Platelet–Neutrophil Association in NETs-Rich Areas in the Retrieved AIS Patient Thrombi
Abstract
1. Introduction
2. Results
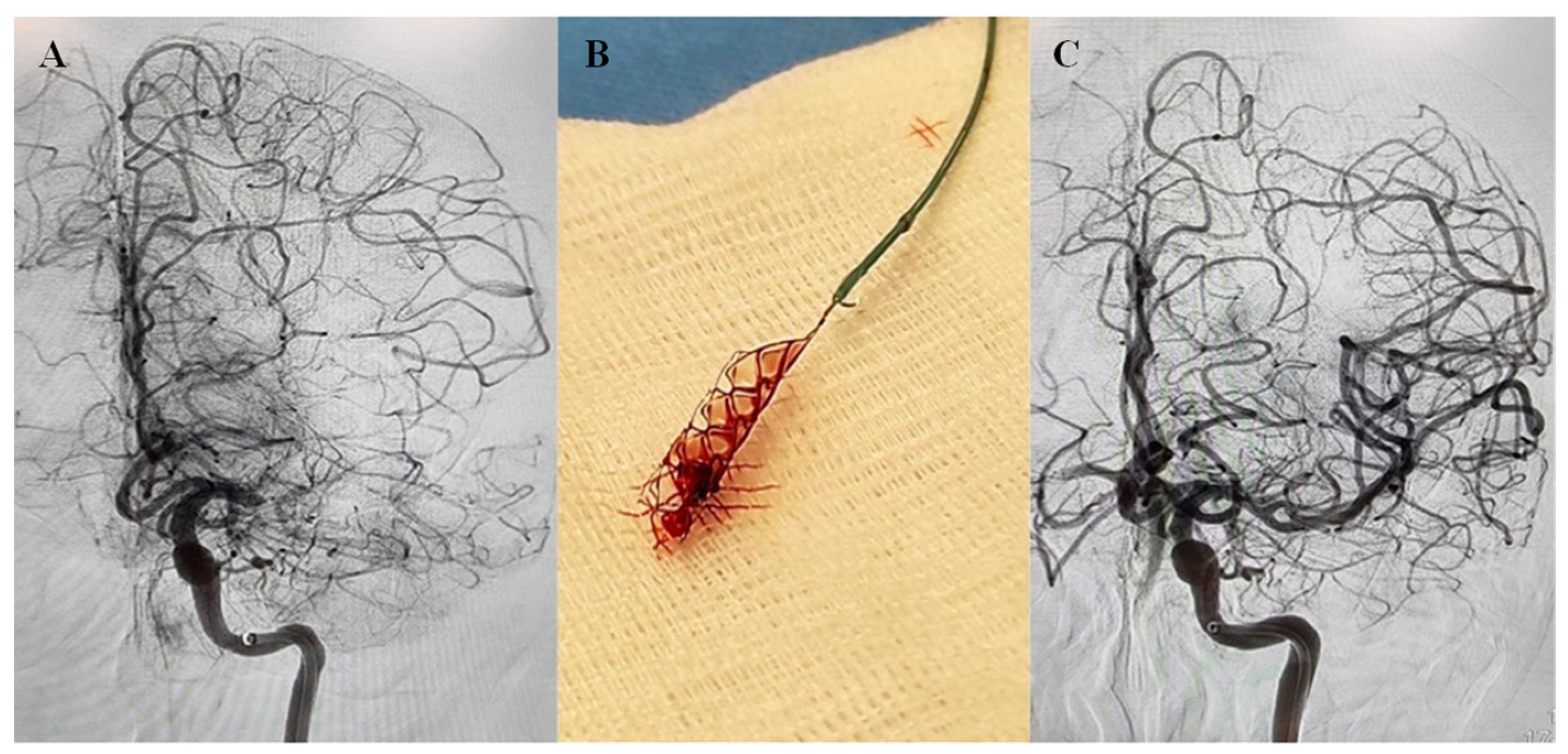
2.1. Clinical Charecteristics of the AIS Patients
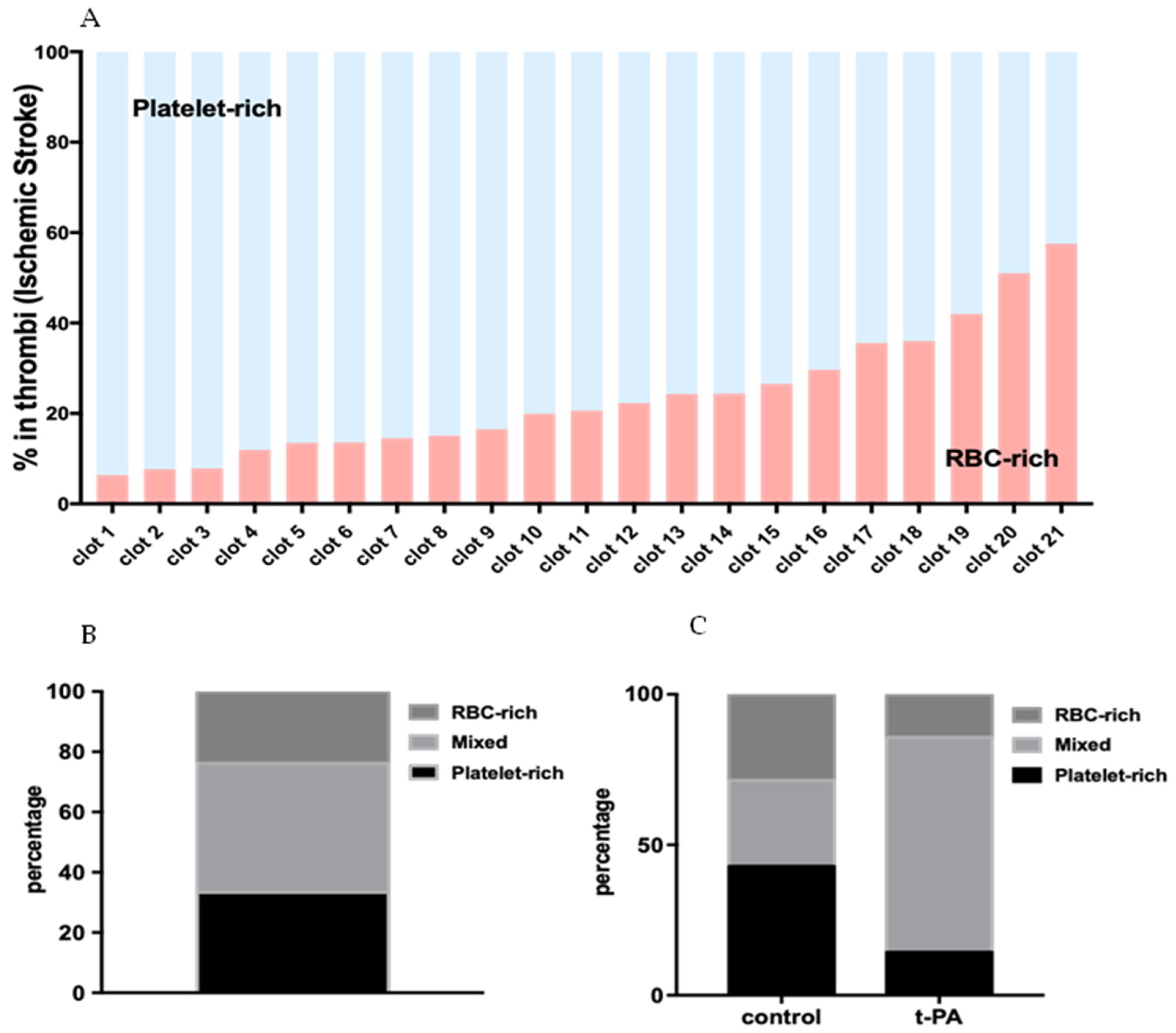
2.2. AIS Patient Thrombi Contain Distinct Patterns of Areas Rich in RBCs and Platelets
2.3. RBC-Rich Areas Show Heavily Populated RBCs Packed in Thin Fibrin Network
2.4. Dense Fibrin Network Associated with VWF and Infiltrated Platelets Define Platelet-Rich Areas
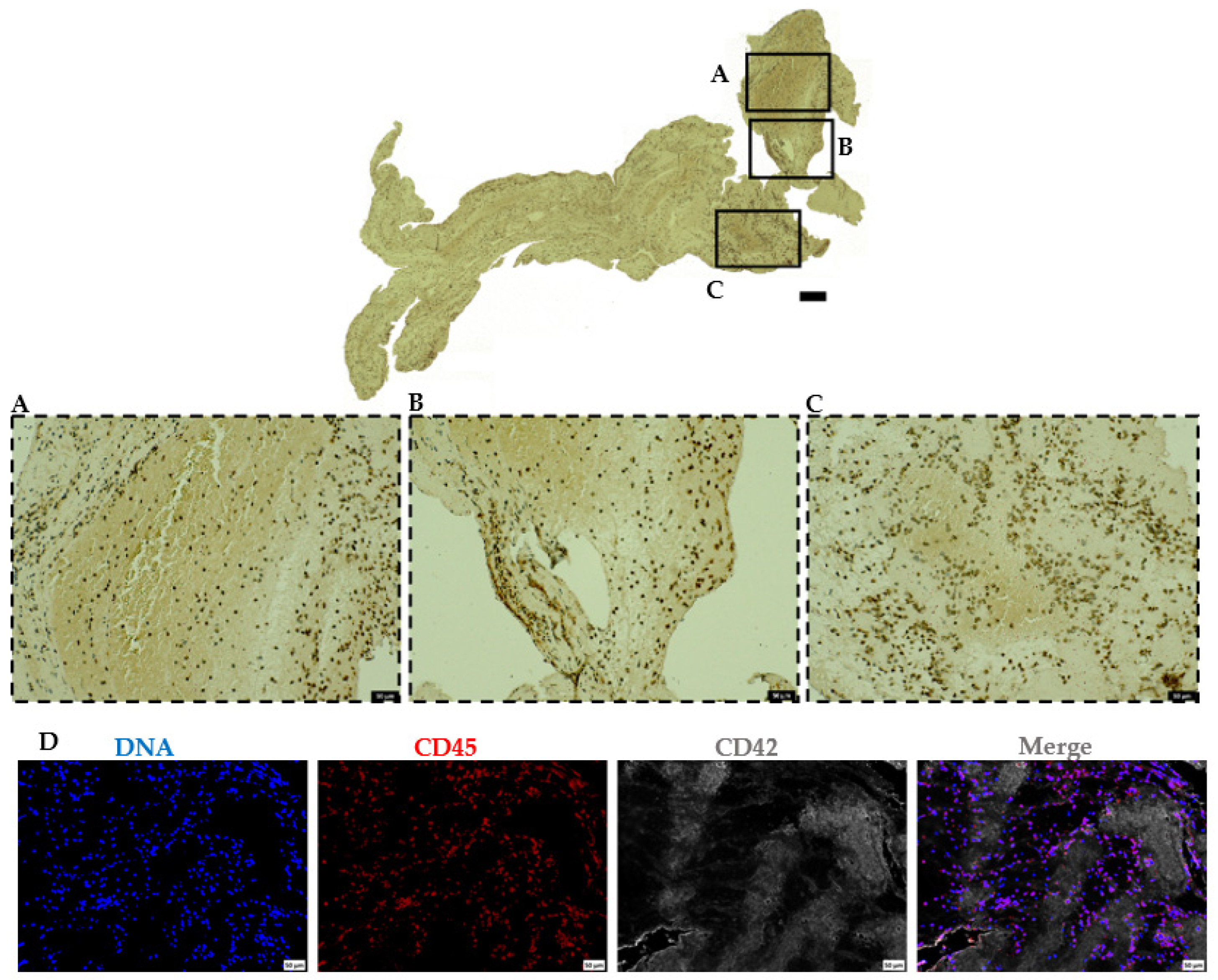
2.5. Leukocytes Majorly Populate the Platelet-Rich Areas in AIS Patient Thrombi
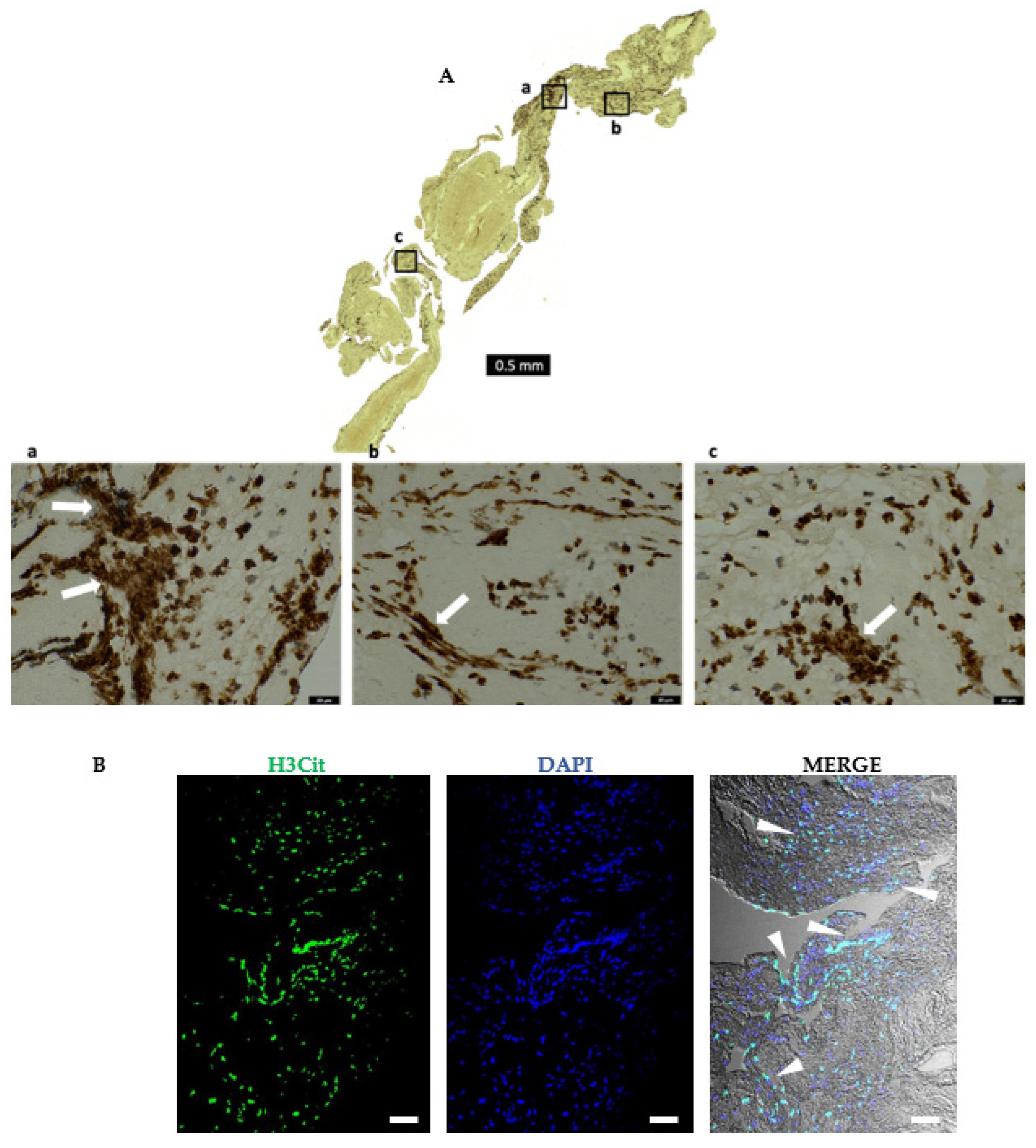
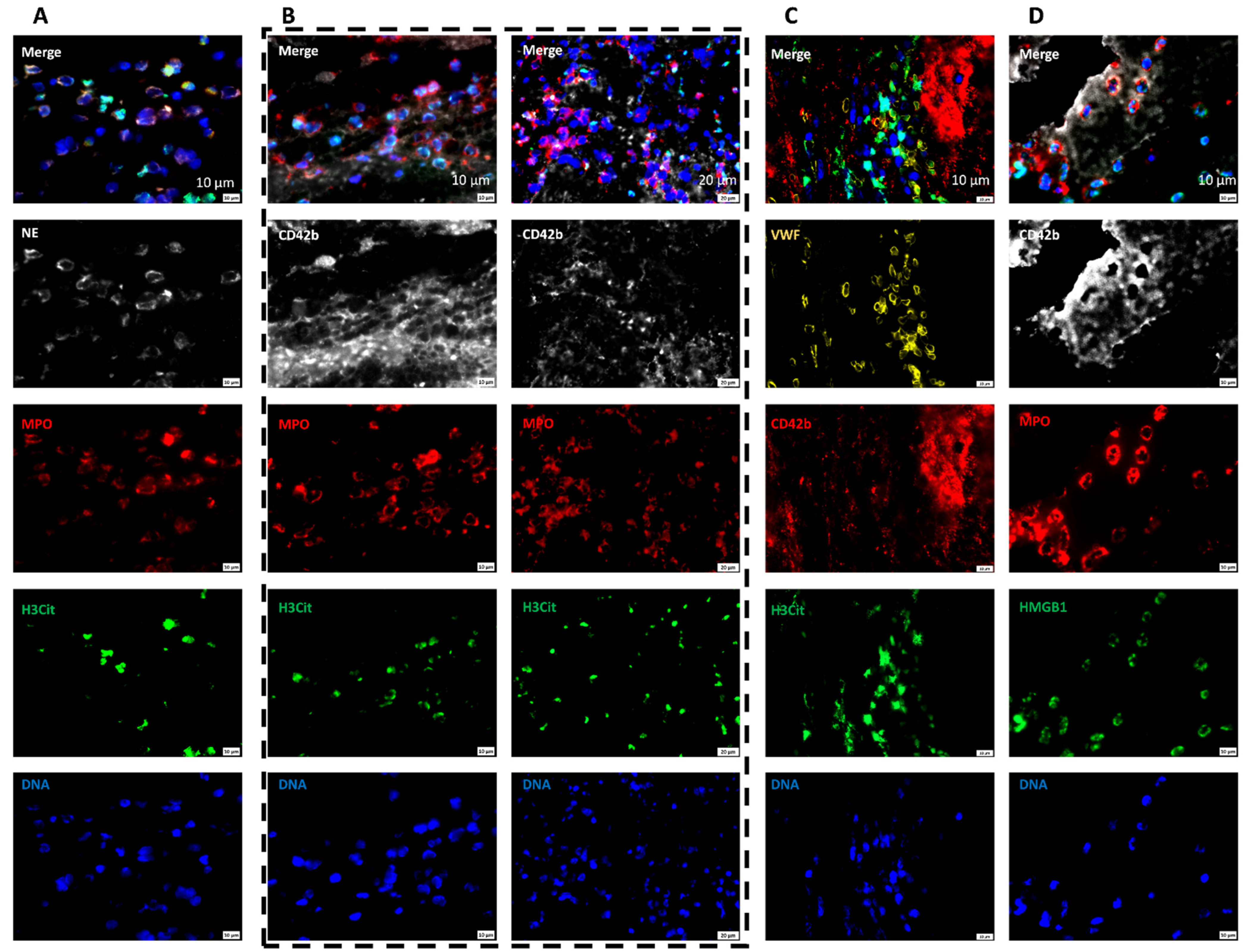
2.6. AIS Patient Thrombi Show Neutrophil Extracellular Traps (NETs) in the Platelet-Rich Areas and at the Interface between RBC-Rich and Platelet-Rich Areas
2.7. Areas Rich in NETs Show Close Association of Platelets and Neutrophils in Thrombi
3. Discussion
4. Materials and Methods
4.1. Thrombi Histology
4.2. Immunofluorescence Staining
4.3. Hematoxylin and Eosin
4.4. Martius Scarlet Blue
4.5. Quantification of RBC-Rich and Platelet-Rich Area
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Marshall, R.S. Progress in Intravenous Thrombolytic Therapy for Acute Stroke. JAMA Neurol. 2015, 72, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, L.; Desilles, J.P.; Mazighi, M.; Ho-Tin-Noé, B. Thrombolysis-resistant intracranial clot. Neurology 2018, 90, 1075. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.K.; Gold, H.K.; Ziskind, A.A.; Fallon, J.T.; Holt, R.E.; Leinbach, R.C.; May, J.W.; Collen, D. Differential sensitivity of erythrocyte-rich and platelet-rich arterial thrombi to lysis with recombinant tissue-type plasminogen activator. A possible explanation for resistance to coronary thrombolysis. Circulation 1989, 79, 920–928. [Google Scholar] [CrossRef]
- Booth, N.A.; Robbie, L.A.; Croll, A.M.; Bennett, B. Lysis of platelet-rich thrombi: The role of PAI-1. Ann. N. Y. Acad. Sci. 1992, 667, 70–80. [Google Scholar] [CrossRef]
- Tomkins, A.J.; Schleicher, N.; Murtha, L.; Kaps, M.; Levi, C.R.; Nedelmann, M.; Spratt, N.J. Platelet rich clots are resistant to lysis by thrombolytic therapy in a rat model of embolic stroke. Exp. Transl. Stroke Med. 2015, 7, 2. [Google Scholar] [CrossRef]
- Denis, C.V.; Wagner, D.D. Platelet adhesion receptors and their ligands in mouse models of thrombosis. Arter. Thromb. Vasc. Biol. 2007, 27, 728–739. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. Von Willebrand factor. Curr. Opin. Hematol. 2003, 10, 142–149. [Google Scholar] [CrossRef]
- Jurk, K.; Clemetson, K.J.; de Groot, P.G.; Brodde, M.F.; Steiner, M.; Savion, N.; Varon, D.; Sixma, J.J.; Van Aken, H.; Kehrel, B.E. Thrombospondin-1 mediates platelet adhesion at high shear via glycoprotein Ib (GPIb): An alternative/backup mechanism to von Willebrand factor. FASEB J. 2003, 17, 1490–1492. [Google Scholar] [CrossRef]
- Ni, H.; Yuen, P.S.; Papalia, J.M.; Trevithick, J.E.; Sakai, T.; Fässler, R.; Hynes, R.O.; Wagner, D.D. Plasma fibronectin promotes thrombus growth and stability in injured arterioles. Proc. Natl. Acad. Sci. USA 2003, 100, 2415–2419. [Google Scholar] [CrossRef]
- Clemetson, K.J.; Clemetson, J.M. Platelet collagen receptors. Thromb. Haemost. 2001, 86, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Piffath, C.L.; Goerge, T.; Cifuni, S.M.; Ruggeri, Z.M.; Ware, J.; Wagner, D.D. The role of platelet adhesion receptor GPIbalpha far exceeds that of its main ligand, von Willebrand factor, in arterial thrombosis. Proc. Natl. Acad. Sci. USA 2006, 103, 16900–16905. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Dopheide, S.M.; Yap, C.L.; Ravanat, C.; Freund, M.; Mangin, P.; Heel, K.A.; Street, A.; Harper, I.S.; Lanza, F.; et al. A revised model of platelet aggregation. J. Clin. Investig. 2000, 105, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Vink, T.; Schiphorst, M.; van Zanten, G.H.; MJ, I.J.; de Groot, P.G.; Sixma, J.J. Platelet thrombus formation on collagen at high shear rates is mediated by von Willebrand factor-glycoprotein Ib interaction and inhibited by von Willebrand factor-glycoprotein IIb/IIIa interaction. Arter. Thromb. Vasc. Biol. 2000, 20, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.T. Fibrinogen and fibrin: Scaffold proteins in hemostasis. Curr. Opin. Hematol. 2007, 14, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M. The role of von Willebrand factor in thrombus formation. Thromb. Res. 2007, 120 (Suppl. S1), S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Ducroux, C.; Di Meglio, L.; Loyau, S.; Delbosc, S.; Boisseau, W.; Deschildre, C.; Ben Maacha, M.; Blanc, R.; Redjem, H.; Ciccio, G.; et al. Thrombus Neutrophil Extracellular Traps Content Impair tPA-Induced Thrombolysis in Acute Ischemic Stroke. Stroke 2018, 49, 754–757. [Google Scholar] [CrossRef]
- Laridan, E.; Denorme, F.; Desender, L.; François, O.; Andersson, T.; Deckmyn, H.; Vanhoorelbeke, K.; De Meyer, S.F. Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 2017, 82, 223–232. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- von Köckritz-Blickwede, M.; Goldmann, O.; Thulin, P.; Heinemann, K.; Norrby-Teglund, A.; Rohde, M.; Medina, E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood 2008, 111, 3070–3080. [Google Scholar] [CrossRef]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef]
- Brinkmann, V.; Zychlinsky, A. Beneficial suicide: Why neutrophils die to make NETs. Nat. Rev. Microbiol. 2007, 5, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.K.; Motto, D.G.; Lamb, C.B.; Bergmeier, W.; Dockal, M.; Plaimauer, B.; Scheiflinger, F.; Ginsburg, D.; Wagner, D.D. Systemic antithrombotic effects of ADAMTS13. J. Exp. Med. 2006, 203, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Langhauser, F.; Desender, L.; Vandenbulcke, A.; Rottensteiner, H.; Plaimauer, B.; François, O.; Andersson, T.; Deckmyn, H.; Scheiflinger, F.; et al. ADAMTS13-mediated thrombolysis of t-PA-resistant occlusions in ischemic stroke in mice. Blood 2016, 127, 2337–2345. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, R.; Orje, J.N.; Capoferri, C.; Remuzzi, G.; Ruggeri, Z.M. Size regulation of von Willebrand factor-mediated platelet thrombi by ADAMTS13 in flowing blood. Blood 2006, 107, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Jin, S.Y.; Xue, J.; Sorvillo, N.; Voorberg, J.; Zheng, X.L. Essential domains of a disintegrin and metalloprotease with thrombospondin type 1 repeats-13 metalloprotease required for modulation of arterial thrombosis. Arter. Thromb. Vasc. Biol. 2011, 31, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Denorme, F.; Portier, I.; Rustad, J.L.; Cody, M.J.; de Araujo, C.V.; Hoki, C.; Alexander, M.D.; Grandhi, R.; Dyer, M.R.; Neal, M.D.; et al. Neutrophil extracellular traps regulate ischemic stroke brain injury. J. Clin. Investig. 2022, 132, e154225. [Google Scholar] [CrossRef]
- Yusuf, S.; Reddy, S.; Ounpuu, S.; Anand, S. Global burden of cardiovascular diseases: Part II: Variations in cardiovascular disease by specific ethnic groups and geographic regions and prevention strategies. Circulation 2001, 104, 2855–2864. [Google Scholar] [CrossRef]
- Wasay, M.; Khatri, I.A.; Kaul, S. Stroke in South Asian countries. Nat. Rev. Neurol. 2014, 10, 135–143. [Google Scholar] [CrossRef]
- Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1736–1788. [CrossRef]
- Kamran, S.; Singh, R.; Akhtar, N.; George, P.; Salam, A.; Babu, B.; Own, A.; Hamid, T.; Perkins, J.D. Left Heart Factors in Embolic Stroke of Undetermined Source in a Multiethnic Asian and North African Cohort. J. Am. Heart Assoc. 2020, 9, e016534. [Google Scholar] [CrossRef]
- Tan, C.T. Neurology in Asia. Neurology 2015, 84, 623–625. [Google Scholar] [CrossRef]
- Gujral, U.P.; Pradeepa, R.; Weber, M.B.; Narayan, K.M.; Mohan, V. Type 2 diabetes in South Asians: Similarities and differences with white Caucasian and other populations. Ann. N. Y. Acad. Sci. 2013, 1281, 51–63. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Kandula, N.R.; Ewing, S.K.; Herrington, D.; Liu, K.; Blaha, M.J.; Srivastava, S.; Dave, S.S.; Budoff, M.J. Comparing coronary artery calcium among U.S. South Asians with four racial/ethnic groups: The MASALA and MESA studies. Atherosclerosis 2014, 234, 102–107. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- De Meyer, S.F.; De Maeyer, B.; Deckmyn, H.; Vanhoorelbeke, K. Von Willebrand factor: Drug and drug target. Cardiovasc. Hematol. Disord. Drug Targets 2009, 9, 9–20. [Google Scholar] [CrossRef]
- De Meyer, S.F.; Stoll, G.; Wagner, D.D.; Kleinschnitz, C. von Willebrand factor: An emerging target in stroke therapy. Stroke 2012, 43, 599–606. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Duerschmied, D.; Schatzberg, D.; Monestier, M.; Myers, D.D., Jr.; Wrobleski, S.K.; Wakefield, T.W.; Hartwig, J.H.; Wagner, D.D. Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 2010, 107, 15880–15885. [Google Scholar] [CrossRef]
- Vogel, S.; Bodenstein, R.; Chen, Q.; Feil, S.; Feil, R.; Rheinlaender, J.; Schäffer, T.E.; Bohn, E.; Frick, J.S.; Borst, O.; et al. Platelet-derived HMGB1 is a critical mediator of thrombosis. J. Clin. Investig. 2015, 125, 4638–4654. [Google Scholar] [CrossRef]
- Maugeri, N.; Campana, L.; Gavina, M.; Covino, C.; De Metrio, M.; Panciroli, C.; Maiuri, L.; Maseri, A.; D’Angelo, A.; Bianchi, M.E.; et al. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 2014, 12, 2074–2088. [Google Scholar] [CrossRef]
- Marder, V.J.; Chute, D.J.; Starkman, S.; Abolian, A.M.; Kidwell, C.; Liebeskind, D.; Ovbiagele, B.; Vinuela, F.; Duckwiler, G.; Jahan, R.; et al. Analysis of thrombi retrieved from cerebral arteries of patients with acute ischemic stroke. Stroke 2006, 37, 2086–2093. [Google Scholar] [CrossRef]
- Staessens, S.; Denorme, F.; Francois, O.; Desender, L.; Dewaele, T.; Vanacker, P.; Deckmyn, H.; Vanhoorelbeke, K.; Andersson, T.; De Meyer, S.F. Structural analysis of ischemic stroke thrombi: Histological indications for therapy resistance. Haematologica 2020, 105, 498–507. [Google Scholar] [CrossRef]
- Kim, S.K.; Yoon, W.; Kim, T.S.; Kim, H.S.; Heo, T.W.; Park, M.S. Histologic Analysis of Retrieved Clots in Acute Ischemic Stroke: Correlation with Stroke Etiology and Gradient-Echo MRI. AJNR Am. J. Neuroradiol. 2015, 36, 1756–1762. [Google Scholar] [CrossRef]
- Niesten, J.M.; van der Schaaf, I.C.; van Dam, L.; Vink, A.; Vos, J.A.; Schonewille, W.J.; de Bruin, P.C.; Mali, W.P.; Velthuis, B.K. Histopathologic composition of cerebral thrombi of acute stroke patients is correlated with stroke subtype and thrombus attenuation. PLoS ONE 2014, 9, e88882. [Google Scholar] [CrossRef]
- Simons, N.; Mitchell, P.; Dowling, R.; Gonzales, M.; Yan, B. Thrombus composition in acute ischemic stroke: A histopathological study of thrombus extracted by endovascular retrieval. J. Neuroradiol. 2015, 42, 86–92. [Google Scholar] [CrossRef]
- Boeckh-Behrens, T.; Kleine, J.F.; Zimmer, C.; Neff, F.; Scheipl, F.; Pelisek, J.; Schirmer, L.; Nguyen, K.; Karatas, D.; Poppert, H. Thrombus Histology Suggests Cardioembolic Cause in Cryptogenic Stroke. Stroke 2016, 47, 1864–1871. [Google Scholar] [CrossRef]
- Turitto, V.T.; Weiss, H.J. Red blood cells: Their dual role in thrombus formation. Science 1980, 207, 541–543. [Google Scholar] [CrossRef]
- Fogelson, A.L.; Neeves, K.B. Fluid Mechanics of Blood Clot Formation. Annu. Rev. Fluid Mech. 2015, 47, 377–403. [Google Scholar] [CrossRef]
- Peyrou, V.; Lormeau, J.C.; Hérault, J.P.; Gaich, C.; Pfliegger, A.M.; Herbert, J.M. Contribution of erythrocytes to thrombin generation in whole blood. Thromb. Haemost. 1999, 81, 400–406. [Google Scholar] [CrossRef]
- Byrnes, J.R.; Wolberg, A.S. Red blood cells in thrombosis. Blood 2017, 130, 1795–1799. [Google Scholar] [CrossRef]
- McEwan, P.A.; Andrews, R.K.; Emsley, J. Glycoprotein Ibalpha inhibitor complex structure reveals a combined steric and allosteric mechanism of von Willebrand factor antagonism. Blood 2009, 114, 4883–4885. [Google Scholar] [CrossRef]
- López, J.A.; Andrews, R.K.; Afshar-Kharghan, V.; Berndt, M.C. Bernard-Soulier syndrome. Blood 1998, 91, 4397–4418. [Google Scholar] [CrossRef]
- Savage, B.; Almus-Jacobs, F.; Ruggeri, Z.M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell 1998, 94, 657–666. [Google Scholar] [CrossRef]
- Momi, S.; Tantucci, M.; Van Roy, M.; Ulrichts, H.; Ricci, G.; Gresele, P. Reperfusion of cerebral artery thrombosis by the GPIb-VWF blockade with the Nanobody ALX-0081 reduces brain infarct size in guinea pigs. Blood 2013, 121, 5088–5097. [Google Scholar] [CrossRef]
- Le Behot, A.; Gauberti, M.; Martinez De Lizarrondo, S.; Montagne, A.; Lemarchand, E.; Repesse, Y.; Guillou, S.; Denis, C.V.; Maubert, E.; Orset, C.; et al. GpIbα-VWF blockade restores vessel patency by dissolving platelet aggregates formed under very high shear rate in mice. Blood 2014, 123, 3354–3363. [Google Scholar] [CrossRef]
- Levy, G.G.; Nichols, W.C.; Lian, E.C.; Foroud, T.; McClintick, J.N.; McGee, B.M.; Yang, A.Y.; Siemieniak, D.R.; Stark, K.R.; Gruppo, R.; et al. Mutations in a member of the ADAMTS gene family cause thrombotic thrombocytopenic purpura. Nature 2001, 413, 488–494. [Google Scholar] [CrossRef]
- Zheng, X.; Chung, D.; Takayama, T.K.; Majerus, E.M.; Sadler, J.E.; Fujikawa, K. Structure of von Willebrand factor-cleaving protease (ADAMTS13), a metalloprotease involved in thrombotic thrombocytopenic purpura. J. Biol. Chem. 2001, 276, 41059–41063. [Google Scholar] [CrossRef]
- Liebeskind, D.S.; Sanossian, N.; Yong, W.H.; Starkman, S.; Tsang, M.P.; Moya, A.L.; Zheng, D.D.; Abolian, A.M.; Kim, D.; Ali, L.K.; et al. CT and MRI early vessel signs reflect clot composition in acute stroke. Stroke 2011, 42, 1237–1243. [Google Scholar] [CrossRef]
- Niesten, J.M.; van der Schaaf, I.C.; van der Graaf, Y.; Kappelle, L.J.; Biessels, G.J.; Horsch, A.D.; Dankbaar, J.W.; Luitse, M.J.; van Seeters, T.; Smit, E.J.; et al. Predictive value of thrombus attenuation on thin-slice non-contrast CT for persistent occlusion after intravenous thrombolysis. CerebroVasc. Dis. 2014, 37, 116–122. [Google Scholar] [CrossRef]
- Meier, T.R.; Myers, D.D., Jr.; Wrobleski, S.K.; Zajkowski, P.J.; Hawley, A.E.; Bedard, P.W.; Ballard, N.E.; Londy, F.J.; Kaila, N.; Vlasuk, G.P.; et al. Prophylactic P-selectin inhibition with PSI-421 promotes resolution of venous thrombosis without anticoagulation. Thromb. Haemost. 2008, 99, 343–351. [Google Scholar] [CrossRef]
- Boeckh-Behrens, T.; Schubert, M.; Förschler, A.; Prothmann, S.; Kreiser, K.; Zimmer, C.; Riegger, J.; Bauer, J.; Neff, F.; Kehl, V.; et al. The Impact of Histological Clot Composition in Embolic Stroke. Clin. Neuroradiol. 2016, 26, 189–197. [Google Scholar] [CrossRef]
- Darbousset, R.; Thomas, G.M.; Mezouar, S.; Frère, C.; Bonier, R.; Mackman, N.; Renné, T.; Dignat-George, F.; Dubois, C.; Panicot-Dubois, L. Tissue factor-positive neutrophils bind to injured endothelial wall and initiate thrombus formation. Blood 2012, 120, 2133–2143. [Google Scholar] [CrossRef]
- von Brühl, M.L.; Stark, K.; Steinhart, A.; Chandraratne, S.; Konrad, I.; Lorenz, M.; Khandoga, A.; Tirniceriu, A.; Coletti, R.; Köllnberger, M.; et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 2012, 209, 819–835. [Google Scholar] [CrossRef]
- Martinod, K.; Wagner, D.D. Thrombosis: Tangled up in NETs. Blood 2014, 123, 2768–2776. [Google Scholar] [CrossRef]
- Urban, C.F.; Ermert, D.; Schmid, M.; Abu-Abed, U.; Goosmann, C.; Nacken, W.; Brinkmann, V.; Jungblut, P.R.; Zychlinsky, A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog. 2009, 5, e1000639. [Google Scholar] [CrossRef]
- Medina, E. Neutrophil extracellular traps: A strategic tactic to defeat pathogens with potential consequences for the host. J. Innate Immun. 2009, 1, 176–180. [Google Scholar] [CrossRef]
- Wartha, F.; Beiter, K.; Normark, S.; Henriques-Normark, B. Neutrophil extracellular traps: Casting the NET over pathogenesis. Curr. Opin. Microbiol. 2007, 10, 52–56. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Simpson, A.J.; Aziz, R.K.; Liu, G.Y.; Kristian, S.A.; Kotb, M.; Feramisco, J.; Nizet, V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 2006, 16, 396–400. [Google Scholar] [CrossRef]
- Mutua, V.; Gershwin, L.J. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin. Rev. Allergy Immunol. 2021, 61, 194–211. [Google Scholar] [CrossRef]
- Grässle, S.; Huck, V.; Pappelbaum, K.I.; Gorzelanny, C.; Aponte-Santamaría, C.; Baldauf, C.; Gräter, F.; Schneppenheim, R.; Obser, T.; Schneider, S.W. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arter. Thromb. Vasc. Biol. 2014, 34, 1382–1389. [Google Scholar] [CrossRef]
- Fuchs, T.A.; Brill, A.; Wagner, D.D. Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arter. Thromb. Vasc. Biol. 2012, 32, 1777–1783. [Google Scholar] [CrossRef] [PubMed]
- Carminita, E.; Crescence, L.; Panicot-Dubois, L.; Dubois, C. Role of Neutrophils and NETs in Animal Models of Thrombosis. Int. J. Mol. Sci. 2022, 23, 1411. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, C.; Varjú, I.; Sótonyi, P.; Szabó, L.; Krumrey, M.; Hoell, A.; Bóta, A.; Varga, Z.; Komorowicz, E.; Kolev, K. Mechanical stability and fibrinolytic resistance of clots containing fibrin, DNA, and histones. J. Biol. Chem. 2013, 288, 6946–6956. [Google Scholar] [CrossRef] [PubMed]
- Bye, A.P.; Unsworth, A.J.; Gibbins, J.M. Platelet signaling: A complex interplay between inhibitory and activatory networks. J. Thromb. Haemost. 2016, 14, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Martinod, K.; Deppermann, C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets 2021, 32, 314–324. [Google Scholar] [CrossRef]
- Denorme, F.; Rustad, J.L.; Campbell, R.A. Brothers in arms: Platelets and neutrophils in ischemic stroke. Curr. Opin. Hematol. 2021, 28, 301–307. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Schattner, M. Platelets: New Bricks in the Building of Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 271. [Google Scholar] [CrossRef]
- Dyer, M.R.; Chen, Q.; Haldeman, S.; Yazdani, H.; Hoffman, R.; Loughran, P.; Tsung, A.; Zuckerbraun, B.S.; Simmons, R.L.; Neal, M.D. Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci. Rep. 2018, 8, 2068. [Google Scholar] [CrossRef]
- Nakazawa, D.; Desai, J.; Steiger, S.; Muller, S.; Devarapu, S.K.; Mulay, S.R.; Iwakura, T.; Anders, H.J. Activated platelets induce MLKL-driven neutrophil necroptosis and release of neutrophil extracellular traps in venous thrombosis. Cell Death Discov. 2018, 4, 6. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pir, G.J.; Parray, A.; Ayadathil, R.; Pananchikkal, S.V.; Mir, F.A.; Muhammad, I.; Abubakar, A.; Amir, N.; Hussain, S.; Haroon, K.H.; et al. Platelet–Neutrophil Association in NETs-Rich Areas in the Retrieved AIS Patient Thrombi. Int. J. Mol. Sci. 2022, 23, 14477. https://doi.org/10.3390/ijms232214477
Pir GJ, Parray A, Ayadathil R, Pananchikkal SV, Mir FA, Muhammad I, Abubakar A, Amir N, Hussain S, Haroon KH, et al. Platelet–Neutrophil Association in NETs-Rich Areas in the Retrieved AIS Patient Thrombi. International Journal of Molecular Sciences. 2022; 23(22):14477. https://doi.org/10.3390/ijms232214477
Chicago/Turabian StylePir, Ghulam Jeelani, Aijaz Parray, Raheem Ayadathil, Sajitha V. Pananchikkal, Fayaz Ahmad Mir, Islam Muhammad, Ahmed Abubakar, Nueman Amir, Sohail Hussain, Khawaja H. Haroon, and et al. 2022. "Platelet–Neutrophil Association in NETs-Rich Areas in the Retrieved AIS Patient Thrombi" International Journal of Molecular Sciences 23, no. 22: 14477. https://doi.org/10.3390/ijms232214477
APA StylePir, G. J., Parray, A., Ayadathil, R., Pananchikkal, S. V., Mir, F. A., Muhammad, I., Abubakar, A., Amir, N., Hussain, S., Haroon, K. H., Muhammad, A., Imam, Y., Patro, S. N., Akhtar, N., Zakaria, A., & Kamran, S. (2022). Platelet–Neutrophil Association in NETs-Rich Areas in the Retrieved AIS Patient Thrombi. International Journal of Molecular Sciences, 23(22), 14477. https://doi.org/10.3390/ijms232214477




