Breed and Feeding System Impact the Bioactive Anti-Inflammatory Properties of Bovine Milk
Abstract
1. Introduction
2. Results
2.1. Influence of Breed on the Biomolecule Content and Antioxidant Activity of Milk and Dairy Products
2.1.1. Average Milk Production
2.1.2. Functional Profile and Antioxidant Activity of Bovine Milk and Dairy Products
2.2. Influence of Feeding System, Limited to Holstein, on the Biomolecule Content and Antioxidant Activity of Milk and Dairy Products
2.2.1. Average Milk Production
2.2.2. Biomolecule Content and Antioxidant Activity of Milk and Dairy Products
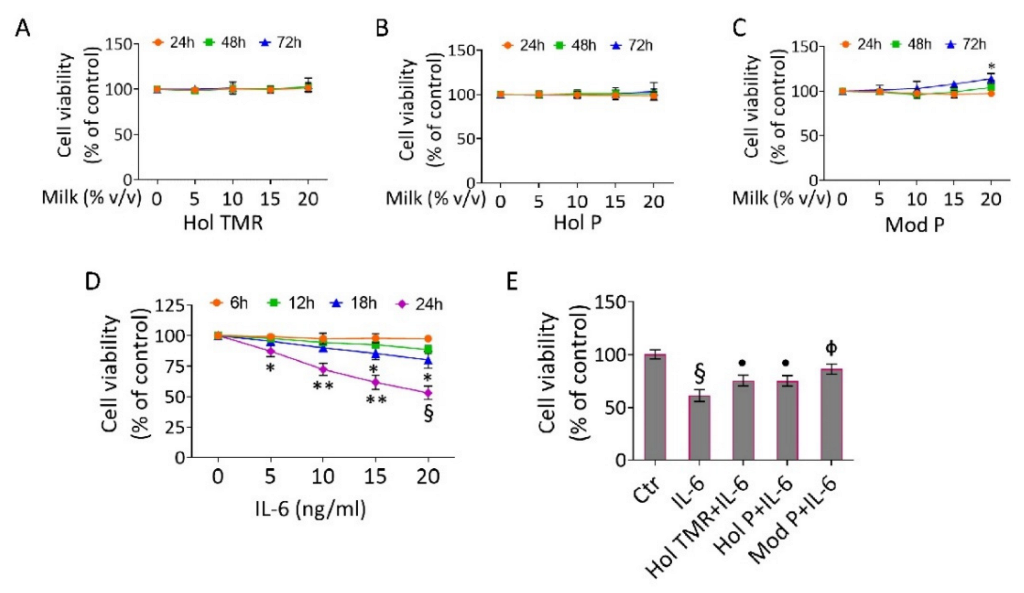
2.3. Milk Extract Effects on Cell Viability
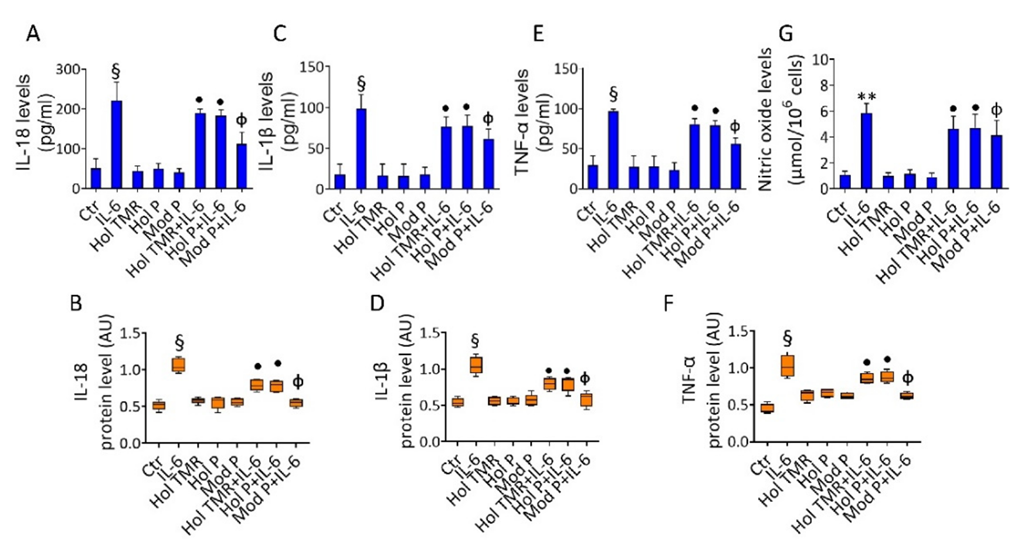
2.4. Milk Extract Effects on Inflammation
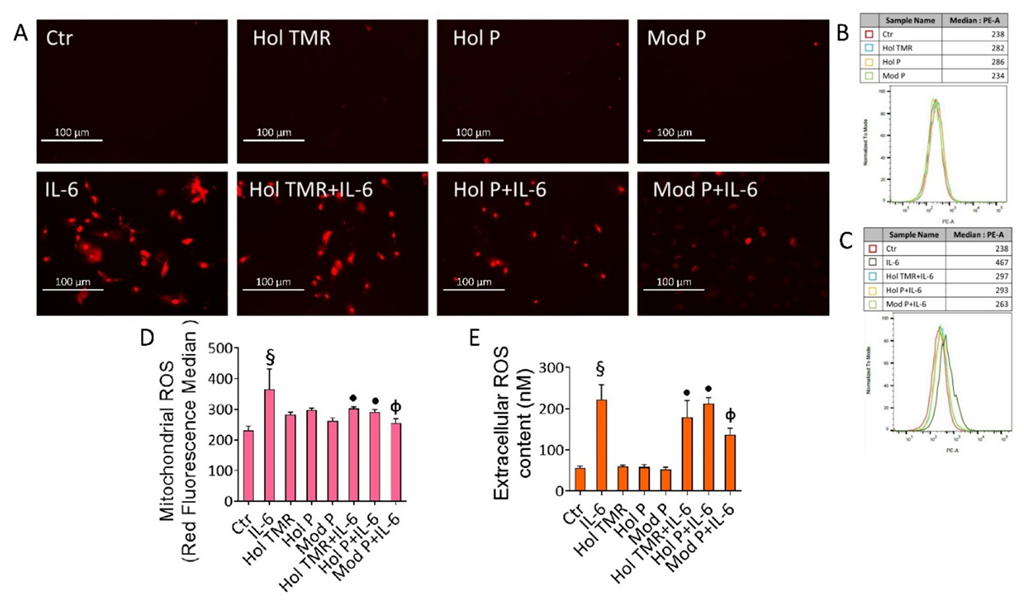
2.5. Milk Extract Effects on Oxidative Stress
2.6. Milk Extract Effects on Cell Death Mechanisms
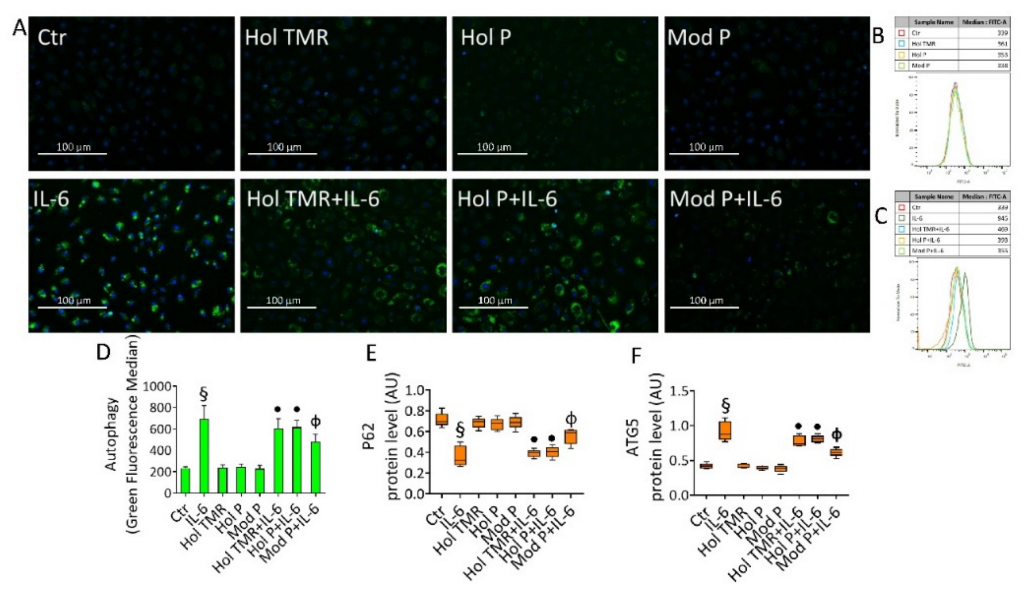
2.7. Milk Extract Effects on Autophagy Induction
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Experimental Design 1—Influence of Breed on the Functional Molecules and Antioxidant Activity of Milk and Dairy Products
4.3. Experimental Design 2—Influence of Feeding System on the Biomolecule Content and Antioxidant Activity of Milk and Dairy Products
4.4. Milk and Dairy Product Sampling and Preparation
4.5. Betaine and Carnitine Profile by HPLC-ESI-MS/MS Analysis
4.6. FRAP and TAC Assay
4.7. Cell Culture and Treatments
4.8. Cell Viability Detection
4.9. Cytokine Level Determination
4.10. Nitric Oxide Level Assessment
4.11. Oxidative Stress Evaluation
4.12. Apoptosis Detection
4.13. Autophagy Assessment
4.14. Cell Lysis and Immunoblotting Analysis
4.15. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mendelsohn, R. The challenge of conserving indigenous domesticated animals. Ecol. Econ. 2003, 45, 501–510. [Google Scholar] [CrossRef]
- Mastrangelo, S.; Ciani, E.; Ajmone Marsan, P.; Bagnato, A.; Battaglini, L.; Bozzi, R.; Carta, A.; Catillo, G.; Cassandro, M.; Casu, S.; et al. Conservation status and historical relatedness of Italian cattle breeds. Genet. Sel. Evol. 2018, 50, 35. [Google Scholar] [CrossRef] [PubMed]
- Valenti, B.; Criscione, A.; Moltisanti, V.; Bordonaro, S.; De Angelis, A.; Marletta, D.; Di Paola, F.; Avondo, M. Genetic polymorphisms at candidate genes affecting fat content and fatty acid composition in Modicana cows: Effects on milk production traits in different feeding systems. Animal 2019, 13, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Bordonaro, S.; Tumino, S.; Marletta, D.; De Angelis, A.; Di Paola, F.; Avondo, M.; Valenti, B. Effect of GH p.L127V Polymorphism and Feeding Systems on Milk Production Traits and Fatty Acid Composition in Modicana Cows. Animals 2020, 10, 1651. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Glasser, F.; Ferlay, A.; Bernard, L.; Rouel, J.; Doreau, M. Diet, rumen biohydrogenation and nutritional quality of cow and goat milk fat. Eur. J. Lipid Sci. Technol. 2007, 109, 828–855. [Google Scholar] [CrossRef]
- Morales-Almaráz, E.; Soldado, A.; González, A.; Martínez-Fernández, A.; Domínguez-Vara, I.; de la Roza-Delgado, B.; Vicente, F. Improving the fatty acid profile of dairy cow milk by combining grazing with feeding of total mixed ration. J. Dairy Res. 2010, 77, 225–230. [Google Scholar] [CrossRef]
- O’Callaghan, T.F.; Hennessy, D.; McAuliffe, S.; Kilcawley, K.N.; O’Donovan, M.; Dillon, P.; Ross, R.P.; Stanton, C. Effect of pasture versus indoor feeding systems on raw milk composition and quality over an entire lactation. J. Dairy Sci. 2016, 99, 9424–9440. [Google Scholar] [CrossRef]
- Marín, M.P.; Meléndez, P.G.; Aranda, P.; Ríos, C. Conjugated linoleic acid content and fatty acids profile of milk from grazing dairy cows in southern Chile fed varying amounts of concentrate. J. Appl. Anim. Res. 2018, 46, 150–154. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Giovane, A.; Casale, R.; Cautela, D.; Castaldo, D.; Iannaccone, F.; Neglia, G.; Campanile, G.; Balestrieri, M.L. Ruminant meat and milk contain δ-valerobetaine, another precursor of trimethylamine N-oxide (TMAO) like γ-butyrobetaine. Food Chem. 2018, 260, 193–199. [Google Scholar] [CrossRef]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 16, 7–30. [Google Scholar] [CrossRef]
- Zhao, G.; He, F.; Wu, C.; Li, P.; Li, N.; Deng, J.; Zhu, G.; Ren, W.; Peng, Y. Betaine in Inflammation: Mechanistic Aspects and Applications. Front. Immunol. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Cacciola, N.A.; Martino, E.; Borrelli, F.; Fiorino, F.; Lombardi, A.; Neglia, G.; Balestrieri, M.L.; Campanile, G. ROS-mediated apoptotic cell death of human colon cancer LoVo cells by milk δ-valerobetaine. Sci. Rep. 2020, 10, 8978. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Mele, L.; Martino, E.; Salzano, A.; Restucci, B.; Cautela, D.; Tatullo, M.; Balestrieri, M.L.; Campanile, G. Synergistic effect of dietary betaines on SIRT1-mediated apoptosis in human oral squamous cell carcinoma Cal 27. Cancers 2020, 12, 2468. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Neglia, G.; Balestrieri, M.L.; Campanile, G. SIRT3 and Metabolic Reprogramming Mediate the Antiproliferative Effects of Whey in Human Colon Cancer Cells. Cancers 2021, 13, 5196. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, N.A.; Salzano, A.; D’Onofrio, N.; Venneri, T.; Cicco, P.D.; Vinale, F.; Petillo, O.; Martano, M.; Maiolino, P.; Neglia, G.; et al. Buffalo Milk Whey Activates Necroptosis and Apoptosis in a Xenograft Model of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 8464. [Google Scholar] [CrossRef]
- Servillo, L.; D’Onofrio, N.; Neglia, G.; Casale, R.; Cautela, D.; Marrelli, M.; Limone, A.; Campanile, G.; Balestrieri, M.L. Carnitine precursors and short-chain acylcarnitines in water buffalo milk. J. Agric. Food Chem. 2018, 66, 8142–8149. [Google Scholar] [CrossRef]
- Sutton, J.D. Altering Milk Composition by Feeding. J. Dairy Sci. 1989, 72, 2801–2814. [Google Scholar] [CrossRef]
- Santos, J.E.P. Feeding for Milk Composition. In Proceedings of the VI International Congress on Bovine Medicine; Spanish Association of Specialists in Bovine Medicine (ANEMBE), Santiago de Compostela, Spain, 29 July–1 August 2002. [Google Scholar]
- Salzano, A.; Licitra, F.; D’Onofrio, N.; Balestrieri, M.L.; Limone, A.; Campanile, G.; D’Occhio, M.J.; Neglia, G. Short communication: Space allocation in intensive Mediterranean buffalo production influences the profile of functional biomolecules in milk and dairy products. J. Dairy Sci. 2019, 102, 7717–7722. [Google Scholar] [CrossRef]
- Salzano, A.; Neglia, G.; D’Onofrio, N.; Balestrieri, M.L.; Limone, A.; Cotticelli, A.; Marrone, R.; Anastasio, A.; D’Occhio, M.J.; Campanile, G. Green feed increases antioxidant and antineoplastic activity of buffalo milk: A globally significant livestock. Food Chem. 2021, 344, 128669. [Google Scholar] [CrossRef]
- Givens, D.I. The Role of Animal Nutrition in Improving the Nutritive Value of Animal-Derived Foods in Relation to Chronic Disease. Proc. Nutr. Soc. 2007, 64, 395–402. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Association of Dairy Intake with Cardiovascular Disease and Mortality in 21 Countries from Five Continents (Pure): A Prospective Cohort Study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef]
- Lamarche, B.; Givens, D.I.; Soedamah-Muthu, S.; Krauss, R.M.; Jakobsen, M.U.; Bischoff-Ferrari, H.; Pan, A.; Després, J. Does Milk Consumption Contribute to Cardiometabolic Health and Overall Diet Quality? Can. J. Cardiol. 2016, 32, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Lovegrovem, A.J.; Givens, D.I. Dairy Food Products: Good or Bad for Cardiometabolic Disease? Nutr. Res. Rev. 2016, 29, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Silveira Rossi, J.L.; Barbalho, S.M.; Reverete de Araujo, R.; Bechara, M.D.; Sloan, K.P.; Sloan, L.A. Metabolic syndrome and cardiovascular diseases: Going beyond traditional risk factors. Diabetes/Metab. Res. Rev. 2022, 38, e3502. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Samouda, H. Clinical and biological risk factors associated with inflammation in patients with type 2 diabetes mellitus. BMC Endocr. Disord. 2022, 22, 16. [Google Scholar] [CrossRef]
- Lecube, A.; López-Cano, C. Obesity, a Diet-Induced Inflammatory Disease. Nutrients 2019, 11, 2284. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Guzzardi, M.A.; Pugliese, G.; Bottiglieri, F.; Pelosini, C.; Muscogiuri, G.; Barrea, L.; Savastano, S.; Colao, A. Obesity-related gut hormones and cancer: Novel insight into the pathophysiology. Int. J. Obes. 2021, 45, 1886–1898. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 5, 1822–1832. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Balestrieri, A.; Neglia, G.; Monaco, A.; Tatullo, M.; Casale, R.; Limone, A.; Balestrieri, M.L.; Campanile, G. Antioxidant and anti-inflammatory activities of buffalo milk δ-Valerobetaine. J. Agric. Food Chem. 2019, 67, 1702–1710. [Google Scholar] [CrossRef]
- Martino, E.; Balestrieri, A.; Anastasio, C.; Maione, M.; Mele, L.; Cautela, D.; Campanile, G.; Balestrieri, M.L.; D’Onofrio, N. SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants 2022, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- Haikonen, R.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K. Diet- and microbiota-related metabolite, 5-aminovaleric acid betaine (5-AVAB), in health and disease. Trends Endocrinol. Metab. 2022, 33, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M.L.; Pastore, A. Where Does Nε-Trimethyllysine for the Carnitine Biosynthesis in Mammals Come from? PLoS ONE 2014, 9, e84589. [Google Scholar] [CrossRef] [PubMed]
- Rebouche, C.J. Kinetics, pharmacokinetics, and regulation of L-carnitine and acetyl-L-carnitine metabolism. Ann. NY Acad. Sci. 2004, 1033, 30–41. [Google Scholar] [CrossRef]
- Rebouche, C.J.; Lehman, L.J.; Olson, L. epsilon-N-trimethyllysine availability regulates the rate of carnitine biosynthesis in the growing rat. J Nutr. 1986, 116, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Licitra, G.; Carpino, S.; Schadt, L.; Avondo, M.; Barresi, S. Forage quality of native pastures in a Mediterranean area. Anim. Feed Sci. Technol. 1997, 69, 315–328. [Google Scholar] [CrossRef]
- AOAC. AOAC Official Methods of Analysis, 13th ed.; Association of Official Analytic Chemists: Washington, DC, USA, 1980. [Google Scholar]
- INRA. Alimentation Des Bovins, Ovins et Caprins–Besoins des Animaux–Valeurs des Aliments–Tables INRA 2007; Editions Quae: Versailles, France, 2007; p. 307. [Google Scholar]
- IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0; IBM Corp: Armonk, NY, USA, 2019. [Google Scholar]

| γ-Butyrobetaine | δ-Valerobetaine | Glycine Betaine | l-Carnitine | Acetyl-l-Carnitine | Propionyl-l-Carnitine | ||
|---|---|---|---|---|---|---|---|
| Milk (mg/L) | Hol P | 1.73 ± 0.08 X | 7.44 ± 0.12 X | 6.76 ± 0.09 X | 17.53 ± 0.24 X | 19.92 ± 0.35 x | 7.12 ± 0.13 X |
| Mod P | 2.18 ± 0.06 Y | 7.89 ± 0.09 Y | 7.11 ± 0.10 Y | 18.80 ± 0.24 Y | 20.91 ± 0.22 y | 7.85 ± 0.11 Y | |
| Whey (mg/Kg) | Hol P | 1.25 ± 0.06 X | 6.02 ± 0.22 X | 5.81 ± 0.10 X | 15.47 ± 0.30 x | 15.21 ± 0.15 x | 5.86 ± 0.17 |
| Mod P | 1.61 ± 0.05 Y | 6.84 ± 0.17 Y | 6.23 ± 0.10 Y | 16.49 ± 0.26 y | 15.75 ± 0.17 y | 6.13 ± 0.11 | |
| Ricotta cheese (mg/Kg) | Hol P | 1.19 ± 0.65 x | 5.59 ± 0.25 | 5.69 ± 0.19 | 14.80 ± 0.43 | 14.43 ± 0.16 x | 5.48 ± 0.09 x |
| Mod P | 1.39 ± 0.48 y | 5.86 ± 0.20 | 5.82 ± 0.17 | 15.20 ± 0.33 | 14.98 ± 0.14 y | 5.88 ± 0.13 y | |
| Mozzarella cheese (mg/Kg) | Hol P | 1.25 ± 0.04 X | 6.24 ± 0.30 | 5.88 ± 0.19 | 15.37 ± 0.36 | 15.53 ± 0.17 | 6.51 ± 0.15 |
| Mod P | 1.51 ± 0.04 Y | 6.38 ± 0.22 | 6.00 ± 0.14 | 15.87 ± 0.25 | 15.83 ± 0.14 | 6.33 ± 0.09 |
| TAC | FRAP | ||
|---|---|---|---|
| Milk (nmol/L) | Hol P | 87.48 ± 2.81 X | 104.94 ± 9.02 X |
| Mod P | 112.46 ± 3.25 Y | 144.97 ± 6.13 Y | |
| Whey (nmol/Kg) | Hol P | 63.97 ± 3.22 X | 53.88 ± 3.10 X |
| Mod P | 89.92 ± 1.87 Y | 72.66 ± 1.69 Y | |
| Ricotta cheese (nmol/Kg) | Hol P | 42.31 ± 1.84 X | 33.97 ± 1.59 X |
| Mod P | 55.34 ± 1.65 Y | 47.85 ± 1.96 Y | |
| Mozzarella cheese (nmol/Kg) | Hol P | 59.82 ± 2.53 X | 50.34 ± 2.28 X |
| Mod P | 77.08 ± 1.75 Y | 66.71 ± 1.67 Y |
| γ-Butyrobetaine | δ-Valerobetaine | Glycine Betaine | l-Carnitine | Acetyl-l-Carnitine | Propionyl-l-Carnitine | ||
|---|---|---|---|---|---|---|---|
| Milk (mg/L) | Hol TMR | 2.08 ± 0.11 X | 7.87 ± 0.19 X | 6.94 ± 0.15 | 18.79 ± 0.43 x | 20.45 ± 0.27 | 7.16 ± 0.18 |
| Hol P | 1.73 ± 0.08 Y | 7.44 ± 0.12 Y | 6.76 ± 0.09 | 17.53 ± 0.24 y | 19.92 ± 0.35 | 7.12 ± 0.13 | |
| Whey (mg/Kg) | Hol TMR | 1.42 ± 0.11 | 7.05 ± 0.35 X | 6.52 ± 0.19 X | 17.68 ± 0.55 X | 16.47 ± 0.28 X | 6.06 ± 0.13 |
| Hol P | 1.25 ± 0.06 | 6.02 ± 0.22 Y | 5.81 ± 0.10 Y | 15.47 ± 0.30 Y | 15.21 ± 0.15 Y | 5.86 ± 0.17 | |
| Ricotta cheese (mg/Kg) | Hol TMR | 1.22 ± 0.07 | 5.63 ± 0.26 | 5.43 ± 0.15 | 14.44 ± 0.43 | 14.20 ± 0.20 | 5.48 ± 0.19 |
| Hol P | 1.19 ± 0.65 | 5.59 ± 0.25 | 5.69 ± 0.19 | 14.80 ± 0.43 | 14.43 ± 0.16 | 5.89 ± 0.15 | |
| Mozzarella cheese (mg/Kg) | Hol TMR | 1.43 ± 0.08 | 6.38 ± 0.26 | 6.04 ± 0.20 | 15.38 ± 0.42 | 15.37 ± 0.26 | 6.22 ± 0.12 |
| Hol P | 1.25 ± 0.04 | 6.24 ± 0.30 | 5.88 ± 0.19 | 15.37 ± 0.36 | 15.53 ± 0.17 | 6.51 ± 0.15 |
| TAC | FRAP | ||
|---|---|---|---|
| Milk (nmol/L) | Hol TMR | 89.39 ± 3.79 | 94.34 ± 9.49 |
| Hol P | 87.48 ± 2.81 | 104.94 ± 9.02 | |
| Whey (nmol/Kg) | Hol TMR | 57.08 ± 4.93 | 47.70 ± 5.13 |
| Hol P | 63.97 ± 3.22 | 53.88 ± 3.10 | |
| Ricotta cheese (nmol/Kg) | Hol TMR | 41.43 ± 3.00 | 33.18 ± 2.83 |
| Hol P | 42.31 ± 1.84 | 33.97 ± 1.59 | |
| Mozzarella cheese (nmol/Kg) | Hol TMR | 59.28 ± 2.70 | 48.87 ± 2.64 |
| Hol P | 59.82 ± 2.53 | 50.34 ± 2.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salzano, A.; Di Meo, M.C.; D’Onofrio, N.; Bifulco, G.; Cotticelli, A.; Licitra, F.; Iraci Fuintino, A.; Cascone, G.; Balestrieri, M.L.; Varricchio, E.; et al. Breed and Feeding System Impact the Bioactive Anti-Inflammatory Properties of Bovine Milk. Int. J. Mol. Sci. 2022, 23, 11088. https://doi.org/10.3390/ijms231911088
Salzano A, Di Meo MC, D’Onofrio N, Bifulco G, Cotticelli A, Licitra F, Iraci Fuintino A, Cascone G, Balestrieri ML, Varricchio E, et al. Breed and Feeding System Impact the Bioactive Anti-Inflammatory Properties of Bovine Milk. International Journal of Molecular Sciences. 2022; 23(19):11088. https://doi.org/10.3390/ijms231911088
Chicago/Turabian StyleSalzano, Angela, Maria Chiara Di Meo, Nunzia D’Onofrio, Giovanna Bifulco, Alessio Cotticelli, Francesca Licitra, Antonio Iraci Fuintino, Giuseppe Cascone, Maria Luisa Balestrieri, Ettore Varricchio, and et al. 2022. "Breed and Feeding System Impact the Bioactive Anti-Inflammatory Properties of Bovine Milk" International Journal of Molecular Sciences 23, no. 19: 11088. https://doi.org/10.3390/ijms231911088
APA StyleSalzano, A., Di Meo, M. C., D’Onofrio, N., Bifulco, G., Cotticelli, A., Licitra, F., Iraci Fuintino, A., Cascone, G., Balestrieri, M. L., Varricchio, E., & Campanile, G. (2022). Breed and Feeding System Impact the Bioactive Anti-Inflammatory Properties of Bovine Milk. International Journal of Molecular Sciences, 23(19), 11088. https://doi.org/10.3390/ijms231911088









