Effects of a 24-Week Exercise Program on Functional Fitness, Oxidative Stress, and Salivary Cortisol Levels in Elderly Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Physical Exercise Intervention Program
2.3. Anthropometric Measurements
2.4. Functional Fitness Tests
- −
- handgrip strength test (HST): upper body strength;
- −
- chair sit-to-stand test (CSST): lower body strength;
- −
- back scratch test (BST): upper body (shoulder) flexibility;
- −
- v-sit and reach test (V-SRT): lower body (lower back and hamstring muscles) flexibility;
- −
- timed up and go test (TUG): dynamic balance
- −
- six-min walk test (6MWT): aerobic endurance.
2.4.1. Handgrip Strength Test (HST)
2.4.2. Chair Sit-to-Stand Test (CSST)
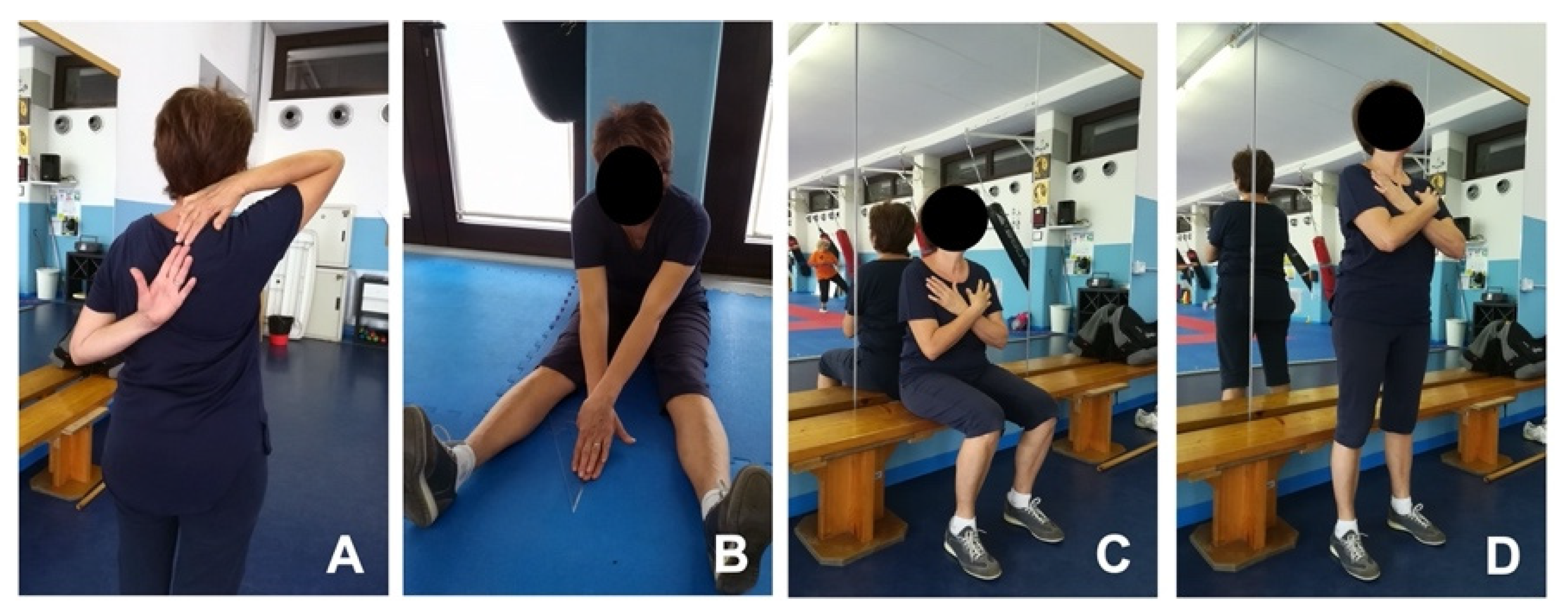
2.4.3. Back Scratch Test (BST)
2.4.4. V-Sit and Reach Test (V-SRT)
2.4.5. Timed Up and Go Test (TUG)
2.4.6. Six-Min Walk Test (6MWT)
2.5. Biochemical Analyses
2.5.1. Stress Hormone
2.5.2. Oxidative Stress
2.6. QoL Questionnaire
2.7. Statistical Analysis
3. Results
3.1. Assessment of Anthropometric Parameters
3.2. Effects of 24-Week Intervention Program on Senior Functional Fitness Tests
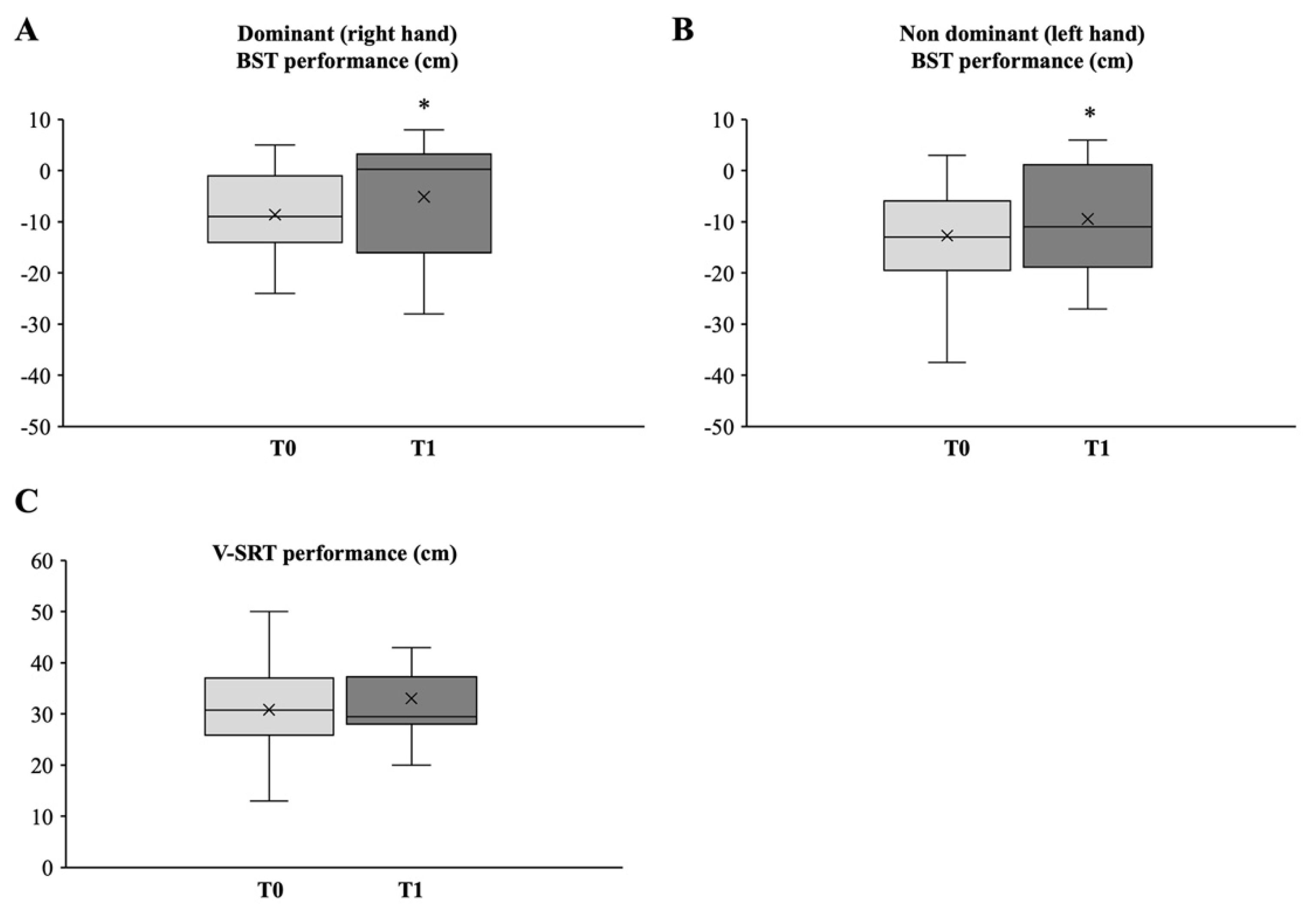
3.2.1. Upper and Lower Body Strength
3.2.2. Upper and Lower Body Flexibility
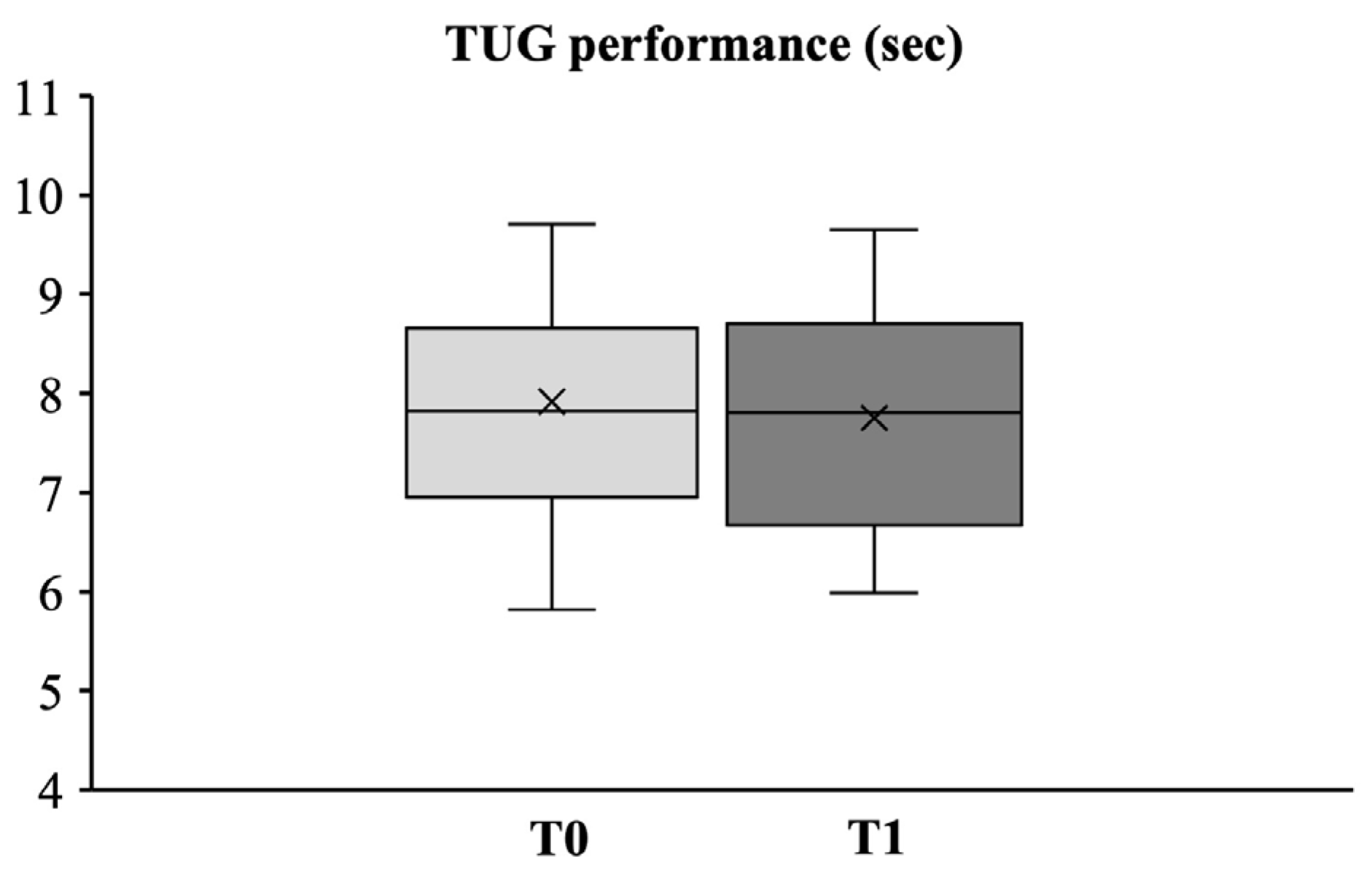
3.2.3. Dynamic Balance
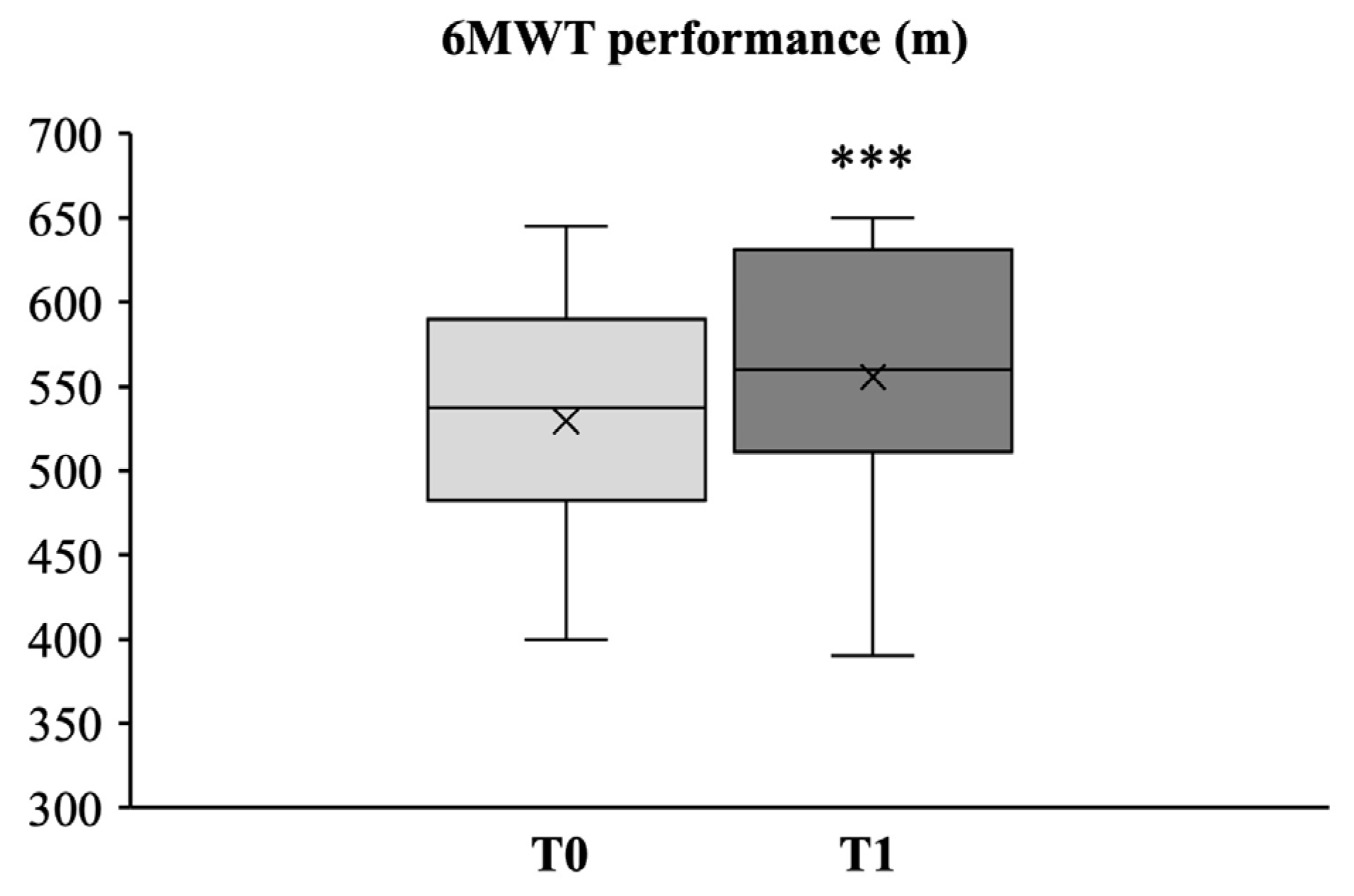
3.2.4. Aerobic Endurance
3.3. Effects of Functional Fitness on Salivary Cortisol and Plasma Oxidative Stress Levels
3.3.1. Stress Hormone
3.3.2. Oxidative Stress
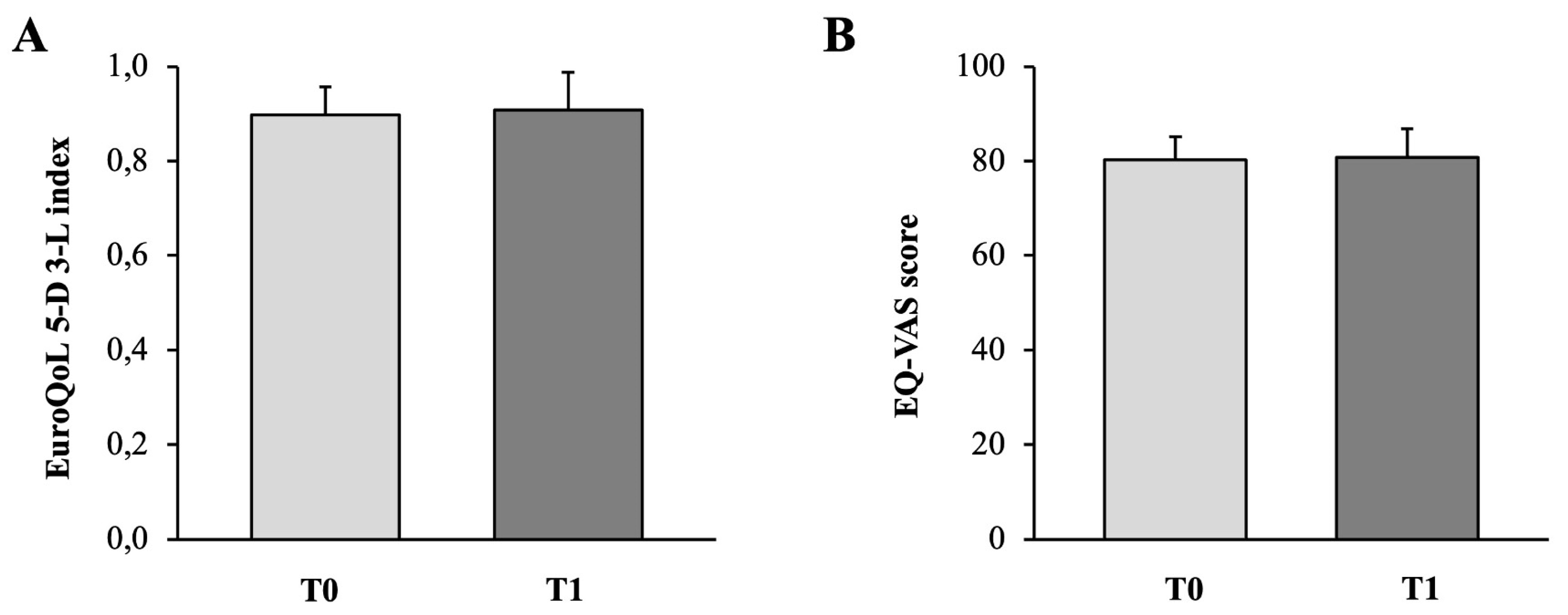
3.4. Quality of Life Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Ukraintseva, S.; Arbeev, K.; Duan, M.; Akushevich, I.; Kulminski, A.; Stallard, E.; Yashin, A. Decline in biological resilience as key manifestation of aging: Potential mechanisms and role in health and longevity. Mech. Ageing Dev. 2021, 194, 111418. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.S.; Singer, B.D.; Vaughan, D.E. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017, 16, 624–633. [Google Scholar] [CrossRef]
- Fingerman, K.L.; Ng, Y.T.; Huo, M.; Birditt, K.S.; Charles, S.T.; Zarit, S. Functional Limitations, Social Integration, and Daily Activities in Late Life. J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 1937–1947, Correction in J. Gerontol. B Psychol. Sci. Soc. Sci. 2021, 76, 2054. [Google Scholar] [CrossRef] [PubMed]
- Siqueira Rodrigues, B.G.; Ali Cader, S.; Bento Torres, N.V.; Oliveira, E.M.; Martin Dantas, E.H. Pilates method in personal autonomy, static balance and quality of life of elderly females. J. Bodyw. Mov. Ther. 2010, 14, 195–202. [Google Scholar] [CrossRef]
- Pernambuco, C.S.; Rodrigues, B.M.; Bezerra, J.C.; Carrielo, A.; Fernandes, A.D.; Vale, R.; Dantas, E. Quality of life, elderly and physical activity. Health 2012, 4, 88–93. [Google Scholar] [CrossRef]
- Moraes, H.; Deslandes, A.; Maciel-Pinheiro Pde, T.; Corrêa, H.; Laks, J. Cortisol, DHEA, and depression in the elderly: The influence of physical capacity. Arq. Neuropsiquiatr. 2016, 74, 456–461. [Google Scholar] [CrossRef]
- Jacomini, A.M.; Dias, D.D.; Brito, J.O.; da Silva, R.F.; Monteiro, H.L.; Llesuy, S.; De Angelis, K.; Amaral, S.L.; Zago, A.S. Influence of estimated training status on anti and pro-oxidant activity, nitrite concentration, and blood pressure in middle-aged and older women. Front. Physiol. 2017, 8, 122. [Google Scholar] [CrossRef]
- Zaleski, A.L.; Taylor, B.A.; Panza, G.A.; Wu, Y.; Pescatello, L.S.; Thompson, P.D.; Fernandez, A.B. Coming of Age: Considerations in the Prescription of Exercise for Older Adults. Methodist Debakey Cardiovasc. J. 2016, 12, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Toraman, N.F. Short term and long term detraining: Is there any difference between young-old and old people? Br. J. Sports Med. 2005, 39, 561–564. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Development and validation of a functional fitness test for community-residing older adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual, 1st ed.; Human Kinetics: Champaign, IL, USA, 2001; pp. 1–176. [Google Scholar]
- Jones, C.J.; Rikli, R.E. Measuring functional fitness of older adults. J. Act. Aging 2002, 1, 24–30. [Google Scholar]
- Stathokostas, L.; McDonald, M.W.; Little, R.M.; Paterson, D.H. Flexibility of older adults aged 55-86 years and the influence of physical activity. J. Aging Res. 2013, 2013, 743843. [Google Scholar] [CrossRef]
- Di Lorito, C.; Long, A.; Byrne, A.; Harwood, R.H.; Gladman, J.R.F.; Schneider, S.; Logan, P.; Bosco, A.; van der Wardt, V. Exercise interventions for older adults: A systematic review of meta-analyses. J. Sport Health Sci. 2021, 10, 29–47. [Google Scholar] [CrossRef]
- Macdonald, S.H.; Travers, J.; Shé, É.N.; Bailey, J.; Romero-Ortuno, R.; Keyes, M.; O’Shea, D.; Cooney, M.T. Primary care interventions to address physical frailty among community-dwelling adults aged 60 years or older: A meta-analysis. PLoS ONE 2020, 15, e0228821. [Google Scholar] [CrossRef]
- Tse, A.C.; Wong, T.W.; Lee, P.H. Effect of Low-intensity Exercise on Physical and Cognitive Health in Older Adults: A Systematic Review. Sports Med. Open. 2015, 1, 37. [Google Scholar] [CrossRef]
- Lee, M.; Lim, T.; Lee, J.; Kim, K.; Yoon, B. Optimal retraining time for regaining functional fitness using multicomponent training after long-term detraining in older adults. Arch. Gerontol. Geriatr. 2017, 73, 227–233. [Google Scholar] [CrossRef]
- Rikli, R.; Jones, C. Senior Fitness Test Manual, 2nd ed.; Editorial Human Kinetics: Fullerton, CA, USA, 2013; pp. 18–21. [Google Scholar]
- Kuzala, E.A.; Vargo, M.C. The relationship between elbow position and grip strength. Am. J. Occup. Ther. 1992, 46, 509–512. [Google Scholar] [CrossRef]
- Häger-Ross, C.; Rösblad, B. Norms for grip strength in children aged 4–16 years. Acta Paediatr. 2002, 91, 617–625. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Functional Fitness Normative Scores for Community-Residing Older Adults, Ages 60–94. J. Aging Phys. Act. 1999, 7, 162–181. [Google Scholar] [CrossRef]
- Heyward, V.H.; Gibson, A. Advanced Fitness Assessment and Exercise Prescription, 7th ed.; Human Kinetics: Champaign, IL, USA, 2014; p. 315. [Google Scholar]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Pacini, S.; Branca, J.J.; Gulisano, M.; Levi Micheli, M.; Ceroti, M.; Ruggiero, M.; Morucci, G. Salivary testosterone and cortisol levels to assess conditioning training program in rugby union players. Med. Sport 2014, 67, 449–463. [Google Scholar]
- Trotti, R.; Carratelli, M.; Barbieri, M. Performance and clinical application of a new, fast method for the detection of hydroperoxides in serum. Panminerva Med. 2002, 44, 37–40. [Google Scholar]
- Cornelli, U.; Belcaro, G.; Cesarone, M.R.; Finco, A. Analysis of oxidative stress during the menstrual cycle. Reprod. Biol. Endocrinol. 2013, 11, 74. [Google Scholar] [CrossRef]
- Mancini, S.; Mariani, F.; Sena, P.; Benincasa, M.; Roncucci, L. Myeloperoxidase expression in human colonic mucosa is related to systemic oxidative balance in healthy subjects. Redox Rep. 2017, 22, 399–407. [Google Scholar] [CrossRef]
- Martinovic, J.; Dopsaj, V.; Dopsaj, M.J.; Kotur-Stevuljevic, J.; Vujovic, A.; Stefanovic, A.; Nesic, G. Long-term effects of oxidative stress in volleyball players. Int. J. Sports Med. 2009, 30, 851–856. [Google Scholar] [CrossRef]
- Fukuda, T.; Kurano, M.; Fukumura, K.; Yasuda, T.; Iida, H.; Morita, T.; Yamamoto, Y.; Takano, N.; Komuro, I.; Nakajima, T. Cardiac rehabilitation increases exercise capacity with a reduction of oxidative stress. Korean Circ. J. 2013, 43, 481–487. [Google Scholar] [CrossRef]
- Scalone, L.; Cortesi, P.A.; Ciampichini, R.; Belisari, A.; D’Angiolella, L.S.; Cesana, G.; Mantovani, L.G. Italian population-based values of EQ-5D health states. Value Health 2013, 16, 814–822. [Google Scholar] [CrossRef]
- National Research Council (US) Committee on Diet and Health. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press: Washington, DC, USA, 1989; PMID:25032333. [Google Scholar]
- Body Composition Data for Individuals 8 Years of Age and Older: U.S. Population, 1999–2004–2010. In Vital and Health Statistics; Series 11; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2010; No. 250.
- Borrud, L.G.; Flegal, K.M.; Looker, A.C.; Everhart, J.E.; Harris, T.B.; Shepherd, J.A. Body composition data for individuals 8 years of age and older: U.S. population, 1999–2004. Vital Health Stat. 11 2010, 250, 1–87. [Google Scholar]
- Activity and Health Research. Allied Dunbar National Fitness Survey: Main Findings; Sports Council and Health Education Authority: London, UK, 1992. [Google Scholar]
- YMCA of the USA. YMCA Fitness Testing and Assessment Manual; Human Kinetics: Champaign, IL, USA, 2000; 315 p. [Google Scholar]
- Monteiro, A.M.; Silva, P.; Forte, P.; Carvalho, J. The Effects of Daily Physical Activity on Functional Fitness, Isokinetic Strength and Body Composition in Elderly Community-Dwelling Women. J. Hum. Sports Exerc. 2019, 14, 385–398. [Google Scholar] [CrossRef]
- Liffiton, J.A.; Horton, S.; Baker, J.; Weir, P.L. Successful aging: How does physical activity influence engagement with life? Eur. Rev. Aging Phys. Act. 2012, 9, 103–108. [Google Scholar] [CrossRef]
- Freiberger, E.; Häberle, L.; Spirduso, W.W.; Zijlstra, G.A.R. Long-Term Effects of Three Multicomponent Exercise Interventions on Physical Performance and Fall-Related Psychological Outcomes in Community-Dwelling Older Adults: A Randomized Controlled Trial. J. Am. Geriatr. Soc. 2012, 60, 437–446. [Google Scholar] [CrossRef]
- Monteiro, A.M.; Forte, P.; Carvalho, J.; Barbosa, T.M.; Morais, J.E. Relationship between Fear of Falling and Balance Factors in Healthy Elderly Women: A Confirmatory Analysis. J. Women Aging 2021, 33, 57–69. [Google Scholar] [CrossRef]
- Monteiro, A.M.; Forte, P.; Carvalho, M.J. The Effect of Three Different Training Programs in Elderly Women’s Isokinetic Strength. Motricidade 2020, 16, 84–93. [Google Scholar]
- de Melo, L.L.; Menec, V.H.; Ready, A.E. Relationship of functional fitness with daily steps in community-dwelling older adults. J. Geriatr. Phys. Ther. 2014, 37, 116–120. [Google Scholar] [CrossRef]
- Chetty, L.; Ramklass, S.; McKune, A. The effects of a structured group exercise programme on functional fitness of older persons living in old-age homes. Ageing Soc. 2019, 39, 1857–1872. [Google Scholar] [CrossRef]
- Yang, Y.-P.; Lin, H.C.; Chen, K.-M. Functional Fitness in Older Adults: A Systematic Review and Meta-analysis. Top. Geriatr. Rehabil. 2019, 35, 238–247. [Google Scholar] [CrossRef]
- Carvalho, M.J.; Marques, E.; Mota, J. Training and Detraining Effects on Functional Fitness after a Multicomponent Training in Older Women. Gerontology 2009, 55, 41–48. [Google Scholar] [CrossRef]
- Monteiro, A.M.; Bartolomeu, R.F.; Forte, P.; Carvalho, J. The effects of three different types of training in functional fitness and body composition in older women. J. Sport Health Res. 2019, 11, 289–304. [Google Scholar]
- Lohne-Seiler, H.; Kolle, E.; Anderssen, S.A.; Hansen, B.H. Musculoskeletal fitness and balance in older individuals (65–85 years) and its association with steps per day: A cross sectional study. BMC Geriatr. 2016, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W. Reference values for the timed up and go test: A descriptive meta-analysis. J. Geriatr. Phys. Ther. 2006, 29, 64–68. [Google Scholar] [CrossRef]
- Rowiński, R.; Kozakiewicz, M.; Kędziora-Kornatowska, K.; Hübner-Woźniak, E.; Kędziora, J. Markers of oxidative stress and erythrocyte antioxidant enzyme activity in older men and women with differing physical activity. Exp. Gerontol. 2013, 48, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Fraile-Bermúdez, A.B.; Kortajarena, M.; Zarrazquin, I.; Maquibar, A.; Yanguas, J.J.; Sánchez-Fernández, C.E.; Gil, J.; Irazusta, A.; Ruiz-Litago, F. Relationship between physical activity and markers of oxidative stress in independent community-living elderly individuals. Exp. Gerontol. 2015, 70, 26–31. [Google Scholar] [CrossRef]
- Gamberi, T.; Gorini, G.; Fiaschi, T.; Morucci, G.; Pratesi, S.; Fittipaldi, L.; Gulisano, M.; Modesti, P.A.; Modesti, A.; Magherini, F. Effect of Functional Fitness on Plasma Oxidation Level in Elders: Reduction of the Plasma Oxidants and Improvement of the Antioxidant Barrier. Am. J. Sports Sci. 2018, 6, 55–64. [Google Scholar] [CrossRef]
- Toraman, N.F.; Ayceman, N. Effects of six weeks of detraining on retention of functional fitness of old people after nine weeks of multicomponent training. Br. J. Sports Med. 2005, 39, 565–568. [Google Scholar] [CrossRef]
- Hallage, T.; Krause, M.P.; Haile, L.; Miculis, C.P.; Nagle, E.F.; Reis, R.S.; Da Silva, S.G. The effects of 12 weeks of step aerobics training on functional fitness of elderly women. J. Strength Cond. Res. 2010, 24, 2261–2266. [Google Scholar] [CrossRef] [PubMed]
- Celestrin, C.P.; Rocha, G.Z.; Stein, A.M.; Guadagnini, D.; Tadelle, R.M.; Saad, M.J.A.; Oliveira, A.G. Effects of a four week detraining period on physical, metabolic, and inflammatory profiles of elderly women who regularly participate in a program of strength training. Eur. Rev. Aging Phys. Act. 2020, 17, 12. [Google Scholar] [CrossRef]
- Silva NDA, Menezes TN De. Functional capacity and its association with age and sex in an elderly population. Rev. Bras. Cineantropom. Desempenho Hum. 2014, 16, 359–370. [Google Scholar]


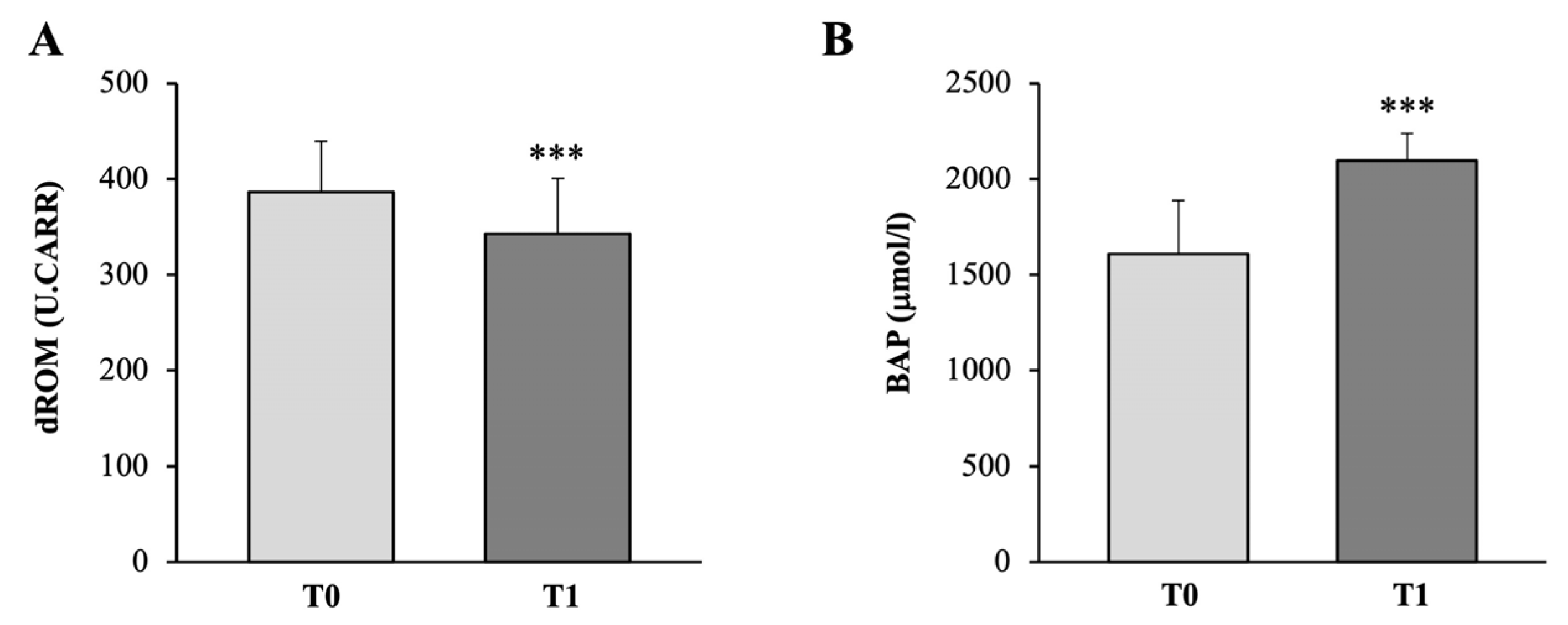
| d-ROMs Test | Standard Value |
|---|---|
| Normal values | 250–299 U.CARR 1 |
| Borderline values | 300–320 |
| Low-level oxidative stress | 321–340 |
| Middle-level oxidative stress | 341–400 |
| High-level oxidative stress | 401–500 |
| Very high-level oxidative stress | >501 |
| BAP Test | Standard Value |
|---|---|
| Normal values | >2200 µmol/L |
| Borderline values | 2200–2000 |
| Slight reduction | 1999–1800 |
| Moderate reduction | 1799–1600 |
| Strong reduction | 1599–1400 |
| Very strong reduction | <1400 |
| Measure | Baseline (T0) | Post-Intervention (T1) | p-Value |
|---|---|---|---|
| Age (years) * | 72.8 ± 7.5 | --- | --- |
| Height (m) * | 1.63 ± 0.08 | --- | --- |
| Weight (kg) * | 68.4 ± 8.9 | 67.6 ± 8.7 | 0.10 |
| BMI (kg/m2) * | 25.8 ± 2.0 | 25.5 ± 2.2 | 0.13 |
| Body fat (%) * | 37.2 ± 5.7 | 35.7 ± 6.1 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morucci, G.; Ryskalin, L.; Pratesi, S.; Branca, J.J.V.; Modesti, A.; Modesti, P.A.; Gulisano, M.; Gesi, M. Effects of a 24-Week Exercise Program on Functional Fitness, Oxidative Stress, and Salivary Cortisol Levels in Elderly Subjects. Medicina 2022, 58, 1341. https://doi.org/10.3390/medicina58101341
Morucci G, Ryskalin L, Pratesi S, Branca JJV, Modesti A, Modesti PA, Gulisano M, Gesi M. Effects of a 24-Week Exercise Program on Functional Fitness, Oxidative Stress, and Salivary Cortisol Levels in Elderly Subjects. Medicina. 2022; 58(10):1341. https://doi.org/10.3390/medicina58101341
Chicago/Turabian StyleMorucci, Gabriele, Larisa Ryskalin, Simone Pratesi, Jacopo J. V. Branca, Alessandra Modesti, Pietro Amedeo Modesti, Massimo Gulisano, and Marco Gesi. 2022. "Effects of a 24-Week Exercise Program on Functional Fitness, Oxidative Stress, and Salivary Cortisol Levels in Elderly Subjects" Medicina 58, no. 10: 1341. https://doi.org/10.3390/medicina58101341
APA StyleMorucci, G., Ryskalin, L., Pratesi, S., Branca, J. J. V., Modesti, A., Modesti, P. A., Gulisano, M., & Gesi, M. (2022). Effects of a 24-Week Exercise Program on Functional Fitness, Oxidative Stress, and Salivary Cortisol Levels in Elderly Subjects. Medicina, 58(10), 1341. https://doi.org/10.3390/medicina58101341











