Age-Related Low Bone Mineral Density in C57BL/6 Mice Is Reflective of Aberrant Bone Morphogenetic Protein-2 Signaling Observed in Human Patients Diagnosed with Osteoporosis
Abstract
1. Introduction
2. Results
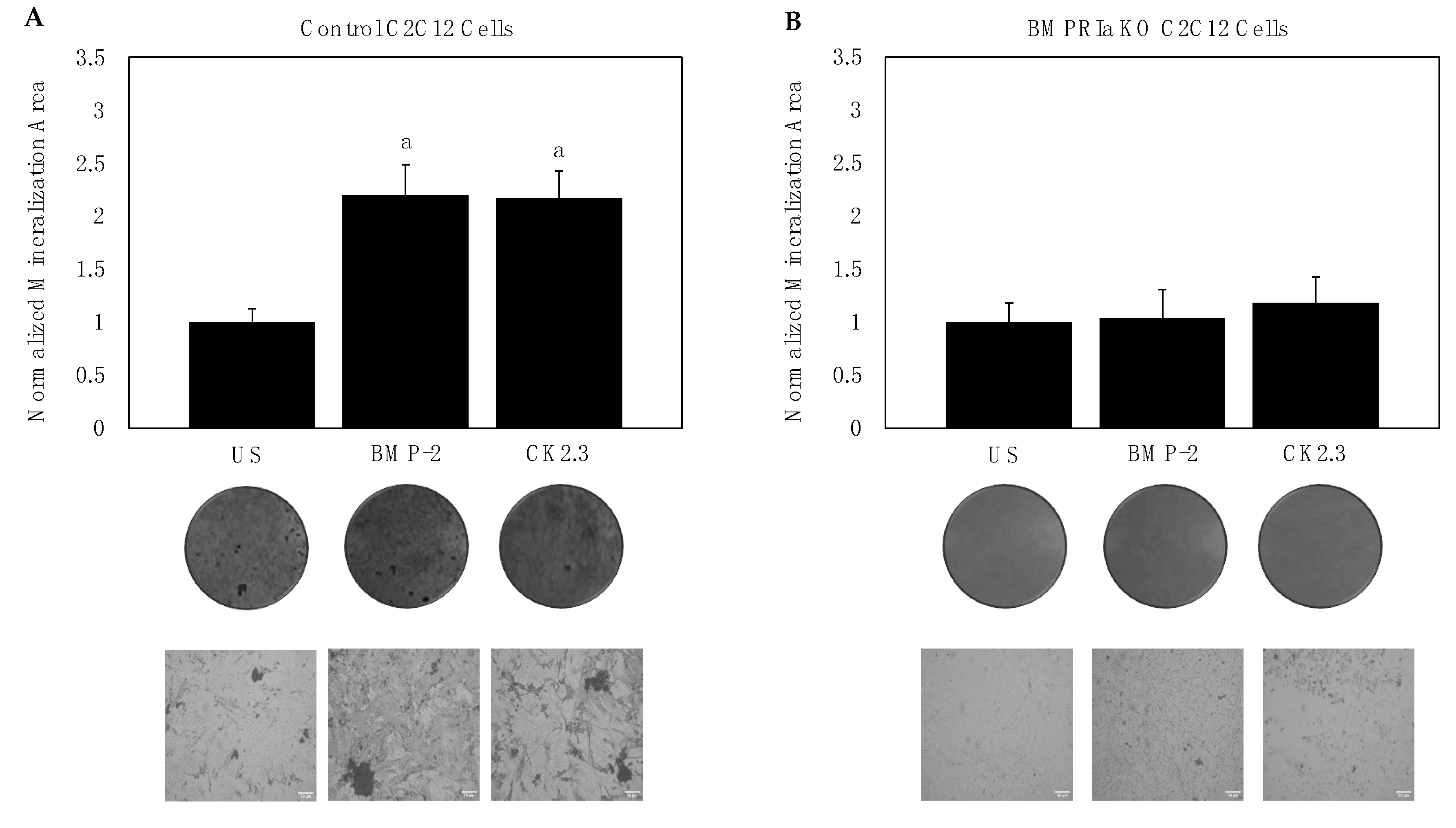
2.1. BMPRIa Is Required for CK2.3-Mediated Osteogenesis in C2C12 Cells
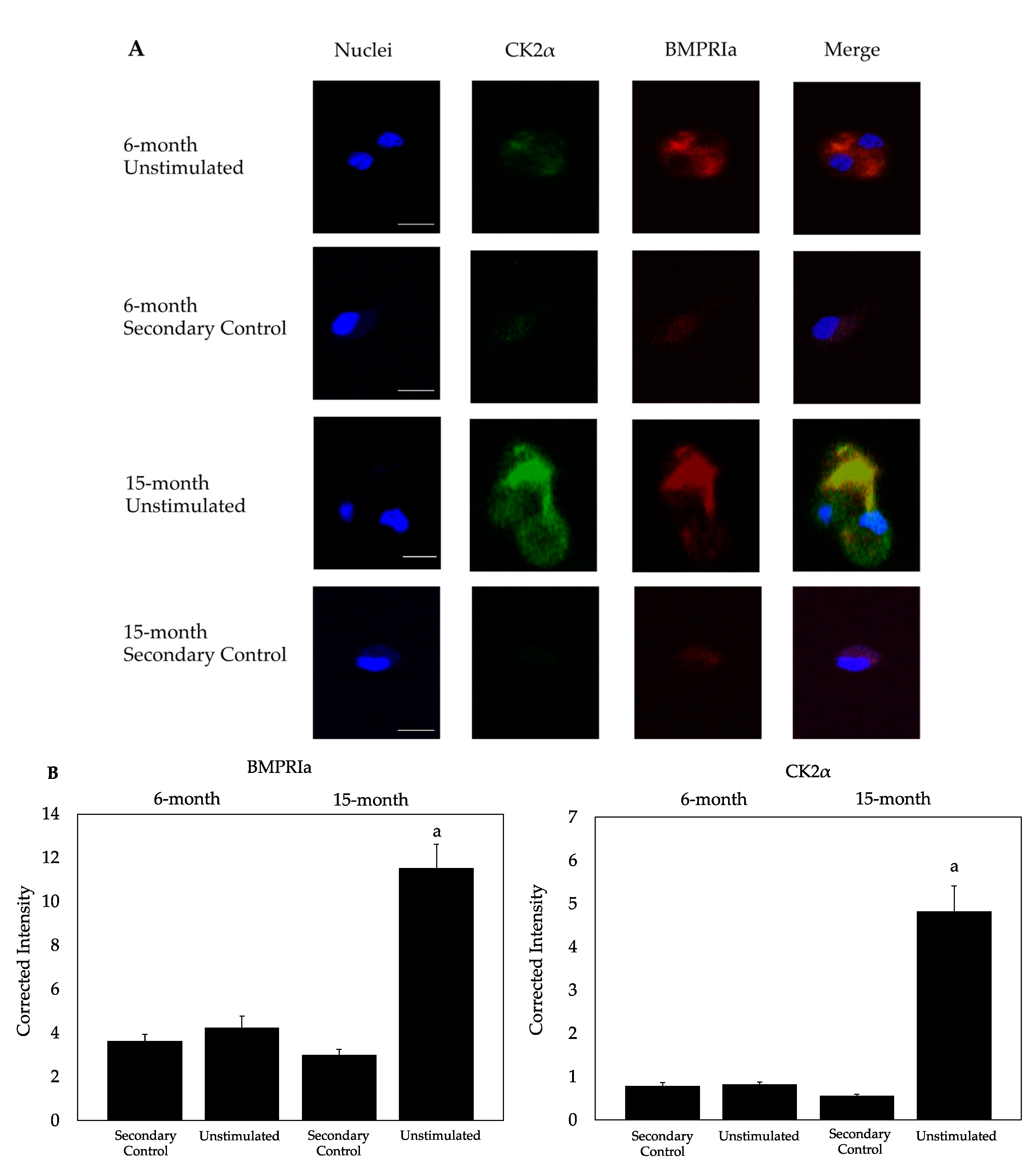
2.2. CK2α and BMPRIa Were Overexpressed in 15-Month Mice
2.3. BMP-2 Did Not Increase BV/TV in 15- and 20-Month-Old Mice upon µCT Analysis
2.4. BMP-2 and CK2.3 Enhanced Bone Mineralization of 6-Month-Old Mice, While Only CK2.3 Increased Mineralization in 15- and 20-Month-Old Mice
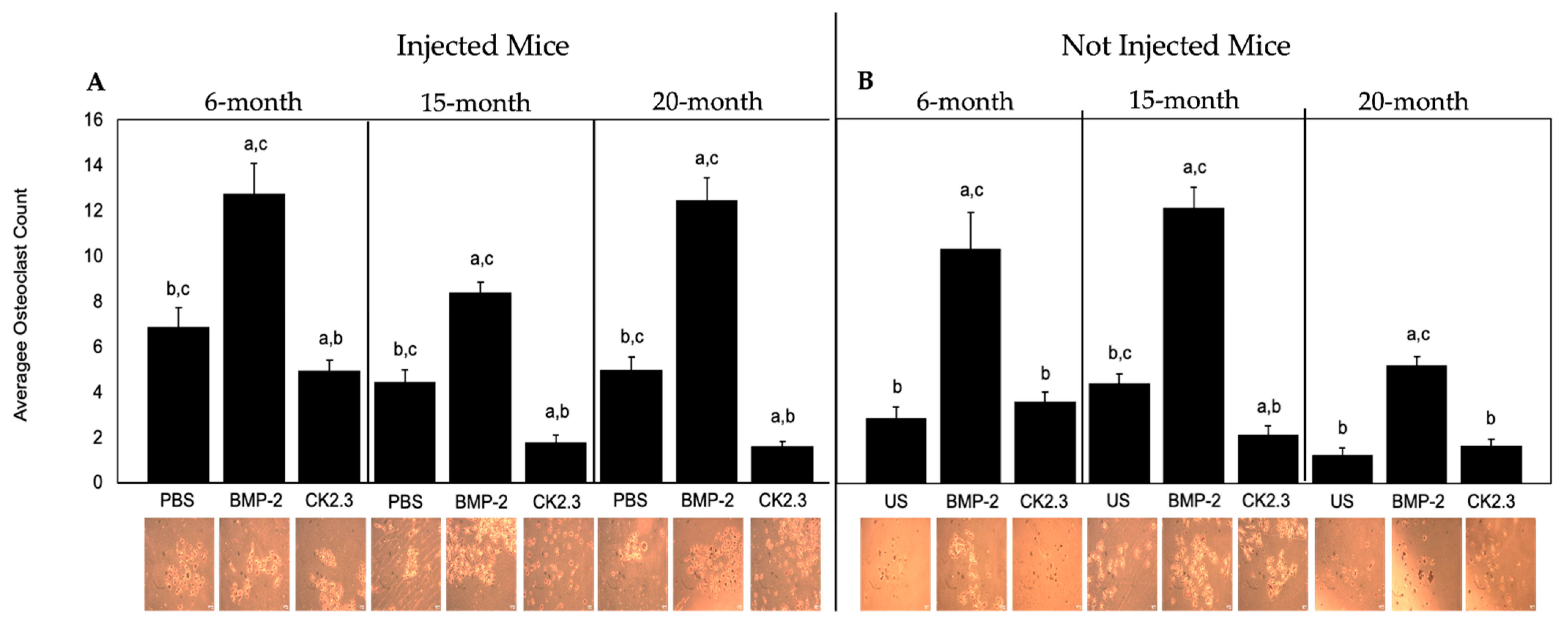
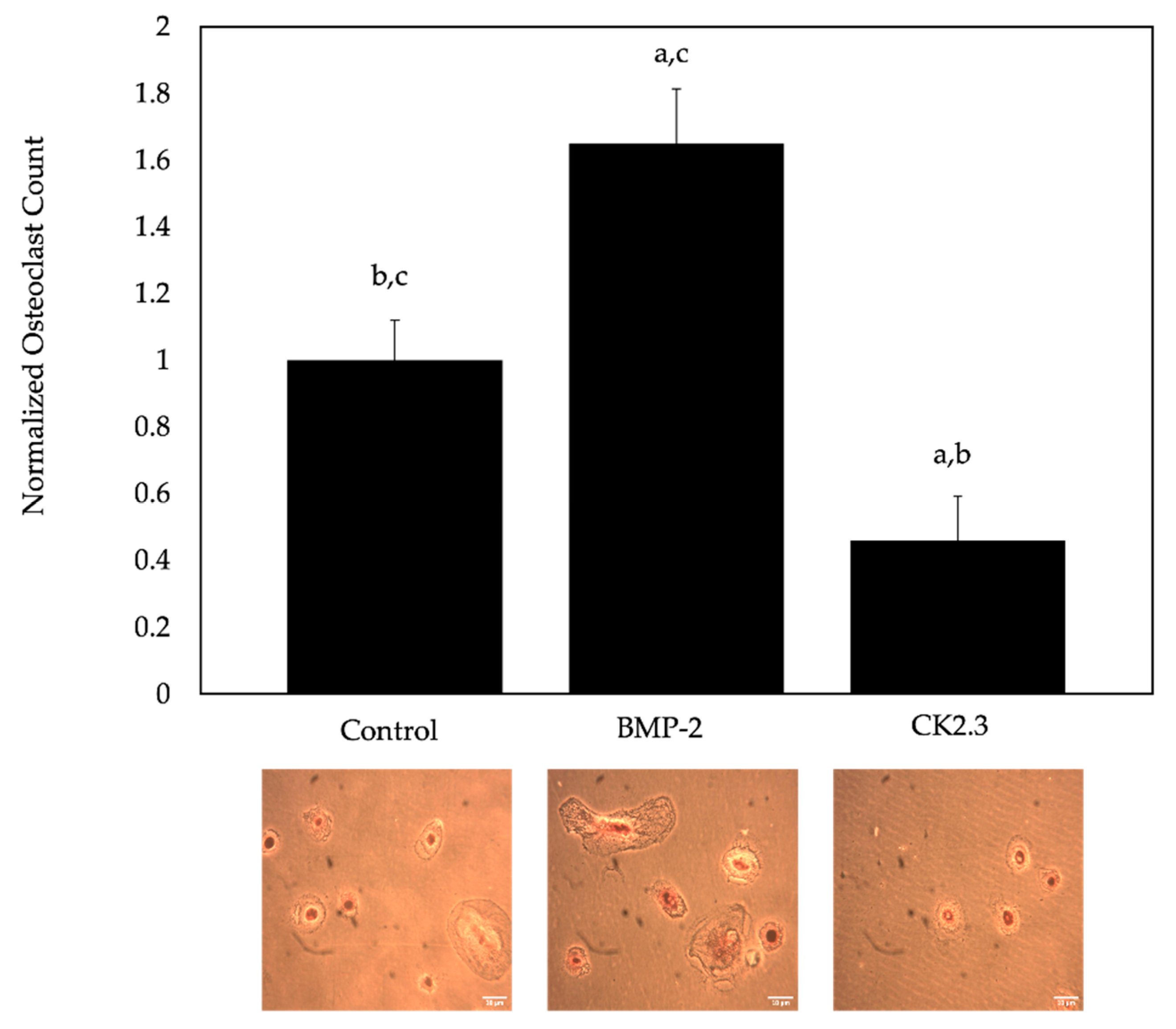
2.5. BMP-2 Increased Osteoclastogenesis, While CK2.3 Decreased Osteoclastogenesis, in All Mice
2.6. BMP-2 Increased Osteoclastogenesis, While CK2.3 Decreased Osteoclastogenesis, in Cells Isolated from Human Femoral Heads of OP Patients
3. Discussion
4. Materials and Methods
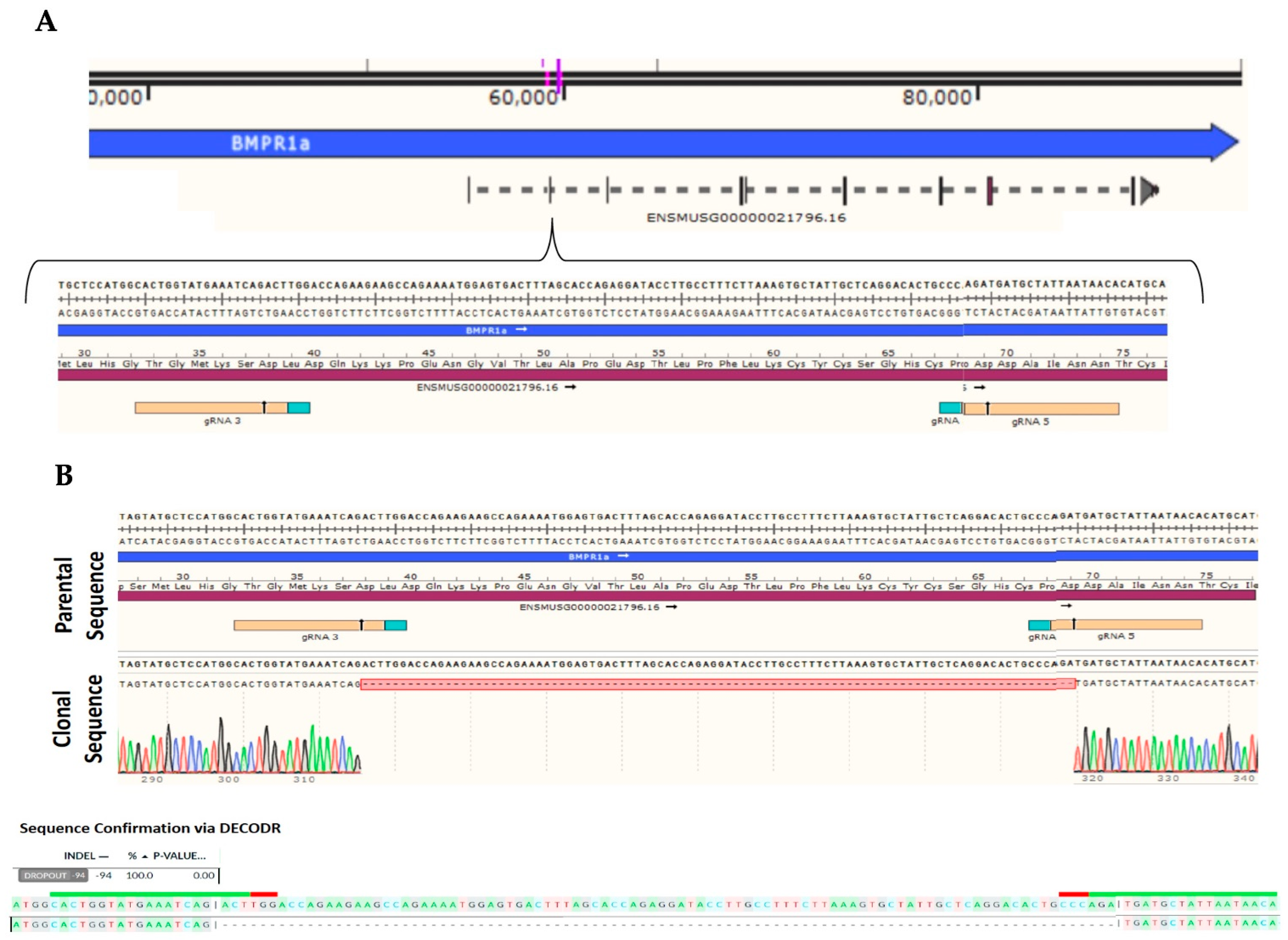
4.1. C2C12 Wildtype and C2C12 BMPRIa Knockout Cell Lines
4.2. C2C12 Cell Culture
4.3. Mice and Ethical Approval
4.4. Mice Injections and Organ Isolation
4.5. Isolation of Bone Marrow Stromal Cells (BMSCs) and Osteoclast Progenitors
4.6. Isolation of Pre-Osteoclasts from Human Femoral Heads
4.7. Immunofluorescent Staining
4.8. Microcomputed Tomography (µCT)
4.9. Von Kossa Assay
4.10. Tartrate Resistant Acid Phosphatase (TRAP) Assay
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponnapakkam, T.; Katikaneni, R.; Sakon, J.; Stratford, R.; Gensure, R.C. Treating osteoporosis by targeting parathyroid hormone to bone. Drug Discov. Today 2014, 19, 204–208. [Google Scholar] [CrossRef]
- Wippert, P.M.; Rector, M.; Kuhn, G.; Wuertz-Kozak, K. Stress and Alterations in Bones: An Interdisciplinary Perspective. Front. Endocrinol. 2017, 8, 96. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Safety and tolerability of denosumab for the treatment of postmenopausal osteoporosis. Drug Healthc. Patient Saf. 2011, 3, 79–91. [Google Scholar] [CrossRef]
- Wright, N.C.; Looker, A.C.; Saag, K.G.; Curtis, J.R.; Delzell, E.S.; Randall, S.; Dawson-Hughes, B. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J. Bone Miner. Res. 2014, 29, 2520–2526. [Google Scholar] [CrossRef]
- Rashki Kemmak, A.; Rezapour, A.; Jahangiri, R.; Nikjoo, S.; Farabi, H.; Soleimanpour, S. Economic burden of osteoporosis in the world: A systematic review. Med. J. Islam. Repub. Iran 2020, 34, 154. [Google Scholar] [CrossRef]
- Ji, M.X.; Yu, Q. Primary osteoporosis in postmenopausal women. Chronic. Dis. Transl. Med. 2015, 1, 9–13. [Google Scholar] [CrossRef]
- Thiruchelvam, N.; Randhawa, J.; Sadiek, H.; Kistangari, G. Teriparatide induced delayed persistent hypercalcemia. Case Rep. Endocrinol. 2014, 2014, 802473. [Google Scholar] [CrossRef]
- Miller, P.D.; Schwartz, E.N.; Chen, P.; Misurski, D.A.; Krege, J.H. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos. Int. 2007, 18, 59–68. [Google Scholar] [CrossRef]
- Nguyen, J.; Kelly, S.; Wood, R.; Heubel, B.; Nohe, A. A Synthetic Peptide, CK2.3, Inhibits RANKL-Induced Osteoclastogenesis through BMPRIa and ERK Signaling Pathway. J. Dev. Biol. 2020, 8, 12. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K. Romosozumab: A novel bone anabolic treatment option for osteoporosis? Wien. Med. Wochenschr. 2019, 170, 124–131. [Google Scholar] [CrossRef]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef] [PubMed]
- Sequeira, L.; Nguyen, J.; Wang, L.; Nohe, A. A Novel Peptide, CK2.3, Improved Bone Formation in Ovariectomized Sprague Dawley Rats. Int. J. Mol. Sci. 2020, 21, 4874. [Google Scholar] [CrossRef] [PubMed]
- Reeve, J.; Meunier, P.J.; Parsons, J.A.; Bernat, M.; Bijvoet, O.L.; Courpron, P.; Edouard, C.; Klenerman, L.; Neer, R.M.; Renier, J.C.; et al. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: A multicentre trial. Br. Med. J. 1980, 280, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Novince, C.M.; Ward, B.B.; McCauley, L.K. Osteonecrosis of the jaw: An update and review of recommendations. Cells Tissues Organs 2009, 189, 275–283. [Google Scholar] [CrossRef]
- Cardwell, C.R.; Abnet, C.C.; Cantwell, M.M.; Murray, L.J. Exposure to oral bisphosphonates and risk of esophageal cancer. JAMA 2010, 304, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Haber, S.L.; McNatty, D. An evaluation of the use of oral bisphosphonates and risk of esophageal cancer. Ann. Pharmacother. 2012, 46, 419–423. [Google Scholar] [CrossRef]
- Khosla, S.; Burr, D.; Cauley, J.; Dempster, D.W.; Ebeling, P.R.; Felsenberg, D.; Gagel, R.F.; Gilsanz, V.; Guise, T.; Koka, S.; et al. Bisphosphonate-associated osteonecrosis of the jaw: Report of a task force of the American Society for Bone and Mineral Research. J. Bone Miner. Res. 2007, 22, 1479–1491. [Google Scholar] [CrossRef]
- Cosman, F.; Crittenden, D.B.; Ferrari, S.; Lewiecki, E.M.; Jaller-Raad, J.; Zerbini, C.; Milmont, C.E.; Meisner, P.D.; Libanati, C.; Grauer, A. Romosozumab FRAME Study: A Post Hoc Analysis of the Role of Regional Background Fracture Risk on Nonvertebral Fracture Outcome. J. Bone Miner. Res. 2018, 33, 1407–1416. [Google Scholar] [CrossRef]
- Langdahl, B.L.; Libanati, C.; Crittenden, D.B.; Bolognese, M.A.; Brown, J.P.; Daizadeh, N.S.; Dokoupilova, E.; Engelke, K.; Finkelstein, J.S.; Genant, H.K.; et al. Romosozumab (sclerostin monoclonal antibody) versus teriparatide in postmenopausal women with osteoporosis transitioning from oral bisphosphonate therapy: A randomised, open-label, phase 3 trial. Lancet 2017, 390, 1585–1594. [Google Scholar] [CrossRef]
- Shakeri, A.; Adanty, C. Romosozumab (sclerostin monoclonal antibody) for the treatment of osteoporosis in postmenopausal women: A review. J. Popul. Ther. Clin. Pharmacol. 2020, 27, e25–e31. [Google Scholar] [CrossRef]
- Geusens, P.; Oates, M.; Miyauchi, A.; Adachi, J.D.; Lazaretti-Castro, M.; Ebeling, P.R.; Perez Niño, C.A.; Milmont, C.E.; Grauer, A.; Libanati, C. The Effect of 1 Year of Romosozumab on the Incidence of Clinical Vertebral Fractures in Postmenopausal Women With Osteoporosis: Results From the FRAME Study. JBMR Plus 2019, 3, e10211. [Google Scholar] [CrossRef] [PubMed]
- Sølling, A.S.K.; Harsløf, T.; Langdahl, B. The clinical potential of romosozumab for the prevention of fractures in postmenopausal women with osteoporosis. Ther. Adv. Musculoskelet. Dis. 2018, 10, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Nickel, J.; Ten Dijke, P.; Mueller, T.D. TGF-β family co-receptor function and signaling. Acta Biochim. Biophys. Sin. 2018, 50, 12–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Olli, K.; Bowen, C.; Bragdon, B.; Coombs, H.; Donahue, L.R.; Duncan, R.; Nohe, A. Systemic injection of CK2.3, a novel peptide acting downstream of bone morphogenetic protein receptor BMPRIa, leads to increased trabecular bone mass. J. Orthop. Res. 2015, 33, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Nohe, A.; Keating, E.; Knaus, P.; Petersen, N.O. Signal transduction of bone morphogenetic protein receptors. Cell. Signal. 2004, 16, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Bonor, J.; Adams, E.L.; Bragdon, B.; Moseychuk, O.; Czymmek, K.J.; Nohe, A. Initiation of BMP2 signaling in domains on the plasma membrane. J. Cell. Physiol. 2012, 227, 2880–2888. [Google Scholar] [CrossRef]
- Gámez, B.; Rodriguez-Carballo, E.; Ventura, F. BMP signaling in telencephalic neural cell specification and maturation. Front. Cell Neurosci. 2013, 7, 87. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Greene, S.B.; Martin, J.F. BMP signaling in congenital heart disease: New developments and future directions. Birth Defects Res. A Clin. Mol. Teratol. 2011, 91, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Karner, C.M.; Lee, S.Y.; Long, F. Bmp Induces Osteoblast Differentiation through both Smad4 and mTORC1 Signaling. Mol. Cell. Biol. 2017, 37, e00253-16. [Google Scholar] [CrossRef]
- Bragdon, B.; D’Angelo, A.; Gurski, L.; Bonor, J.; Schultz, K.L.; Beamer, W.G.; Rosen, C.J.; Nohe, A. Altered plasma membrane dynamics of bone morphogenetic protein receptor type Ia in a low bone mass mouse model. Bone 2012, 50, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Gaussin, V.; Morley, G.E.; Cox, L.; Zwijsen, A.; Vance, K.M.; Emile, L.; Tian, Y.; Liu, J.; Hong, C.; Myers, D.; et al. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ. Res. 2005, 97, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Sountoulidis, A.; Stavropoulos, A.; Giaglis, S.; Apostolou, E.; Monteiro, R.; Chuva de Sousa Lopes, S.M.; Chen, H.; Stripp, B.R.; Mummery, C.; Andreakos, E.; et al. Activation of the canonical bone morphogenetic protein (BMP) pathway during lung morphogenesis and adult lung tissue repair. PLoS ONE 2012, 7, e41460. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Song, T.J.; Li, X.; Hu, L.; He, Q.; Liu, M.; Lane, M.D.; Tang, Q.Q. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. USA 2009, 106, 12670–12675. [Google Scholar] [CrossRef]
- Bragdon, B.; Thinakaran, S.; Bonor, J.; Underhill, T.M.; Petersen, N.O.; Nohe, A. FRET reveals novel protein-receptor interaction of bone morphogenetic proteins receptors and adaptor protein 2 at the cell surface. Biophys. J. 2009, 97, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Nohe, A.; Keating, E.; Underhill, T.M.; Knaus, P.; Petersen, N.O. Effect of the distribution and clustering of the type I A BMP receptor (ALK3) with the type II BMP receptor on the activation of signalling pathways. J. Cell Sci. 2003, 116, 3277–3284. [Google Scholar] [CrossRef] [PubMed]
- Nohe, A.; Keating, E.; Underhill, T.M.; Knaus, P.; Petersen, N.O. Dynamics and interaction of caveolin-1 isoforms with BMP-receptors. J. Cell Sci. 2005, 118, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Bonor, J.; Shultz, K.L.; Beamer, W.G.; Rosen, C.J.; Nohe, A. Bone morphogenetic protein receptor type Ia localization causes increased BMP2 signaling in mice exhibiting increased peak bone mass phenotype. J. Cell. Physiol. 2012, 227, 2870–2879. [Google Scholar] [CrossRef]
- Gilboa, L.; Nohe, A.; Geissendörfer, T.; Sebald, W.; Henis, Y.I.; Knaus, P. Bone morphogenetic protein receptor complexes on the surface of live cells: A new oligomerization mode for serine/threonine kinase receptors. Mol. Biol. Cell. 2000, 11, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.; Weidner, H.; Schell, L.M.; Sequeira, L.; Kabrick, R.; Dharmadhikari, S.; Coombs, H.; Duncan, R.L.; Wang, L.; Nohe, A. Synthetic Peptide CK2.3 Enhances Bone Mineral Density in Senile Mice. J. Bone Res. 2018, 6, 190. [Google Scholar] [CrossRef] [PubMed]
- Weidner, H.; Yuan Gao, V.; Dibert, D.; McTague, S.; Eskander, M.; Duncan, R.; Wang, L.; Nohe, A. CK2.3, a Mimetic Peptide of the BMP Type I Receptor, Increases Activity in Osteoblasts over BMP2. Int. J. Mol. Sci. 2019, 20, 5877. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; Gurski, L.; Bonor, J.; Price, C.; Wang, L.; Beamer, W.G.; Nohe, A. Casein kinase 2 regulates in vivo bone formation through its interaction with bone morphogenetic protein receptor type Ia. Bone 2011, 49, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Bragdon, B.; Thinakaran, S.; Moseychuk, O.; King, D.; Young, K.; Litchfield, D.W.; Petersen, N.O.; Nohe, A. Casein kinase 2 beta-subunit is a regulator of bone morphogenetic protein 2 signaling. Biophys. J. 2010, 99, 897–904. [Google Scholar] [CrossRef]
- Halloran, D.; Pandit, V.; Nohe, A. The Role of Protein Kinase CK2 in Development and Disease Progression: A Critical Review. J. Dev. Biol. 2022, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Vrathasha, V.; Weidner, H.; Nohe, A. Mechanism of CK2.3, a Novel Mimetic Peptide of Bone Morphogenetic Protein Receptor Type IA, Mediated Osteogenesis. Int. J. Mol. Sci. 2019, 20, 2500. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 in Development and Bone Homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Vrathasha, V.; Durbano, H.W.; Nohe, A. Bone Morphogenetic Protein-2 Conjugated to Quantum Dot. Nanomaterials 2020, 10, 1208. [Google Scholar] [CrossRef] [PubMed]
- Moseychuk, O.; Akkiraju, H.; Dutta, J.; D’Angelo, A.; Bragdon, B.; Duncan, R.L.; Nohe, A. Inhibition of CK2 binding to BMPRIa induces C2C12 differentiation into osteoblasts and adipocytes. J. Cell Commun. Signal. 2013, 7, 265–278. [Google Scholar] [CrossRef]
- Hindoyan, K.; Tilan, J.; Buser, Z.; Cohen, J.R.; Brodke, D.S.; Youssef, J.A.; Park, J.B.; Yoon, S.T.; Meisel, H.J.; Wang, J.C. A Retrospective Analysis of Complications Associated With Bone Morphogenetic Protein 2 in Anterior Lumbar Interbody Fusion. Glob. Spine J. 2017, 7, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Cyriac, M.; Kyhos, J.; Iweala, U.; Lee, D.; Mantell, M.; Yu, W.; O’Brien, J.R. Anterior Lumbar Interbody Fusion with Cement Augmentation without Posterior Fixation to Treat Isthmic Spondylolisthesis in an Osteopenic Patient—A Surgical Technique. Int. J. Spine Surg. 2018, 12, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Herford, A.S. The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillofacial trauma. Chin. J. Traumatol. 2017, 20, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Hao, X.; Xu, F.; Liu, J.; Luo, E.; Meng, G. Effects of local delivery of BMP2, zoledronate and their combination on bone microarchitecture, biomechanics and bone turnover in osteoporotic rabbits. Sci. Rep. 2016, 6, 28537. [Google Scholar] [CrossRef] [PubMed]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the Clinical Side Effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B Rev. 2016, 22, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef] [PubMed]
- Tannoury, C.A.; An, H.S. Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J. 2014, 14, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.D.; Gum, J.L.; Crawford, C.H.; Shields, C.B.; Carreon, L.Y. Complications with recombinant human bone morphogenetic protein-2 in posterolateral spine fusion associated with a dural tear. Spine J. 2011, 11, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Comer, G.C.; Smith, M.W.; Hurwitz, E.L.; Mitsunaga, K.A.; Kessler, R.; Carragee, E.J. Retrograde ejaculation after anterior lumbar interbody fusion with and without bone morphogenetic protein-2 augmentation: A 10-year cohort controlled study. Spine J. 2012, 12, 881–890. [Google Scholar] [CrossRef]
- Heubel, B.; Nohe, A. The Role of BMP Signaling in Osteoclast Regulation. J. Dev. Biol. 2021, 9, 24. [Google Scholar] [CrossRef]
- Halloran, D.R.; Heubel, B.; MacMurray, C.; Root, D.; Eskander, M.; McTague, S.P.; Pelkey, H.; Nohe, A. Differentiation of Cells Isolated from Human Femoral Heads into Functional Osteoclasts. J. Dev. Biol. 2022, 10, 6. [Google Scholar] [CrossRef]
- Durbano, H.W.; Halloran, D.; Nguyen, J.; Stone, V.; McTague, S.; Eskander, M.; Nohe, A. Aberrant BMP2 Signaling in Patients Diagnosed with Osteoporosis. Int. J. Mol. Sci. 2020, 21, 6909. [Google Scholar] [CrossRef] [PubMed]
- Vrathasha, V.; Booksh, K.; Duncan, R.L.; Nohe, A. Mechanisms of Cellular Internalization of Quantum Dot® Conjugated Bone Formation Mimetic Peptide CK2.3. Nanomaterials 2018, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Bonor, J.; Nohe, A. CK2.1, a novel peptide, induces articular cartilage formation in vivo. J. Orthop. Res. 2017, 35, 876–885. [Google Scholar] [CrossRef]
- Wu, X.; Gehring, W. Cellular uptake of the Antennapedia homeodomain polypeptide by macropinocytosis. Biochem. Biophys. Res. Commun. 2014, 443, 1136–1140. [Google Scholar] [CrossRef]
- Derossi, D.; Calvet, S.; Trembleau, A.; Brunissen, A.; Chassaing, G.; Prochiantz, A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 1996, 271, 18188–18193. [Google Scholar] [CrossRef] [PubMed]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Roman-Roman, S. BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef]
- Pujari-Palmer, M.; Pujari-Palmer, S.; Lu, X.; Lind, T.; Melhus, H.; Engstrand, T.; Karlsson-Ott, M.; Engqvist, H. Pyrophosphate Stimulates Differentiation, Matrix Gene Expression and Alkaline Phosphatase Activity in Osteoblasts. PLoS ONE 2016, 11, e0163530. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Pham, L.; Billington, C.J.; Espe, K.; Carlson, A.E.; Westendorf, J.J.; Petryk, A.; Gopalakrishnan, R.; Mansky, K. Bone morphogenic protein 2 directly enhances differentiation of murine osteoclast precursors. J. Cell. Biochem. 2010, 109, 672–682. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halloran, D.; Pandit, V.; MacMurray, C.; Stone, V.; DeGeorge, K.; Eskander, M.; Root, D.; McTague, S.; Pelkey, H.; Nohe, A. Age-Related Low Bone Mineral Density in C57BL/6 Mice Is Reflective of Aberrant Bone Morphogenetic Protein-2 Signaling Observed in Human Patients Diagnosed with Osteoporosis. Int. J. Mol. Sci. 2022, 23, 11205. https://doi.org/10.3390/ijms231911205
Halloran D, Pandit V, MacMurray C, Stone V, DeGeorge K, Eskander M, Root D, McTague S, Pelkey H, Nohe A. Age-Related Low Bone Mineral Density in C57BL/6 Mice Is Reflective of Aberrant Bone Morphogenetic Protein-2 Signaling Observed in Human Patients Diagnosed with Osteoporosis. International Journal of Molecular Sciences. 2022; 23(19):11205. https://doi.org/10.3390/ijms231911205
Chicago/Turabian StyleHalloran, Daniel, Venu Pandit, Connor MacMurray, Victoria Stone, Kailey DeGeorge, Mark Eskander, Denise Root, Sean McTague, Heather Pelkey, and Anja Nohe. 2022. "Age-Related Low Bone Mineral Density in C57BL/6 Mice Is Reflective of Aberrant Bone Morphogenetic Protein-2 Signaling Observed in Human Patients Diagnosed with Osteoporosis" International Journal of Molecular Sciences 23, no. 19: 11205. https://doi.org/10.3390/ijms231911205
APA StyleHalloran, D., Pandit, V., MacMurray, C., Stone, V., DeGeorge, K., Eskander, M., Root, D., McTague, S., Pelkey, H., & Nohe, A. (2022). Age-Related Low Bone Mineral Density in C57BL/6 Mice Is Reflective of Aberrant Bone Morphogenetic Protein-2 Signaling Observed in Human Patients Diagnosed with Osteoporosis. International Journal of Molecular Sciences, 23(19), 11205. https://doi.org/10.3390/ijms231911205





