiae as Fermenting Yeast with Focus on Fermentation PH. Int. J. Eng. Res. Technol. 2015, 4, 356–360. [Google Scholar]
- Tropea, A.; Wilson, D.; Torre, L.G.l.; Curto, R.B.L.; Saugman, P.; Troy-Davies, P.; Dugo, G.; Waldron, K.W. Bioethanol Production from Pineapple Wastes. J. Food Res. 2014, 3, 60–70. [Google Scholar] [CrossRef]
- Praveena, R.J.; Estherlydia, D. Comparative Study of Phytochemical Screening and Antioxidant Capacities of Vinegar Made from Peel and Fruit of Pineapple (Ananas comosus L.). Int. J. Pharma Bio Sci. 2014, 5, 394–403. [Google Scholar]
- Tanamool, V.; Chantarangsee, M.; Soemphol, W. Simultaneous Vinegar Fermentation from a Pineapple By-Product Using the Co-Inoculation of Yeast and Thermotolerant Acetic Acid Bacteria and Their Physiochemical Properties. 3 Biotech 2020, 10, 115. [Google Scholar] [CrossRef]
- Joy, P.P. Benefits and Uses of Pineapple. Pineapple Res. Stn. (Kerala Agric. Univ.) 2010, 686670. [Google Scholar] [CrossRef]
- Shu, H.; Sun, W.; Xu, G.; Zhan, R.; Chang, S. The Situation and Challenges of Pineapple Industry in China. Agric. Sci. 2019, 10, 683–688. [Google Scholar] [CrossRef]
- Williams, P.A.; Crespo, O.; Atkinson, C.J.; Essegbey, G.O. Impact of Climate Variability on Pineapple Production in Ghana. Agric. Food Secur. 2017, 6, 26. [Google Scholar] [CrossRef]
- Hamzah, A.F.A.; Hamzah, M.H.; Man, H.C.; Jamali, N.S.; Siajam, S.I.; Ismail, M.H. Recent Updates on the Conversion of Pineapple Waste (Ananas comosus) to Value-Added Products, Future Perspectives and Challenges. Agronomy 2021, 11, 2221. [Google Scholar] [CrossRef]
- Banerjee, S.; Ranganathan, V.; Patti, A.; Arora, A. Valorisation of Pineapple Wastes for Food and Therapeutic Applications. Trends Food Sci. Technol. 2018, 82, 60–70. [Google Scholar] [CrossRef]
- Iwuchukwu, J.C.; Nwobodo Cynthia, E.; Udoye, C.E. Problems and Prospects of Pineapple Production in Enugu State, Nigeria. J. Agric. Ext. 2017, 21, 167–180. [Google Scholar] [CrossRef][Green Version]
| Adsorbent | Preparation Method | Dye Removed | pH | Adsorption Capacity (mg g−1) | Isotherm | References |
|---|---|---|---|---|---|---|
| Titanium dioxide nano bio-adsorbent (TiO2L) based on Ananas comosus leaf extract | Sol gel | Victoria blue | 6 | 83 | Langmuir | [51] |
| Pineapple leaf fibers-cl-poly (acrylic acid-co-2-dimethyl amino ethyl acrylate) | Hydrogel | Methyl violet | 3 | 625 | Freundlich | [52] |
| Pineapple stem | Powder | Methylene blue | 2–4 | 119.05 | Langmuir | [53] |
| Activated carbon pineapple crown | Chemical activation using NaOH and pyrolysis | Methylene blue | 6 | 292 | Langmuir | [54] |
| Pineapple leaf | Powder | Remazol brilliant blue R | N/A | 9.58 | Langmuir | [55] |
| Pineapple bark | Powder | Congo red | 9.8 | N/A | N/A | [56] |
| Pineapple bark | Powder | Brilliant green | 9.8 | N/A | N/A | [56] |
| Pineapple bark | Powder | Methylene blue | 9.8 | N/A | N/A | [56] |
| Cellulose acetate from ananas cosmus leave | Ethanol and toluene (1:2) | Direct red | N/A | 71.43 | Freundlich | [57] |
| Pineapple peel | Powder | Eosin yellow | N/A | 11.76 | Langmuir | [58] |
| Pineapple leaf powder | Powder | Methylene blue | 4 | 52.6 | Langmuir | [59] |
| Surfactant-modified pineapple leaf powder | Hydrogel | Methylene blue | 4 | 52.6 | Langmuir | [59] |
| Pineapple leaf powder | Powder | Methylene orange | 3 | 47.6 | Langmuir | [59] |
| Surfactant-modified pineapple leaf powder | Hydrogel | Methylene orange | 3 | 47.6 | Langmuir | [59] |
| Adsorbents | Heavy Metal Adsorbed | pH | Maximum Adsorption Capacity (mg g−1) | Isotherm Representing Best the Adsorption | References |
|---|---|---|---|---|---|
| KMnO4 modified carbon from pineapple leaf fiber waste | Fe3+ | 4.7 | 25.25 | Langmuir-Freundlich | [66] |
| APF | Cr (VI) | 3 | 133 | Langmuir | [67] |
| APF-PEI | Cr (VI) | 3 | 222 | Langmuir | [67] |
| PCL | Cr(VI) | 1.5 | Liu model | [68] | |
| Cr (III) | 5 | ||||
| APF-PEI | Cu2+ | 5 | 237 | Langmuir | [69] |
| Pb2+ | 5 | 165 | |||
| MPPF-DM70 | Cu2+ | 5.5 | 65.98 | Langmuir | [70] |
| Cd2+ | 7.5 | 102.92 | |||
| Pb2+ | 5.5 | 111.41 | |||
| Pineapple leaf fiber | Pb2+ | 6 | 63.92 | Langmuir | [71] |
| Cd2+ | 6 | 48.02 | |||
| Chemically oxidized pineapple fruit peel | Cd2+ | 4 | 42.10 | Langmuir | [72] |
| Pb2+ | 28.55 | ||||
| Natural pineapple plant stem | Pb2+ | 5 | 14.25 | Langmuir | [73] |
| Oxylic acid pineapple plant stem | Pb2+ | 4 | 30.47 | Langmuir | [73] |
| DETA coated pineapple aerogel | Ni2+ | N/A | 49.00 | Langmuir-Freundlich | [74] |
| Calcium | Energy | Carbohydrates | Dietary Fiber | Iron | Magnesium | Protein | Phosphorous |
|---|---|---|---|---|---|---|---|
| 16 mg | 52 Calories | 13.7 gm | 1.4 gm | 0.28 mg | 12 mg | 0.54 g | 11 mg |
| Potassium | Vitamin A | Vitamin B1 | Vitamin B2 | Vitamin C | Vitamin B3 | Vitamin B6 | Zinc |
| 150 mg | 130 I.U | 0.079 mg | 0.031 mg | 24 mg | 0.489 mg | 0.110 mg | 0.10 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fouda-Mbanga, B.G.; Tywabi-Ngeva, Z. Application of Pineapple Waste to the Removal of Toxic Contaminants: A Review. Toxics 2022, 10, 561. https://doi.org/10.3390/toxics10100561
Fouda-Mbanga BG, Tywabi-Ngeva Z. Application of Pineapple Waste to the Removal of Toxic Contaminants: A Review. Toxics. 2022; 10(10):561. https://doi.org/10.3390/toxics10100561
Chicago/Turabian StyleFouda-Mbanga, Bienvenu Gael, and Zikhona Tywabi-Ngeva. 2022. "Application of Pineapple Waste to the Removal of Toxic Contaminants: A Review" Toxics 10, no. 10: 561. https://doi.org/10.3390/toxics10100561
APA StyleFouda-Mbanga, B. G., & Tywabi-Ngeva, Z. (2022). Application of Pineapple Waste to the Removal of Toxic Contaminants: A Review. Toxics, 10(10), 561. https://doi.org/10.3390/toxics10100561