Lithium Treatment Improves Cardiac Dysfunction in Rats Deprived of Rapid Eye Movement Sleep
Abstract
1. Introduction
2. Results
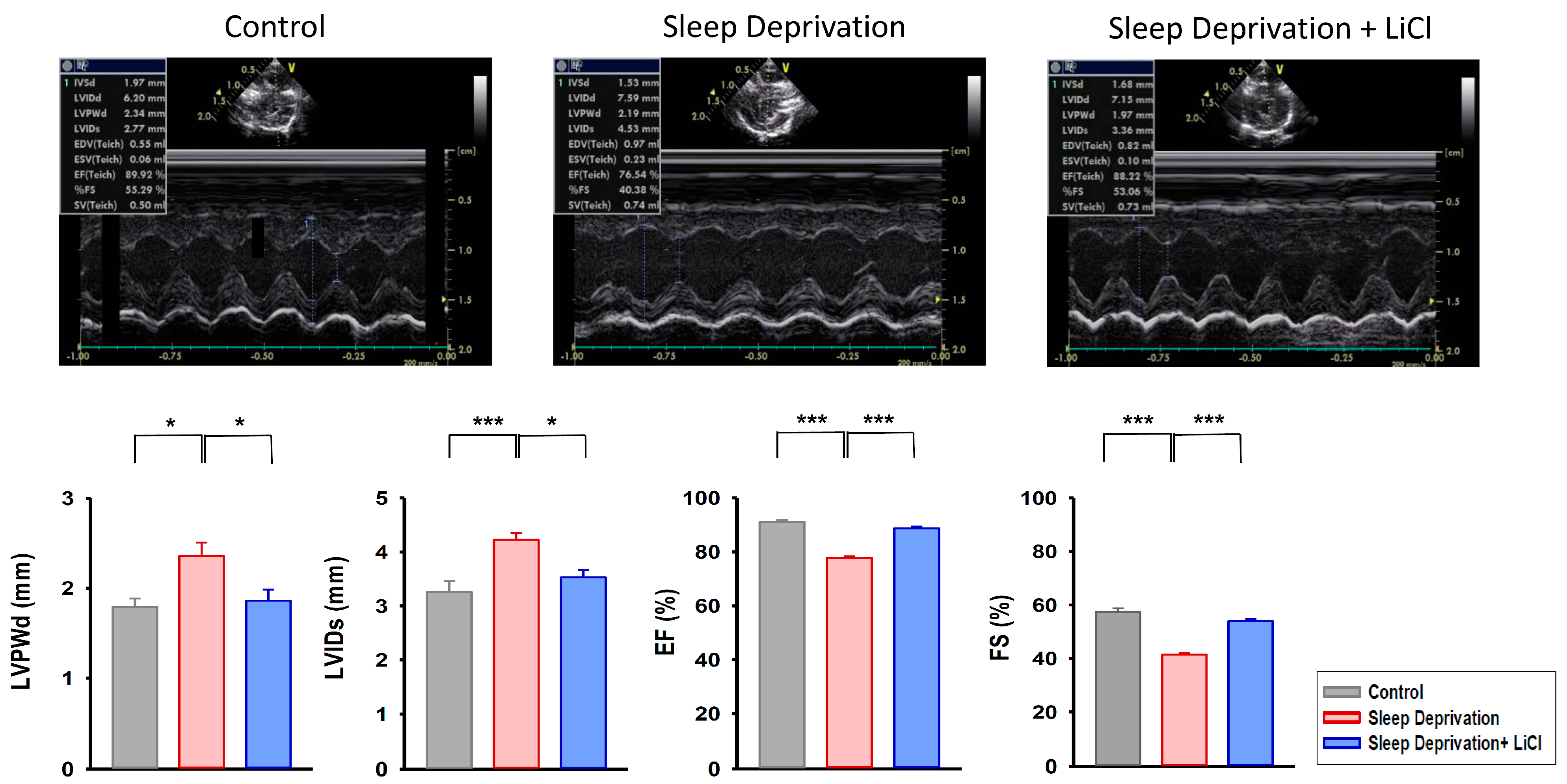
2.1. Effect of Lithium Treatment on Cardiac Function in REM Sleep-Deprived Rats
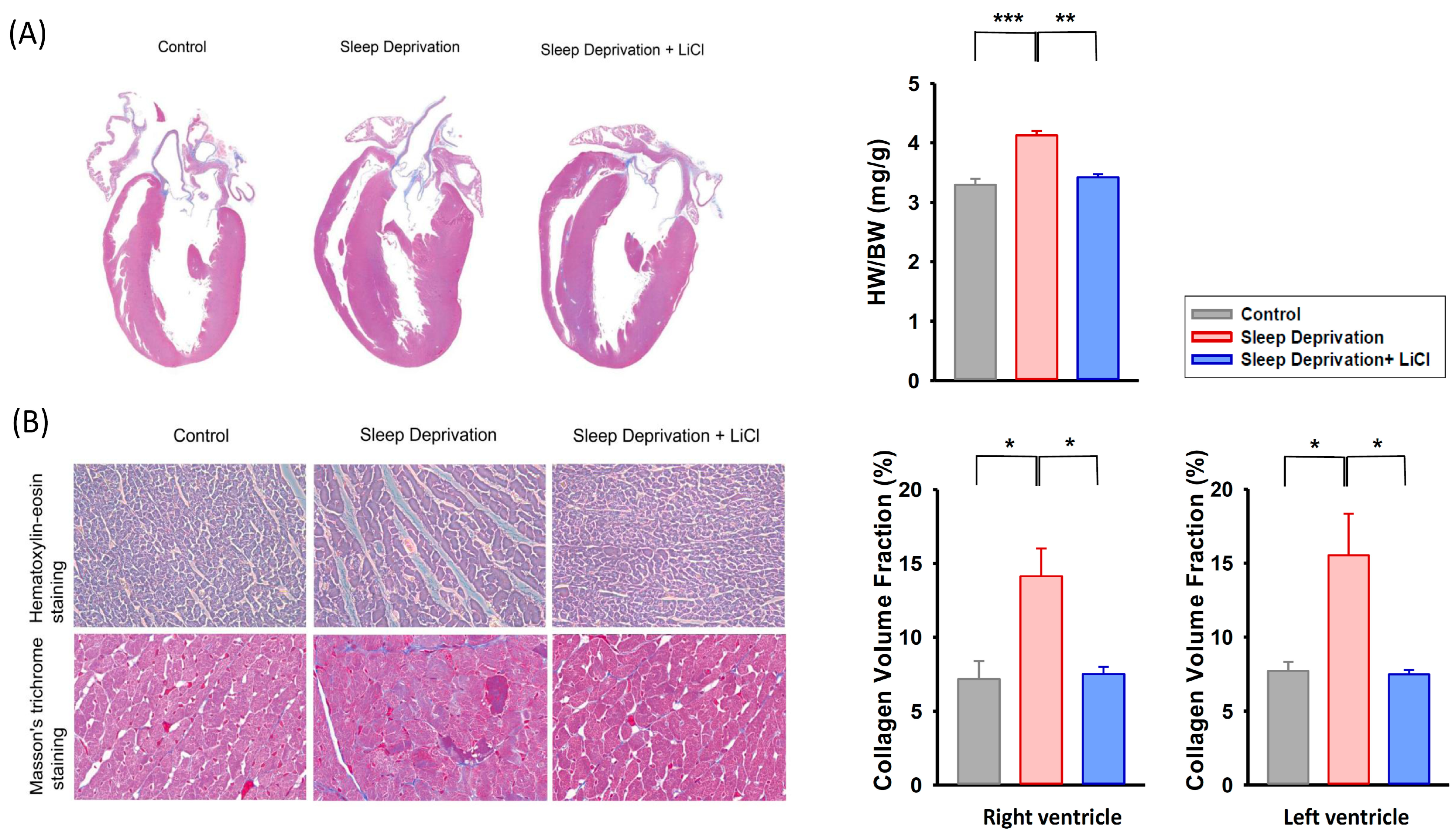
2.2. Effect of Lithium Treatment on Cardiac Fibrosis in REM Sleep-Deprived Rats
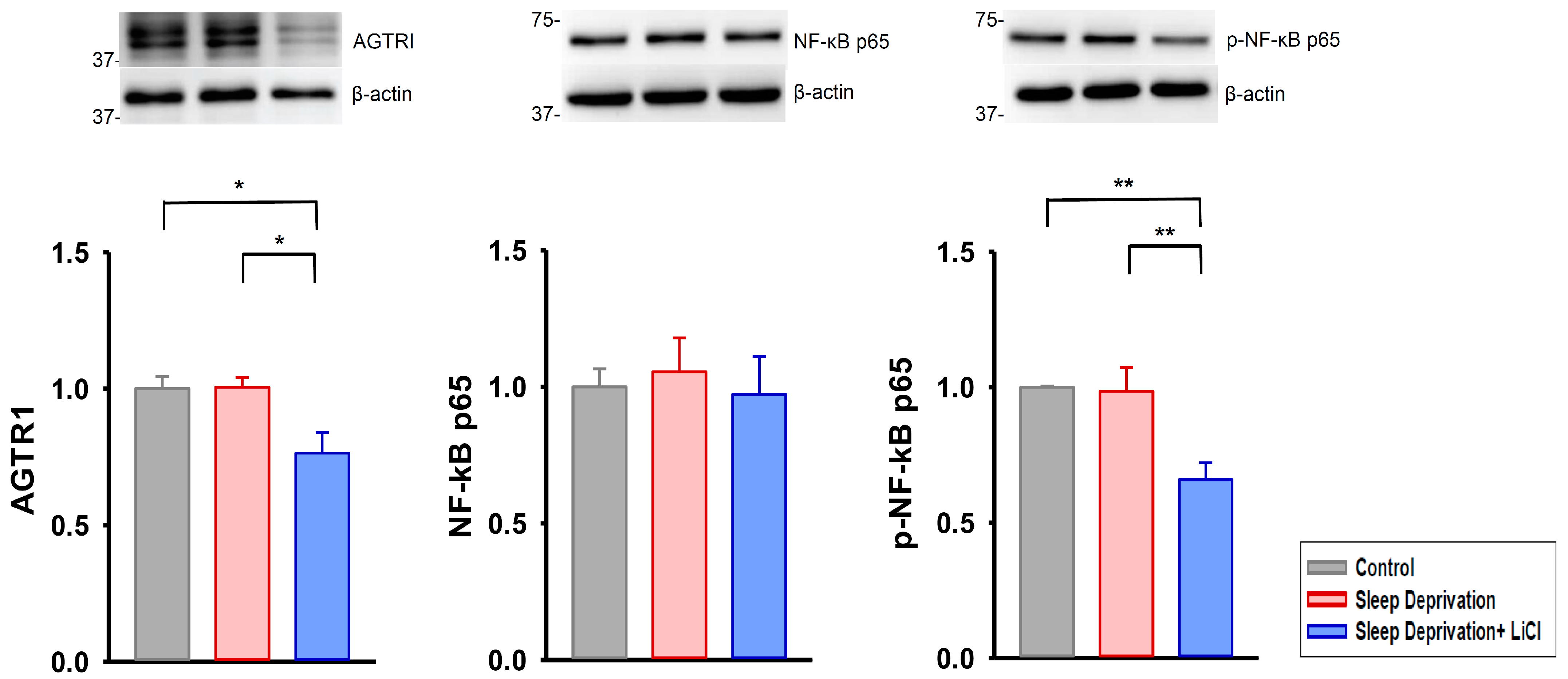
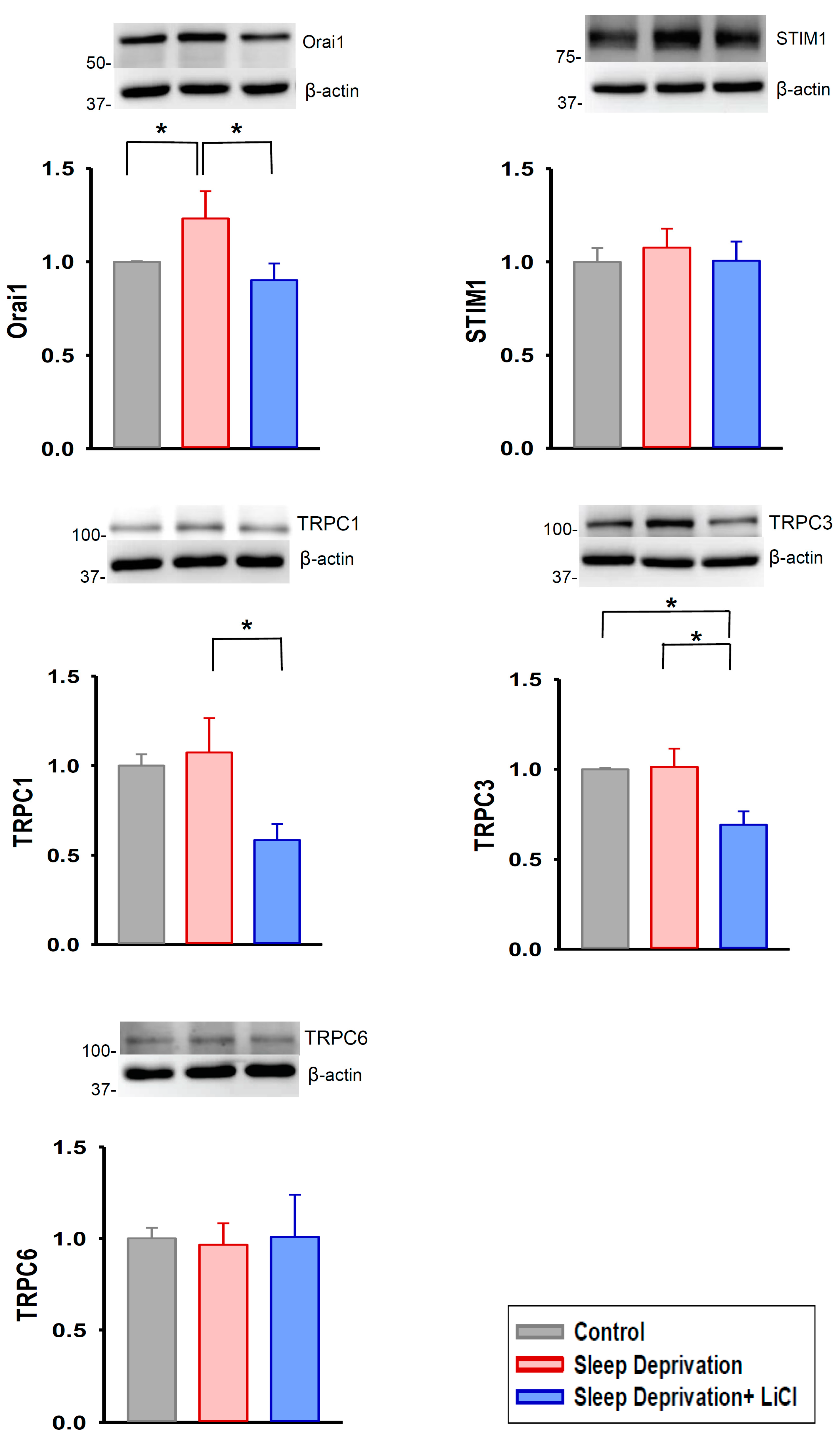
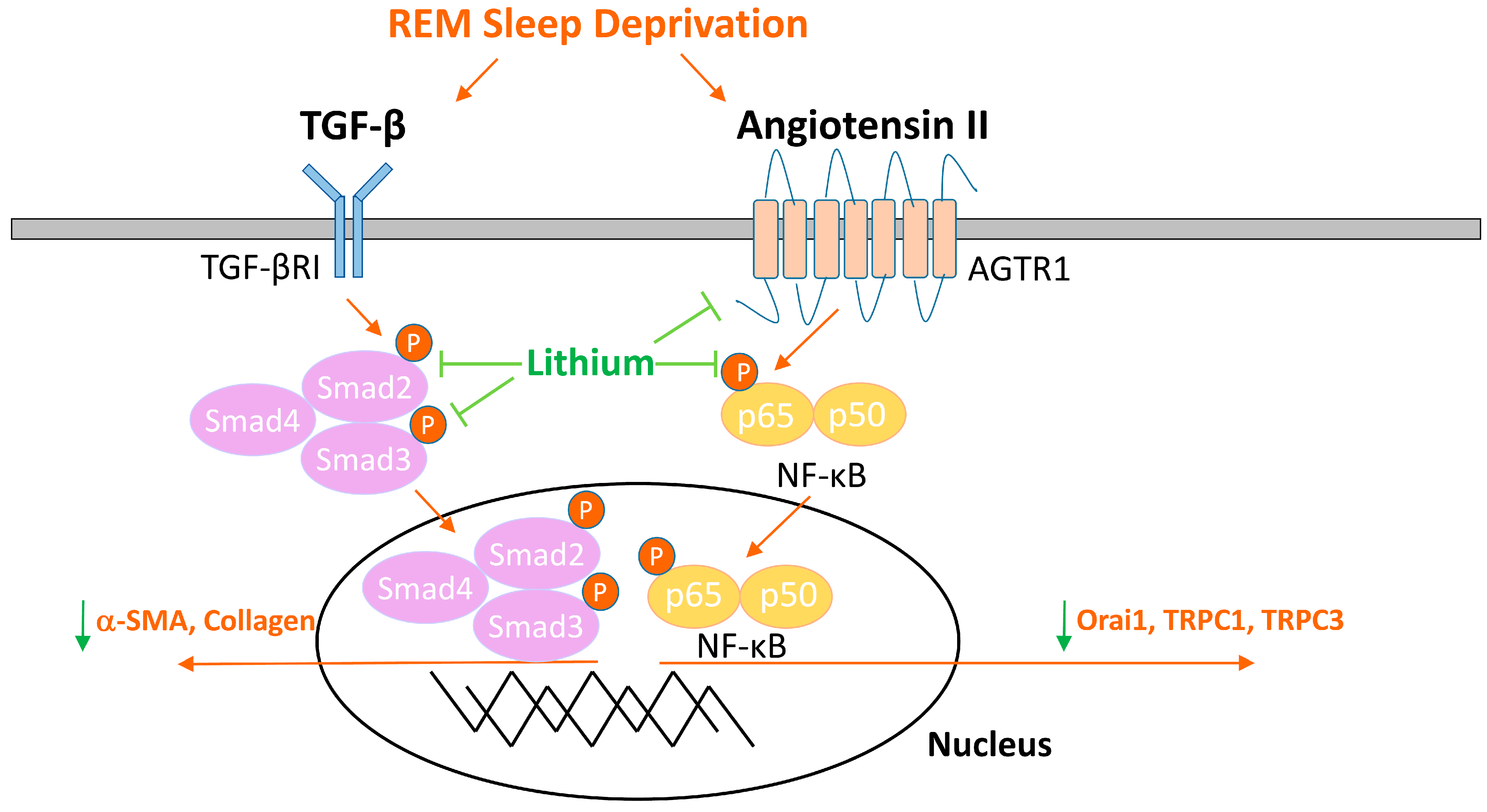
2.3. Lithium Downregulates Profibrotic Signaling in Rats Deprived of REM Sleep
3. Discussion
4. Materials and Methods
4.1. Animal Housing and Care
4.2. REM Sleep Deprivation Procedure and Lithium Treatment
4.3. Transthoracic Echocardiographic Examination
4.4. Histological Study
4.5. Western Blotting
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melo, M.C.A.; Abreu, R.L.C.; Linhares Neto, V.B.; de Bruin, P.F.C.; de Bruin, V.M.S. Chronotype and circadian rhythm in bipolar disorder: A systematic review. Sleep Med. Rev. 2017, 34, 46–58. [Google Scholar] [CrossRef]
- Gold, A.K.; Kinrys, G. Treating circadian rhythm disruption in bipolar disorder. Curr. Psychiatry Rep. 2019, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Zangani, C.; Casetta, C.; Saunders, A.S.; Donati, F.; Maggioni, E.; D’Agostino, A. Sleep abnormalities across different clinical stages of bipolar disorder: A review of EEG studies. Neurosci. Biobehav. Rev. 2020, 118, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Gessa, G.L.; Pani, L.; Serra, G.; Fratta, W. Animal models of mania. Adv. Biochem. Psychopharmacol. 1995, 49, 43–66. [Google Scholar] [PubMed]
- Valvassori, S.S.; Resende, W.R.; Dal-Pont, G.; Sangaletti-Pereira, H.; Gava, F.F.; Peterle, B.R.; Carvalho, A.F.; Varela, R.B.; Dal-Pizzol, F.; Quevedo, J. Lithium ameliorates sleep deprivation-induced mania-like behavior, hypothalamic-pituitary-adrenal (HPA) axis alterations, oxidative stress and elevations of cytokine concentrations in the brain and serum of mice. Bipolar Disord. 2017, 19, 246–258. [Google Scholar] [CrossRef]
- Andrabi, M.; Andrabi, M.M.; Kunjunni, R.; Sriwastva, M.K.; Bose, S.; Sagar, R.; Srivastava, A.K.; Mathur, R.; Jain, S.; Subbiah, V. Lithium acts to modulate abnormalities at behavioral, cellular, and molecular levels in sleep deprivation-induced mania-like behavior. Bipolar Disord. 2020, 22, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Dumaine, J.E.; Ashley, N.T. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R1062–R1069. [Google Scholar] [CrossRef]
- Giampá, S.Q.; Mônico-Neto, M.; de Mello, M.T.; Souza, H.S.; Tufik, S.; Lee, K.S.; Koike, M.K.; Dos Santos, A.A.; Antonio, E.L.; Serra, A.J.; et al. Paradoxical sleep deprivation causes cardiac dysfunction and the impairment is attenuated by resistance training. PLoS ONE 2016, 11, e0167029. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Mu, Y.; Xue, B.; Zhao, A.; Wang, D.; Chang, D.; Pan, Y.; Liu, J. Proteomic analysis of rat serum revealed the effects of chronic sleep deprivation on metabolic, cardiovascular and nervous system. PLoS ONE 2018, 13, e0199237. [Google Scholar] [CrossRef]
- Galvez-Contreras, A.Y.; Campos-Ordonez, T.; Lopez-Virgen, V.; Gomez-Plascencia, J.; Ramos-Zuniga, R.; Gonzalez-Perez, O. Growth factors as clinical biomarkers of prognosis and diagnosis in psychiatric disorders. Cytokine Growth Factor Rev. 2016, 32, 85–96. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Cardiac fibrosis. Cardiovasc. Res. 2021, 117, 1450–1488. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac fibrosis: The fibroblast awakens. Circ. Res. 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Chung, C.C.; Lin, Y.F.; Kao, Y.H.; Chen, Y.J. Lithium reduces migration and collagen synthesis activity in human cardiac fibroblasts by inhibiting store-operated Ca2+ entry. Int. J. Mol. Sci. 2021, 22, 842. [Google Scholar] [CrossRef] [PubMed]
- Prosser, J.M.; Fieve, R.R. Patients receiving lithium therapy have a reduced prevalence of neurological and cardiovascular disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 71, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Hsiao, C.Y.; Chiang, S.J.; Shen, R.S.; Lin, Y.K.; Chung, K.H.; Tsai, S.Y. Cardioprotective potential of lithium and role of fractalkine in euthymic patients with bipolar disorder. Aust. N. Z. J. Psychiatry 2021. accepted. [Google Scholar] [CrossRef]
- Chen, P.H.; Chiang, S.J.; Hsiao, C.Y.; Shen, R.S.; Lin, Y.K.; Chung, K.H.; Tsai, S.Y. Incidence and risk factors of sudden cardiac death in bipolar disorder across the lifespan. J. Affect. Disord. 2020, 274, 210–217. [Google Scholar] [CrossRef]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Yeh, Y.H.; Chen, Y.J. Calcium regulation on the atrial regional difference of collagen production activity in atrial fibrogenesis. Biomedicines 2021, 9, 686. [Google Scholar] [CrossRef]
- Bezchlibnyk, Y.B.; Wang, J.F.; McQueen, G.M.; Young, L.T. Gene expression differences in bipolar disorder revealed by cDNA array analysis of post-mortem frontal cortex. J. Neurochem. 2001, 79, 826–834. [Google Scholar] [CrossRef]
- Barbosa, I.G.; Ferreira, G.C.; Andrade Júnior, D.F.; Júnior, D.F.; Januário, C.R.; Belisário, A.R.; Bauer, M.E.; Simões ESilva, A.C. The renin angiotensin system and bipolar disorder: A systematic review. Protein Pept. Lett. 2020, 27, 520–528. [Google Scholar] [CrossRef]
- Harrison, P.J.; Hall, N.; Mould, A.; Al-Juffali, N.; Tunbridge, E.M. Cellular calcium in bipolar disorder: Systematic review and meta-analysis. Mol. Psychiatry 2021, 26, 4106–4116. [Google Scholar] [CrossRef]
- Wasserman, M.J.; Corson, T.W.; Sibony, D.; Cooke, R.G.; Parikh, S.V.; Pennefather, P.S.; Li, P.P.; Warsh, J.J. Chronic lithium treatment attenuates intracellular calcium mobilization. Neuropsychopharmacology 2004, 29, 759–769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Eder, P. Cardiac remodeling and disease: SOCE and TRPC signaling in cardiac pathology. Adv. Exp. Med. Biol. 2017, 993, 505–521. [Google Scholar]
- Ross, G.R.; Bajwa, T., Jr.; Edwards, S.; Emelyanova, L.; Rizvi, F.; Holmuhamedov, E.L.; Werner, P.; Downey, F.X.; Tajik, A.J.; Jahangir, A. Enhanced store-operated Ca2+ influx and ORAI1 expression in ventricular fibroblasts from human failing heart. Biol. Open 2017, 6, 326–332. [Google Scholar] [CrossRef]
- Mai, X.; Shang, J.; Liang, S.; Yu, B.; Yuan, J.; Lin, Y.; Luo, R.; Zhang, F.; Liu, Y.; Lv, X.; et al. Blockade of Orai1 store-operated calcium entry protects against renal fibrosis. J. Am. Soc. Nephrol. 2016, 27, 3063–3078. [Google Scholar] [CrossRef]
- Ong, H.L.; de Souza, L.B.; Ambudkar, I.S. Role of TRPC channels in store-operated calcium entry. Adv. Exp. Med. Biol. 2016, 898, 87–109. [Google Scholar]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Andreopoulos, S.; Wasserman, M.; Woo, K.; Li, P.P.; Warsh, J.J. Chronic lithium treatment of B lymphoblasts from bipolar disorder patients reduces transient receptor potential channel 3 levels. Pharmacogenomics J. 2004, 4, 365–373. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaeri, S.; Farjadian, S.; Emamghoreishi, M. Decreased levels of canonical transient receptor potential channel 3 protein in the rat cerebral cortex after chronic treatment with lithium or valproate. Res. Pharm. Sci. 2015, 10, 397–406. [Google Scholar]
- Du, T.; Rong, Y.; Feng, R.; Verkhratsky, A.; Peng, L. Chronic treatment with anti-bipolar drugs down-regulates gene expression of TRPC1 in neurons. Front. Cell. Neurosci. 2017, 10, 305. [Google Scholar] [CrossRef]
- Liao, Y.; Erxleben, C.; Yildirim, E.; Abramowitz, J.; Armstrong, D.L.; Birnbaumer, L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc. Natl. Acad. Sci. USA 2007, 104, 4682–4687. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, J.; Tong, X. Cross-talk between mechanosensitive ion channels and calcium regulatory proteins in cardiovascular health and disease. Int. J. Mol. Sci. 2021, 22, 8782. [Google Scholar] [CrossRef] [PubMed]
- Brasier, A.R.; Jamaluddin, M.; Han, Y.; Patterson, C.; Runge, M.S. Angiotensin II induces gene transcription through cell-type-dependent effects on the nuclear factor-kappaB (NF-kappaB) transcription factor. Mol. Cell. Biochem. 2000, 212, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.S.; Pan, C.H. Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell. Mol. Life Sci. 2008, 65, 1489–1508. [Google Scholar] [CrossRef] [PubMed]
- Young, C.N.; Davisson, R.L. Angiotensin-II, the brain, and hypertension: An update. Hypertension 2015, 66, 920–936. [Google Scholar] [CrossRef]
- Yang, L.X.; Guo, R.W.; Liu, B.; Wang, X.M.; Qi, F.; Guo, C.M.; Shi, Y.K.; Wang, H. Role of TRPC1 and NF-kappaB in mediating angiotensin II-induced Ca2+ entry and endothelial hyperpermeability. Peptides 2009, 30, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Cobos, J.C.; Trebak, M. TRPC channels in smooth muscle cells. Front. Biosci. Landmark Ed. 2010, 15, 1023–1039. [Google Scholar] [CrossRef]
- Eylenstein, A.; Schmidt, S.; Gu, S.; Yang, W.; Schmid, E.; Schmidt, E.M.; Alesutan, I.; Szteyn, K.; Regel, I.; Shumilina, E.; et al. Transcription factor NF-κB regulates expression of pore-forming Ca2+ channel unit, Orai1, and its activator, STIM1, to control Ca2+ entry and affect cellular functions. J. Biol. Chem. 2012, 287, 2719–2730. [Google Scholar] [CrossRef]
- Niemeyer, B.A. Changing calcium: CRAC channel (STIM and Orai) expression, splicing, and posttranslational modifiers. Am. J. Physiol. Cell. Physiol. 2016, 310, C701–C709. [Google Scholar] [CrossRef]
- Nassar, A.; Azab, A.N. Effects of lithium on inflammation. ACS Chem. Neurosci. 2014, 5, 451–458. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lee, F.L.; Peng, S.F.; Lin, K.H.; Chen, R.J.; Ho, T.J.; Tsai, F.J.; Padma, V.V.; Kuo, W.W.; Huang, C.Y. HSF1 phosphorylation by ERK/GSK3 suppresses RNF126 to sustain IGF-IIR expression for hypertension-induced cardiomyocyte hypertrophy. J. Cell. Physiol. 2018, 233, 979–989. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signaling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y. TGF-β in fibrosis by acting as a conductor for contractile properties of myofibroblasts. Cell. Biosci. 2019, 9, 98. [Google Scholar] [CrossRef] [PubMed]
- Ehanire, T.; Ren, L.; Bond, J.; Medina, M.; Li, G.; Bashirov, L.; Chen, L.; Kokosis, G.; Ibrahim, M.; Selim, A.; et al. Angiotensin II stimulates canonical TGF-β signaling pathway through angiotensin type 1 receptor to induce granulation tissue contraction. J. Mol. Med. Berl. 2015, 93, 289–302. [Google Scholar] [CrossRef]
- Ma, Z.G.; Yuan, Y.P.; Wu, H.M.; Zhang, X.; Tang, Q.Z. Cardiac fibrosis: New insights into the pathogenesis. Int. J. Biol. Sci. 2018, 14, 1645–1657. [Google Scholar] [CrossRef]
- Malhi, G.S.; Gessler, D.; Outhred, T. The use of lithium for the treatment of bipolar disorder: Recommendations from clinical practice guidelines. J. Affect. Disord. 2017, 217, 266–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.M.; Lin, S.Z.; Chang, N.C. Antiarrhythmic effect of lithium in rats after myocardial infarction by activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 77, 71–81. [Google Scholar] [CrossRef]
- Lee, T.M.; Lin, S.Z.; Chang, N.C. Effect of lithium on ventricular remodelling in infarcted rats via the Akt/mTOR signalling pathways. Biosci. Rep. 2017, 37, BSR20160257. [Google Scholar] [CrossRef]
- Kao, Y.H.; Liou, J.P.; Chung, C.C.; Lien, G.S.; Kuo, C.C.; Chen, S.A.; Chen, Y.J. Histone deacetylase inhibition improved cardiac functions with direct antifibrotic activity in heart failure. Int. J. Cardiol. 2013, 168, 4178–4183. [Google Scholar] [CrossRef]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Yeh, Y.H.; Chen, Y.J. Factor Xa inhibition by rivaroxaban regulates fibrogenesis in human atrial fibroblasts with modulation of nitric oxide synthesis and calcium homeostasis. J. Mol. Cell. Cardiol. 2018, 123, 128–138. [Google Scholar] [CrossRef]
- Lee, T.I.; Chen, Y.C.; Lin, Y.K.; Chung, C.C.; Lu, Y.Y.; Kao, Y.H.; Chen, Y.J. Empagliflozin attenuates myocardial sodium and calcium dysregulation and reverses cardiac remodeling in streptozotocin-induced diabetic rats. Int. J. Mol. Sci. 2019, 20, 1680. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Chung, C.-C.; Liu, S.-H.; Kao, Y.-H.; Chen, Y.-J. Lithium Treatment Improves Cardiac Dysfunction in Rats Deprived of Rapid Eye Movement Sleep. Int. J. Mol. Sci. 2022, 23, 11226. https://doi.org/10.3390/ijms231911226
Chen P-H, Chung C-C, Liu S-H, Kao Y-H, Chen Y-J. Lithium Treatment Improves Cardiac Dysfunction in Rats Deprived of Rapid Eye Movement Sleep. International Journal of Molecular Sciences. 2022; 23(19):11226. https://doi.org/10.3390/ijms231911226
Chicago/Turabian StyleChen, Pao-Huan, Cheng-Chih Chung, Shuen-Hsin Liu, Yu-Hsun Kao, and Yi-Jen Chen. 2022. "Lithium Treatment Improves Cardiac Dysfunction in Rats Deprived of Rapid Eye Movement Sleep" International Journal of Molecular Sciences 23, no. 19: 11226. https://doi.org/10.3390/ijms231911226
APA StyleChen, P.-H., Chung, C.-C., Liu, S.-H., Kao, Y.-H., & Chen, Y.-J. (2022). Lithium Treatment Improves Cardiac Dysfunction in Rats Deprived of Rapid Eye Movement Sleep. International Journal of Molecular Sciences, 23(19), 11226. https://doi.org/10.3390/ijms231911226




