Click Chemistry of Selenium Dihalides: Novel Bicyclic Organoselenium Compounds Based on Selenenylation/Bis-Functionalization Reactions and Evaluation of Glutathione Peroxidase-like Activity
Abstract
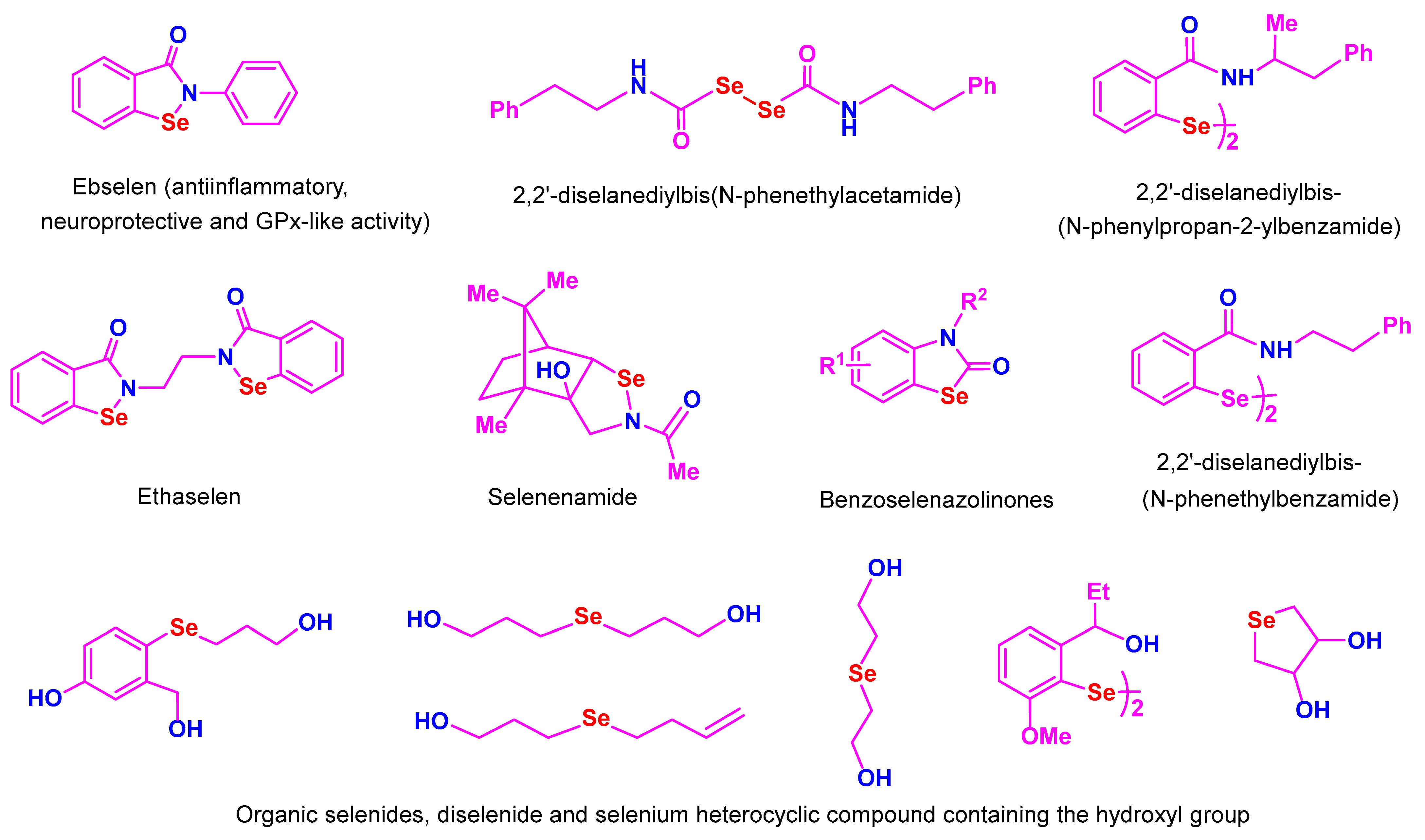
1. Introduction
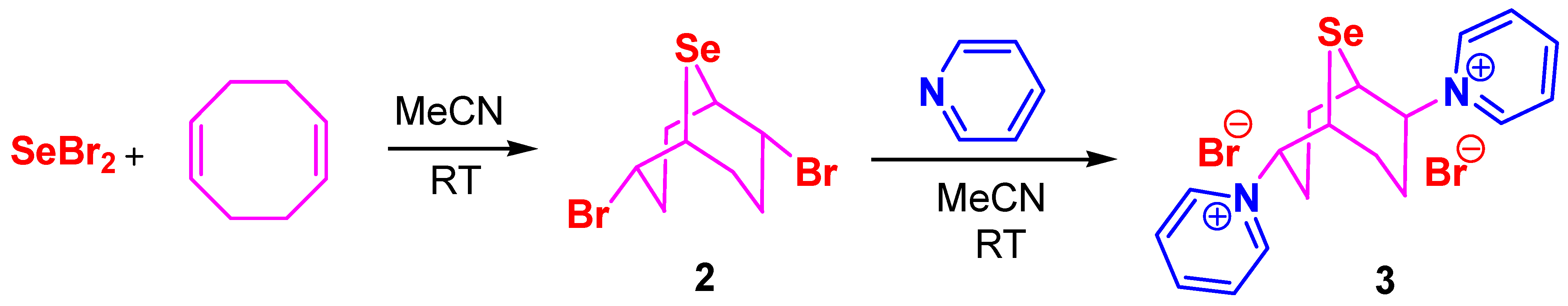
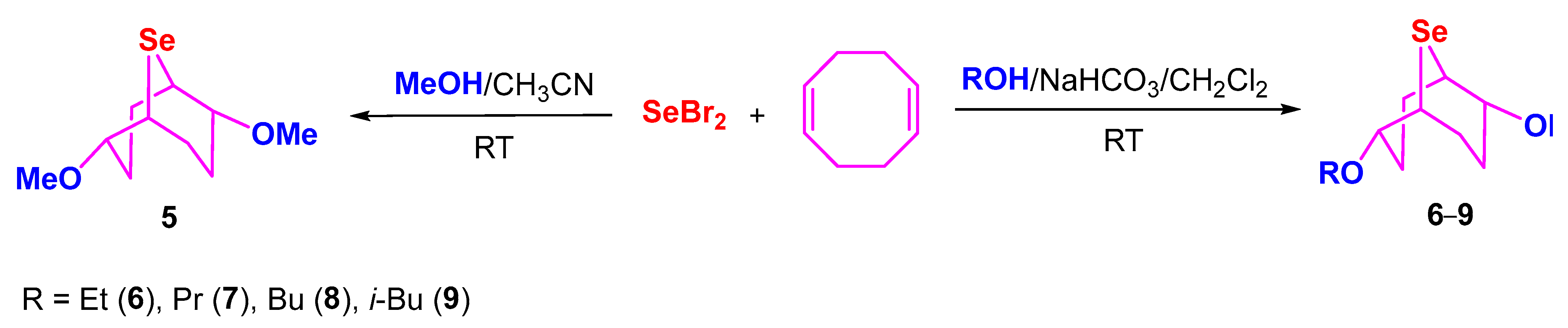
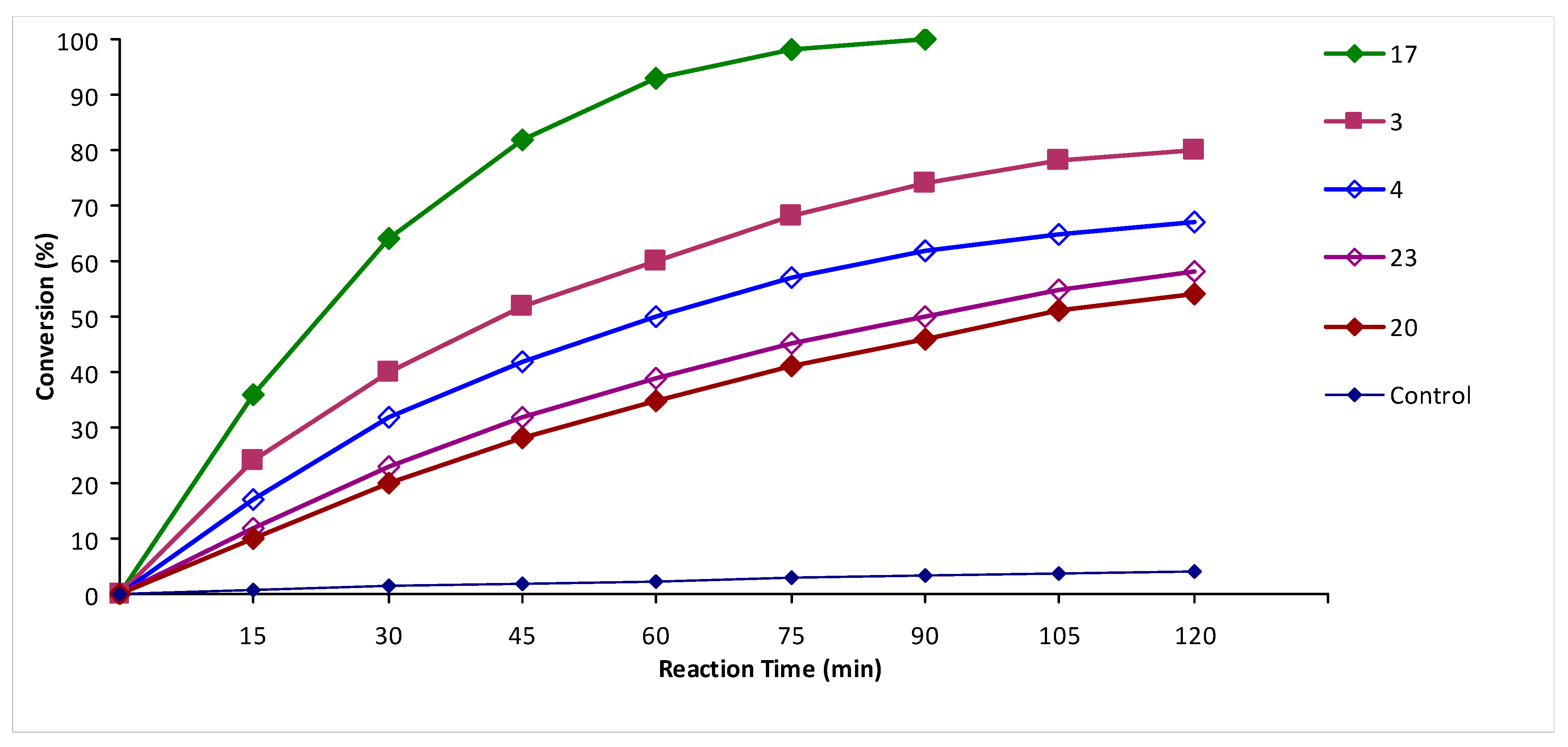
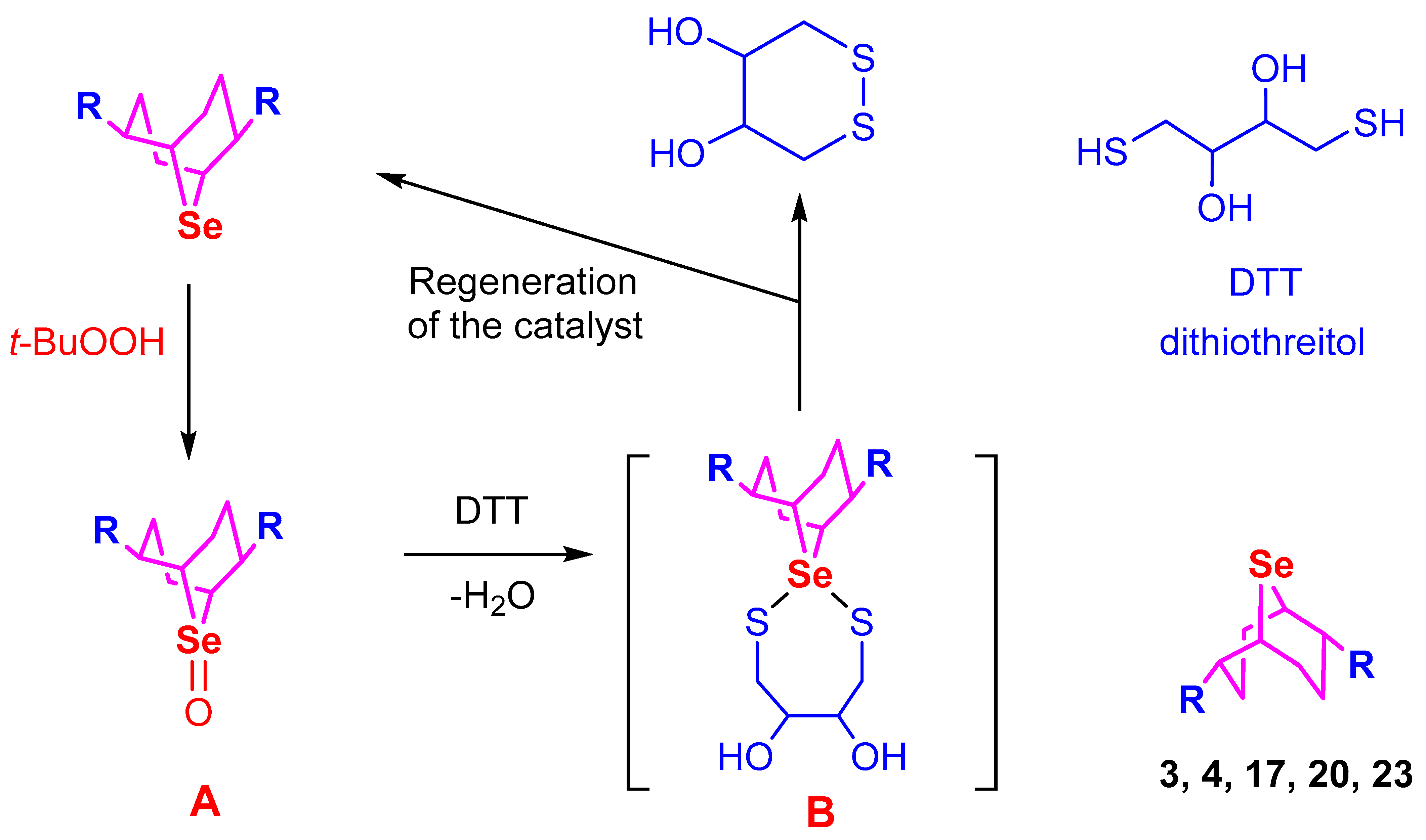
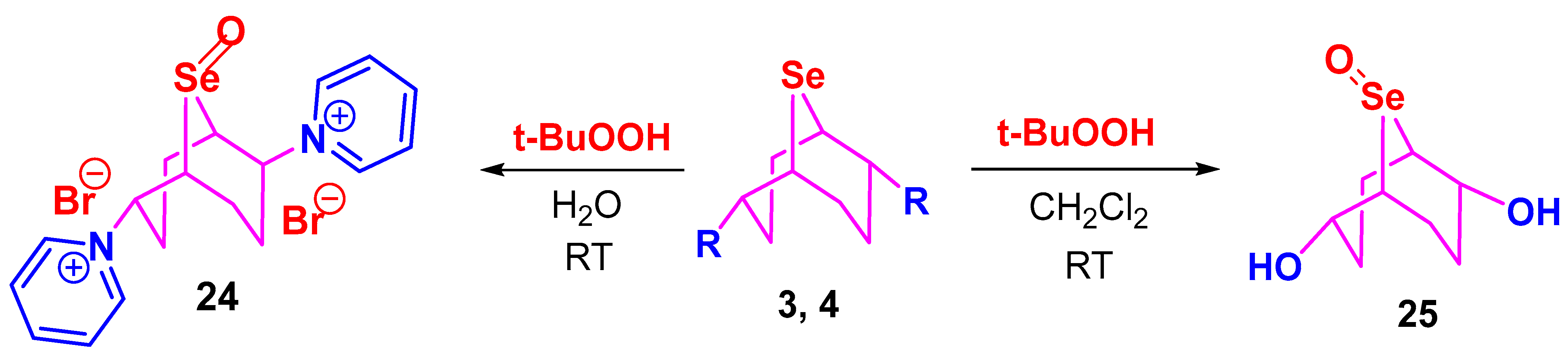
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of Compounds 4–9
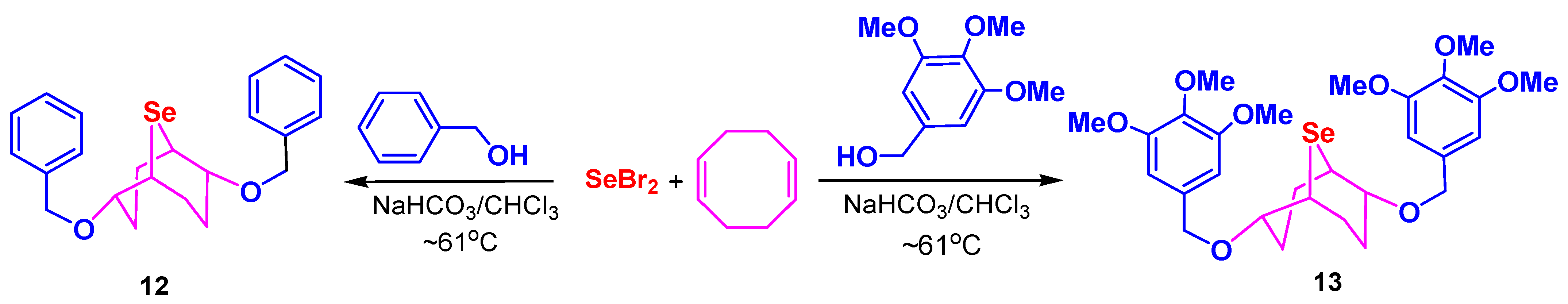
3.3. Synthesis of Compounds 10–13
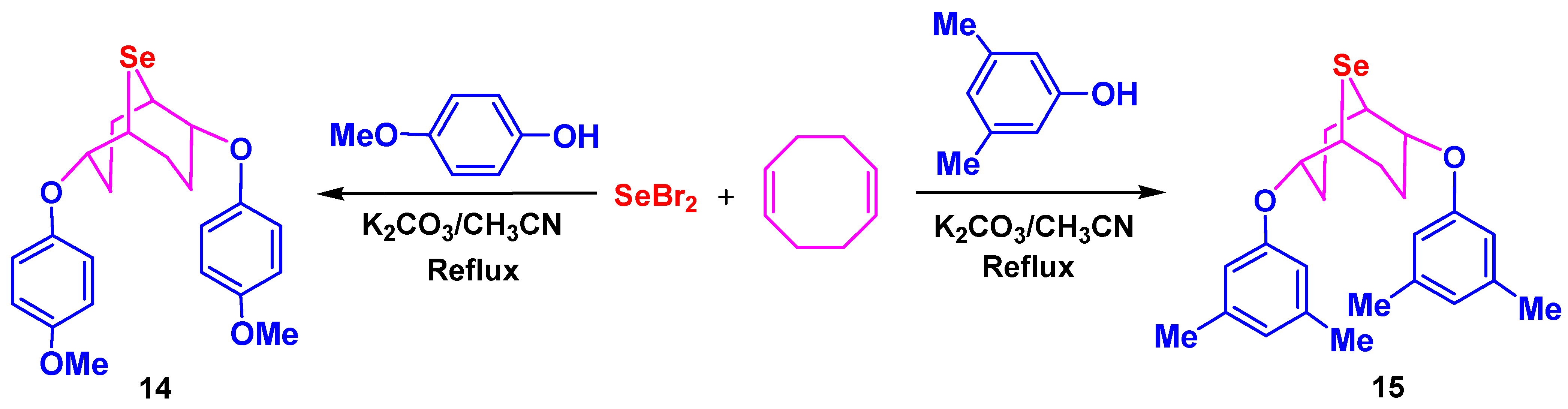
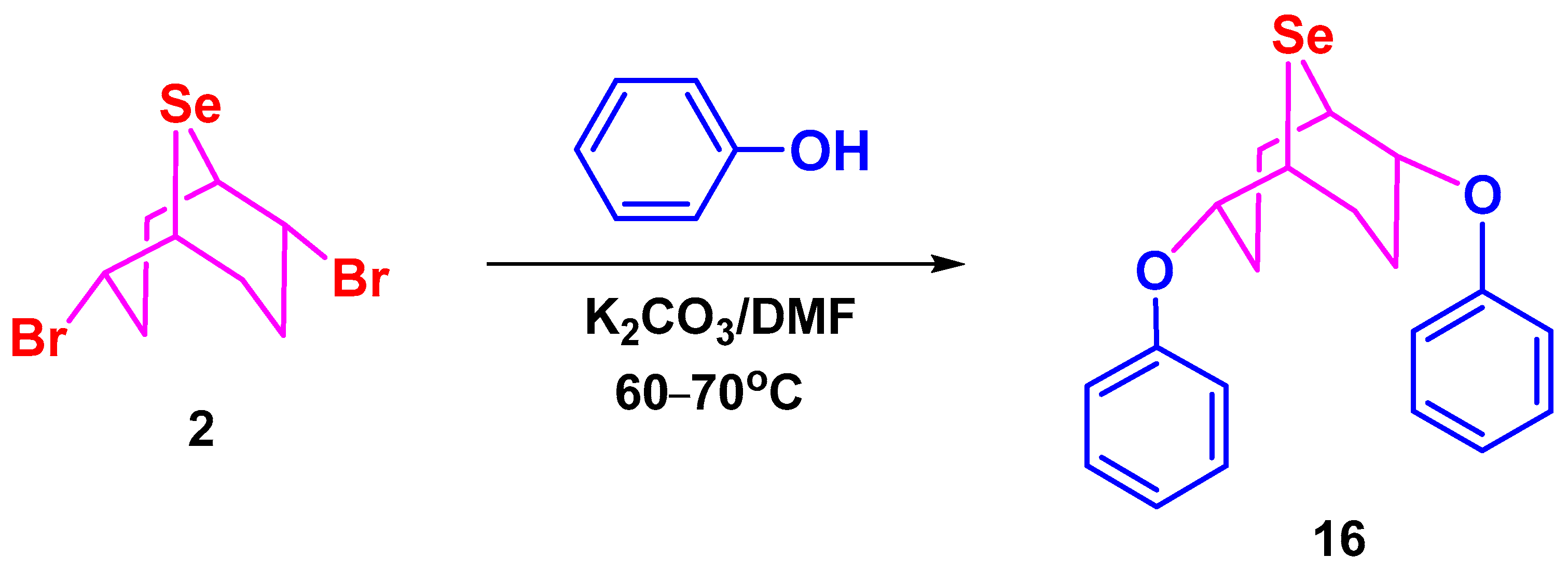
3.4. Synthesis of Phenol Derivatives 14–16
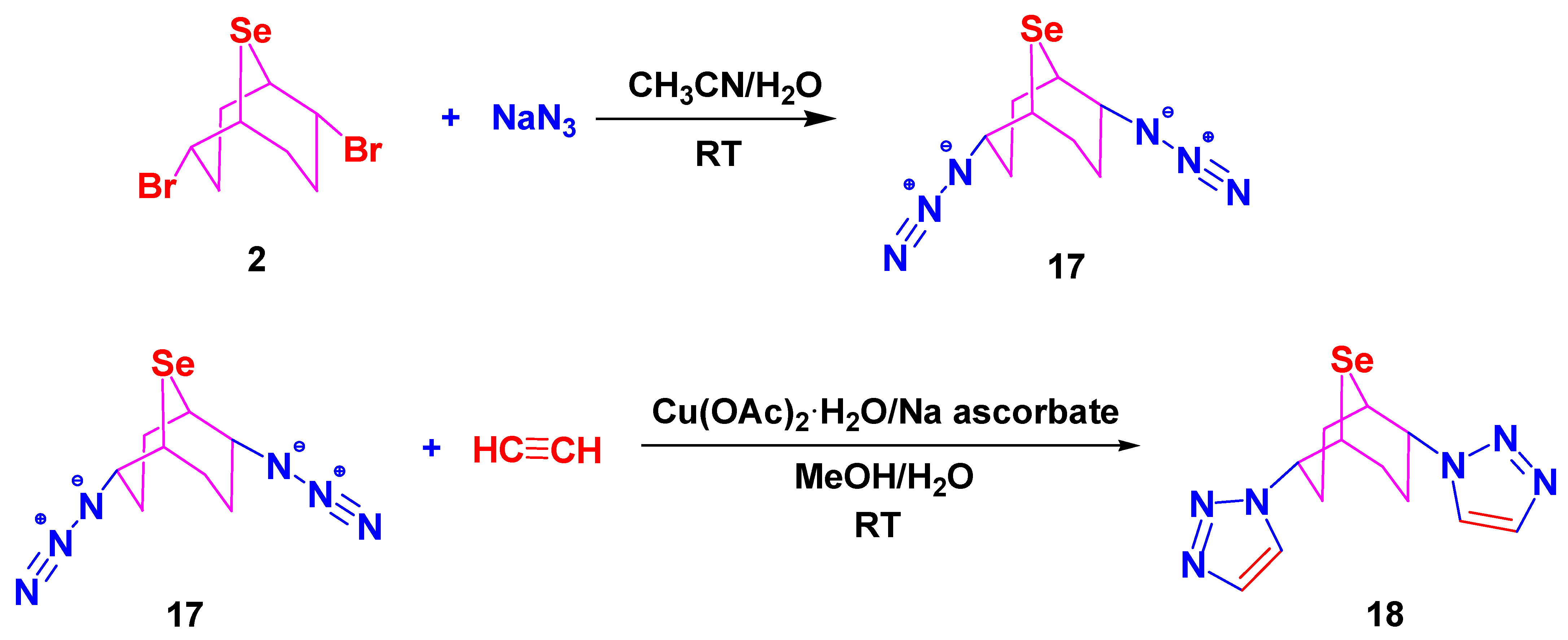
3.5. Synthesis of Compounds 17–19
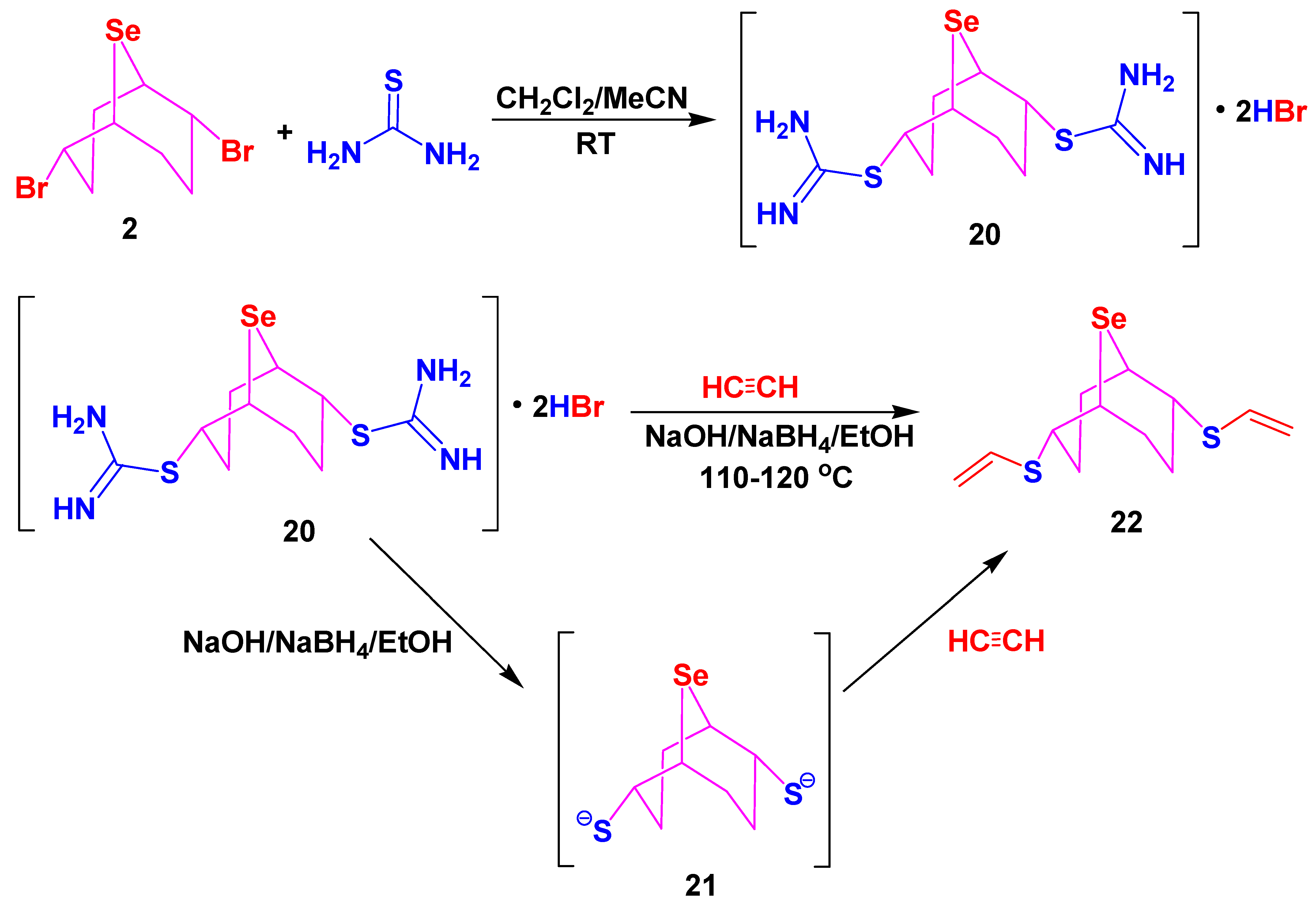
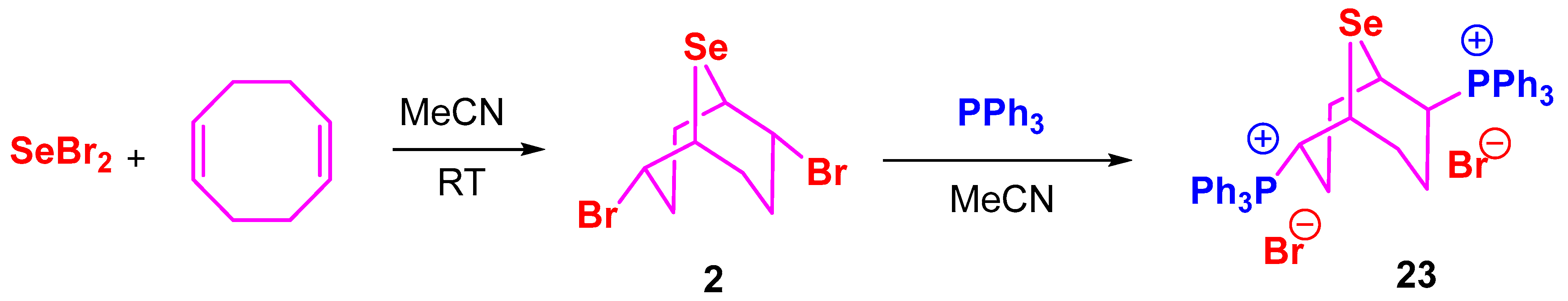
3.6. Synthesis of Compounds 20–23
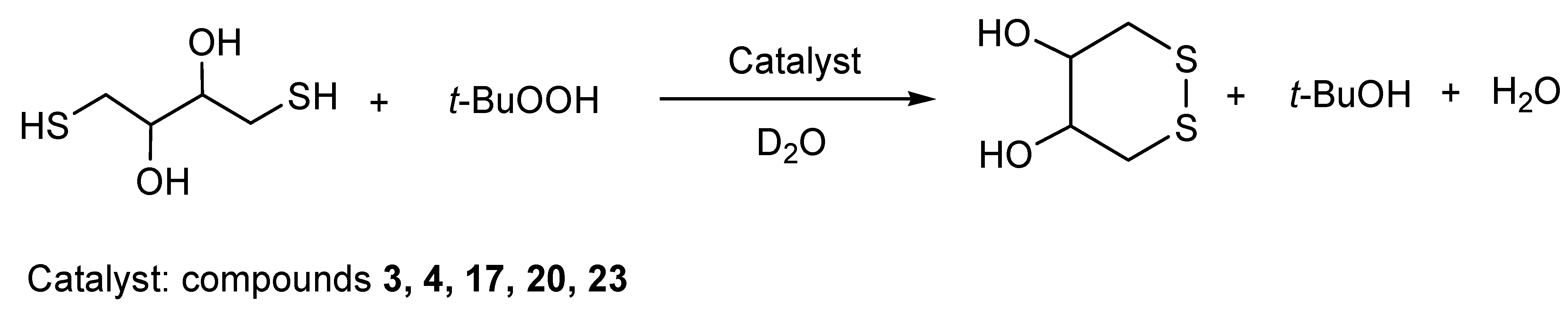
3.7. Synthesis of Selenoxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Petasi, N.A. Selenium in Natural Products Synthesis; CIS: Philadelphia, PA, USA, 1984; 300p. [Google Scholar]
- Wirth, T. (Ed.) Organoselenium Chemistry: Synthesis and Reactions; Wiley-VCH: Weinheim, Germany, 2012; 462p. [Google Scholar]
- Wirth, T. Organoselenium Chemistry—Modern Developments in Organic Synthesis, Topics in Current Chemistry, 208; Spring: Heidelberg, Germany, 2000; 260p. [Google Scholar]
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Pergamon: Oxford, UK, 1986; 463p. [Google Scholar]
- Back, T.G. Organoselenium Chemistry: A Practical Approach; Oxford University Press: Oxford, UK, 1999; 295p. [Google Scholar]
- Nogueira, C.W.; Zeni, G.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. [Google Scholar] [CrossRef] [PubMed]
- Santi, C. (Ed.) Organoselenium Chemistry: Between Synthesis and Biochemistry; Bentham Science Publishers: Sharjah, United Arab Emirates, 2014; p. 563. [Google Scholar]
- Tiekink, E.R.T. Therapeutic potential of selenium and tellurium compounds: Opportunities yet unrealized. Dalton Trans. 2012, 41, 6390–6395. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Braga, A.L.; Rafique, J. Synthesis of biologically relevant small molecules containing selenium. Part B. Anti-infective and anticancer compounds. In Patai’s Chemistry of Functional Groups. Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; John Wiley and Sons: Chichester, UK, 2013; Volume 4, pp. 1053–1117. [Google Scholar]
- Al-Rubaie, A.Z.; Al-Jadaan, S.A.S.; Muslim, S.K.; Saeed, E.A.; Ali, E.T.; Al-Hasani, A.K.J.; Al-Salman, H.N.K.; Al-Fadal, S.A.M. Synthesis, characterization and antibacterial activity of some new ferrocenyl selenazoles and 3,5-diferrocenyl-1,2,4-selenadiazole. J. Organomet. Chem. 2014, 774, 43–47. [Google Scholar] [CrossRef]
- Dhau, J.S.; Singh, A.; Singh, A.; Sooch, B.S.; Brandão, P.; Félix, V. Synthesis and antibacterial activity of pyridylselenium compounds: Self-assembly of bis(3-bromo-2-pyridyl)diselenide via intermolecular secondary and π⋯π stacking interactions. J. Organomet. Chem. 2014, 766, 57–66. [Google Scholar] [CrossRef]
- Angeli, A.; Tanini, D.; Capperucci, A.; Supuran, C.T. Synthesis of novel selenides bearing benzenesulfonamide moieties as carbonic anhydrase I, II, IV, VII, and IX inhibitors. ASC Med. Chem. Lett. 2017, 8, 1213–1217. [Google Scholar] [CrossRef]
- Banerjee, B.; Koketsy, M. Recent developments in the synthesis of biologically relevant selenium-containing scaffolds. Coord. Chem. Rev. 2017, 339, 104–127. [Google Scholar] [CrossRef]
- Elsherbini, M.; Hamama, W.S.; Zoorob, H.H. Recent advances in the chemistry of selenium-containing heterocycles: Five-membered ring systems. Coord. Chem. Rev. 2016, 312, 149–177. [Google Scholar] [CrossRef]
- Ninomiya, M.; Garud, D.R.; Koketsu, M. Biologically significant selenium-containing heterocycles. Coord. Chem. Rev. 2011, 255, 2968–2990. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, R.A.; Ninomiya, M.N.; Koketsu, M. Synthesis of seleno-heterocycles via electrophilic/radical cyclization of alkyne containing heteroatoms. Adv. Synth. Catal. 2020, 362, 3485–3515. [Google Scholar] [CrossRef]
- Ruberte, A.C.; Sanmartin, C.; Aydillo, C.; Sharma, A.K.; Plano, D. Development and Therapeutic Potential of Selenazo Compounds. J. Med. Chem. 2020, 63, 1473–1489. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-C.; Kuhn, H.; Daniliuc, C.-G.; Ivanov, I.; Jones, P.G.; du Mont, W.-W. 5-Selenization of salicylic acid derivatives yielded isoform-specific 5-lipoxygenase inhibitors. Org. Biomol. Chem. 2010, 8, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Koketsu, M.; Yang, H.; Kim, Y.M.; Ichihash, M.; Ishihara, H. Preparation of 1,4-Oxaselenin from AgNO3/LDA-Assisted Reaction of 3-Selena-4-pentyn-1-one as Potential Antitumor Agents. Org. Lett. 2001, 3, 1705–1707. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.K.; Priyadarsini, K.I. (Eds.) Organoselenium Compounds in Biology and Medicine: Synthesis, Biological and Therapeutic Treatments; RSC: London, UK, 2017; 458p. [Google Scholar]
- Azad, G.K.; Tomar, R.S. Ebselen, a promising antioxidant drug: Mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef]
- Weglarz-Tomczak, E.; Tomczak, J.M.; Talma, M.; Burda-Grabowska, M.; Giurg, M.; Brul, S. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Sci. Rep. 2021, 11, 3640. [Google Scholar] [CrossRef]
- Selvakumar, K.; Shah, P.; Singh, H.B.; Butcher, R.J. Synthesis, Structure, and Glutathione Peroxidase-Like Activity of Amino Acid Containing Ebselen Analogues and Diaryl Diselenides. Chem.-Eur. J. 2011, 17, 12741–12755. [Google Scholar] [CrossRef]
- Sarma, B.K.; Mugesh, G. Glutathione Peroxidase (GPx)-like Antioxidant Activity of the Organoselenium Drug Ebselen: Unexpected Complications with Thiol Exchange Reactions. J. Am. Chem. Soc. 2005, 127, 11477–11485. [Google Scholar] [CrossRef]
- Kumakura, F.; Mishra, B.; Priyadarsini, K.I.; Iwaoka, M. A Water-Soluble Cyclic Selenide with Enhanced Glutathione Peroxidase-Like Catalytic Activities. Eur. J. Org. Chem. 2010, 2010, 440–445. [Google Scholar] [CrossRef]
- Back, T.G.; Dyck, B.P. A Novel Camphor-Derived Selenenamide That Acts as a Glutathione Peroxidase Mimetic. J. Am. Chem. Soc. 1997, 119, 2079–2083. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Remarkable Activity of a Novel Cyclic Seleninate Ester as a Glutathione Peroxidase Mimetic and Its Facile in Situ Generation from Allyl 3-Hydroxypropyl. J. Am. Chem. Soc. 2002, 124, 12104–12105. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Moussa, Z. Diselenides and Allyl Selenides as Glutathione Peroxidase Mimetics. Remarkable Activity of Cyclic Seleninates Produced in Situ by the Oxidation of Allyl ω-Hydroxyalkyl Selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Braverman, S.; Cherkinsky, M.; Kalendar, Y.; Jana, R.; Sprecher, M.; Goldberg, I. Synthesis of water-soluble vinyl selenides and their high glutathione peroxidase (GPx)-like antioxidant activity. Synthesis 2014, 46, 119–125. [Google Scholar] [CrossRef]
- McNeil, N.M.R.; Press, D.J.; Mayder, D.M.; Garnica, P.; Doyle, L.M.; Back, T.G. Enhanced Glutathione Peroxidase Activity of Water-Soluble and Polyethylene Glycol-Supported Selenides, Related Spirodioxyselenuranes, and Pincer Selenuranes. J. Org. Chem. 2016, 81, 7884–7897. [Google Scholar] [CrossRef]
- Flohe, L.; Gunzler, W.A.; Schock, H.H. Glutathione peroxidase: A selenoenzyme. FEBS Lett. 1973, 32, 132–134. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Gladyshev, V.N.; Hatfield, D.L. Selenocysteine-Containing Proteins in Mammals. J. Biomed. Sci. 1999, 6, 151–160. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Elmoselhi, A.B.; Hata, T.; Makino, N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 2000, 47, 446–456. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, J.; Li, H. Selenium, aging and aging-related diseases. Aging Clin. Exp. Res. 2019, 31, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Alehagen, U.; Opstad, T.B.; Alexander, J.; Larsson, A.; Aaseth, J. Impact of Selenium on Biomarkers and Clinical Aspects Related to Ageing. A Review. Biomolecules 2021, 11, 1478. [Google Scholar] [CrossRef] [PubMed]
- Artem’ev, A.V.; Gusarova, N.K.; Malysheva, S.F.; Kraikivskii, P.B.; Belogorlova, N.A.; Trofimov, B.A. Efficient General Synthesis of Alkylammonium Diselenophosphinates via Multicomponent One-Pot Reaction of Secondary Phosphines with Elemental Selenium and Amines. Synthesis 2010, 21, 3724–3730. [Google Scholar] [CrossRef]
- Jimoh, Y.A.; LawalIge, A.O.; Kade, I.J.; Olatunde, D.M.; Oluwayomi, O. Diphenyl diselenide modulates antioxidant status, inflammatory and redox-sensitive genes in diesel exhaust particle-induced neurotoxicity. Chem.-Biol. Interact. 2022, 367, 110196. [Google Scholar] [CrossRef] [PubMed]
- Lorenzoni, S.; Cerra, S.; Angulo-Elizari, E.; Salamone, T.A.; Battocchio, C.; Marsotto, M.; Scaramuzzo, F.A.; Sanmartín, C.; Plano, D.; Fratoddi, I. Organoselenium compounds as functionalizing agents for gold nanoparticles in cancer therapy. Colloids Surf. B Biointerfaces 2022, 2019, 112828. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, S.F.; Gusarova, N.K.; Artem’ev, A.V.; Belogorlova, N.A.; Albanov, A.I.; Borodina, T.N.; Smirnov, V.I.; Trofimov, B.A. Facile Non-Catalyzed Synthesis of Tertiary Phosphine Sulfides by Regioselective Addition of Secondary Phosphine Sulfides to Alkenes. Eur. J. Org. Chem. 2014, 2014, 2516–2521. [Google Scholar] [CrossRef]
- Chuai, H.; Zhang, S.-Q.; Bai, H.; Li, J.; Wang, Y.; Sun, J.; Wen, E.; Zhang, J.; Xin, M. Small molecule selenium-containing compounds: Recent development and therapeutic applications. Eur. J. Med. Chem. 2021, 223, 113621. [Google Scholar] [CrossRef]
- Sonawane, A.D.; Sonawane, A.R.; Ninomiya, M.; Koketsu, M. Diorganyl diselenides: A powerful tool for the construction of selenium containing scaffolds. Dalton Trans. 2021, 50, 12764–12790. [Google Scholar] [CrossRef]
- Trost, B.M.; Tang, W.; Toste, F.D. Divergent Enantioselective Synthesis of (−)-Galanthamine and (−)-Morphine. J. Am. Chem. Soc. 2005, 127, 14785–14803. [Google Scholar] [CrossRef]
- Shin, H.S.; Jung, Y.G.; Cho, H.K.; Park, Y.G.; Cho, C.G. Total Synthesis of (±)-Lycorine from the Endo-Cycloadduct of 3,5-Dibromo-2-pyrone and (E)-β-Borylstyrene. Org. Lett. 2014, 16, 5718–5720. [Google Scholar] [CrossRef]
- Gao, Y.; Wei, Y.; Ma, D. Synthetic Studies toward Plumisclerin A. Org. Lett. 2019, 21, 1384–1387. [Google Scholar] [CrossRef] [PubMed]
- Wirth, T.; Häuptli, S.; Leuenberger, M. Catalytic asymmetric oxyselenenylation–elimination reactions using chiral selenium compounds. Tetrahedron Asymmetry 1998, 9, 547–550. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V. New Methods for Preparation of Organoselenium and Organotellurium Compounds from Elemental Chalcogens. Russ. J. Org. Chem. 2003, 39, 1373–1380. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V.; Musalova, M.V.; Amosova, S.V. Recent Advances in Organochalcogen Synthesis Based on Reactions of Chalcogen Halides with Alkynes and Alkenes. Curr. Org. Chem. 2016, 20, 136–145. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A. Selenium dihalides: New possibilities for the synthesis of selenium-containing heterocycles. Chem. Heterocycl. Comp. 2017, 53, 150–152. [Google Scholar] [CrossRef]
- Abakumov, G.A.; Piskunov, A.V.; Cherkasov, V.K.; Fedushkin, I.L.; Ananikov, V.P.; Eremin, D.B.; Gordeev, E.G.; Beletskaya, I.P.; Averin, A.D.; Bochkarev, M.N.; et al. Organoelement chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2018, 87, 393–507. [Google Scholar] [CrossRef]
- Musalov, M.V.; Yakimov, V.A.; Potapov, V.A.; Amosova, S.V.; Borodina, T.N.; Zinchenko, S.V. A novel methodology for the synthesis of condensed selenium heterocycles based on the annulation and annulation–methoxylation reactions of selenium dihalides. New J. Chem. 2019, 43, 18476–18483. [Google Scholar] [CrossRef]
- Accurso, A.A.; Cho, S.-H.; Amin, A.; Potapov, V.A.; Amosova, S.V.; Finn, M.G. Thia-, Aza-, and Selena[3.3.1]bicyclononane Dichlorides: Rates vs Internal Nucleophile in Anchimeric Assistance. J. Org. Chem. 2011, 76, 4392–4395. [Google Scholar] [CrossRef]
- Potapov, V.A.; Amosova, S.V.; Abramova, E.V.; Musalov, M.V.; Lyssenko, K.A.; Finn, M.G. 2,6-Dihalo-9-selenabicyclo[3.3.1]nonanes and their complexes with selenium dihalides: Synthesis and structural characterization. New J. Chem. 2015, 39, 8055–8059. [Google Scholar] [CrossRef]
- Abramova, E.V.; Sterkhova, I.V.; Molokeev, M.S.; Potapov, V.A.; Amosova, S.V. First coordination compounds of SeBr2 with selenium ligands: X-ray structural determination. Mendeleev Commun. 2016, 26, 532–534. [Google Scholar] [CrossRef]
- Potapov, V.A.; Musalov, M.V. Triple-Click Chemistry of Selenium Dihalides: Catalytic Regioselective and Highly Efficient Synthesis of Bis-1,2,3-Triazole Derivatives of 9-Selenabicyclo[3.3.1]nonane. Catalysts 2022, 12, 1032. [Google Scholar] [CrossRef]
- Musalov, M.V.; Potapov, V.A.; Amosova, S.V. Efficient Synthesis of a New Family of 2,6-Disulfanyl-9-Selenabicyclo[3.3.1]Nonanes. Molecules 2021, 26, 2849. [Google Scholar] [CrossRef] [PubMed]
- Yurieva, O.V.; Dubrovina, V.I.; Potapov, V.A.; Musalov, M.V.; Starovoitova, T.P.; Ivanova, T.A.; Gromova, A.V.; Shkaruba, T.T.; Balakhonov, S.V. Effect of Synthetic Organoselenium Drug on the Degree of Pathological Changes in the Organs of White Mice Immunized with Tularemia and Brucellosis Vaccines. Bull. Exp. Biol. Med. 2019, 168, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Bozorov, K.; Zhao, J.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef]
- Jain, A.; Piplani, P. Exploring the Chemistry and Therapeutic Potential of Triazoles: A Comprehensive Literature Review. Mini Rev. Med. Chem. 2019, 19, 1298–1368. [Google Scholar] [CrossRef]
- Xu, M.; Peng, Y.; Zhu, L.; Wang, S.; Ji, J.; Rakesh, K.P. Triazole derivatives as inhibitors of Alzheimer’s disease: Current developments and structure-activity relationships. Eur. J. Med. Chem. 2019, 180, 656–672. [Google Scholar] [CrossRef] [PubMed]
- Agalave, S.G.; Maujan, S.R.; Pore, V.S. Click Chemistry: 1,2,3-Triazoles as Pharmacophores. Chem. Asian J. 2011, 6, 2696–2718. [Google Scholar] [CrossRef]
- Kashyap, S.J.; Garg, V.K.; Sharma, P.K.; Kumar, N.; Dudhe, R.; Gupta, J.K. Thiazoles: Having diverse biological activities. Med. Chem. Res. 2012, 21, 2123–2132. [Google Scholar] [CrossRef]
- Fabbrizzi, P.; Menchi, G.; Guarna, A.; Trabocchi, A. Use of Click-Chemistry in the Development of Peptidomimetic Enzyme Inhibitors. Curr. Med. Chem. 2014, 21, 1467–1477. [Google Scholar] [CrossRef]
- Bonandi, E.; Fumagalli, G.; Perdicchia, D.; Christodoulou, M.S.; Rastelli, G.; Passarella, D. The 1,2,3-triazole ring as a bioisostere in medicinal chemistry. Drug Discov. Today 2017, 22, 1572–1581. [Google Scholar] [CrossRef] [PubMed]
- Doiron, J.E.; Ody, B.K.; Brace, J.B.; Post, S.J.; Thacker, N.L.; Hill, H.M.; Breton, G.W.; Turlington, M.; Le Christina, A.; Aller, S.G.; et al. Evaluation of 1,2,3-Triazoles as Amide Bioisosteres in Cystic Fibrosis Transmembrane Conductance Regulator Modulators VX-770 and VX-809. Chem. Eur. J. 2019, 25, 3662–3674. [Google Scholar] [CrossRef] [PubMed]
- Kharb, R.; Sharma, P.C.; Yar, M.S. Pharmacological significance of triazole scaffold. J. Enzym. Inhib. Med. Chem. 2011, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.; Sodero, A.C.; Cardoso, M.F.; Lima, E.S.; Kaiser, C.R.; Silva, F.P.; Ferreira, V.F. Synthesis, Biological Activity, and Molecular Modeling Studies of 1H-1,2,3-Triazole Derivatives of Carbohydrates as α-Glucosidases Inhibitors. J. Med. Chem. 2010, 53, 2364–2375. [Google Scholar] [CrossRef]
- Devender, N.; Gunjan, S.; Chhabra, S.; Singh, K.; Pasam, V.R.; Shukla, S.K.; Sharma, A.; Jaiswal, S.; Singh, S.K.; Kumar, Y.; et al. Identification of β-Amino alcohol grafted 1,4,5 trisubstituted 1,2,3-triazoles as potent antimalarial agents. Eur. J. Med. Chem. 2016, 109, 187–198. [Google Scholar] [CrossRef]
- Lee, T.; Cho, M.; Ko, S.-Y.; Youn, H.-J.; Baek, D.J.; Cho, W.-J.; Kang, C.-Y.; Kim, S. Synthesis and Evaluation of 1,2,3-Triazole Containing Analogues of the Immunostimulant α-GalCer. J. Med. Chem. 2007, 50, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Raj, R.; Kumar, V.; Mahajan, M.P.; Bedi, P.M.; Kaur, T.; Saxena, A.K. 1,2,3-Triazole tethered β-lactam-Chalcone bifunctional hybrids: Synthesis and anticancer evaluation. Eur. J. Med. Chem. 2012, 47, 594–600. [Google Scholar] [CrossRef]
- Ganesh, A. Potential biological activity of 1,4-sustituted-1H-[1,2,3]triazoles. Int. J. Chem. Sci. 2013, 11, 573–578. [Google Scholar]
- Dheer, D.; Singh, V.; Shankar, R. Medicinal attributes of 1,2,3-triazoles: Current developments. Bioorg. Chem. 2017, 71, 30–54. [Google Scholar] [CrossRef]
- Dhall, E.; Sain, S.; Jain, S.; Dwivedi, J. Synthesis of Triazole Derivatives Manifesting Antimicrobial and Anti-Tubercular Activities. Mini-Rev. Org. Chem. 2018, 15, 291–314. [Google Scholar] [CrossRef]
- Lauria, A.; Delisi, R.; Mingoia, F.; Terenzi, A.; Martorana, A.; Barone, G.; Almerico, A.M. 1,2,3-Triazole in Heterocyclic Compounds, Endowed with Biological Activity, through 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2014, 2014, 3289–3306. [Google Scholar] [CrossRef]
- Amblard, F.; Cho, J.H.; Schinazi, R.F. Cu(I)-catalyzed Huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry. Chem. Rev. 2009, 109, 4207–4220. [Google Scholar] [CrossRef] [PubMed]
- Librando, I.L.; Mahmoud, A.G.; Carabineiro, S.A.C.; Guedes da Silva, M.F.C.; Geraldes, C.F.G.C.; Pombeiro, A.J.L. The Catalytic Activity of Carbon-Supported Cu(I)-Phosphine Complexes for the Microwave-Assisted Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 185. [Google Scholar] [CrossRef]
- Pucci, A.; Albano, G.; Pollastrini, M.; Lucci, A.; Colalillo, M.; Oliva, F.; Evangelisti, C.; Marelli, M.; Santalucia, D.; Mandoli, A. Supported Tris-Triazole Ligands for Batch and Continuous-Flow Copper-Catalyzed Huisgen 1,3-Dipolar Cycloaddition Reactions. Catalysts 2020, 10, 434. [Google Scholar] [CrossRef]
- Himo, F.; Lovell, T.; Hilgraf, R.; Rostovtsev, V.V.; Noodleman, L.; Sharpless, K.B.; Fokin, V.V. Copper(I)-Catalyzed Synthesis of Azoles. DFT Study Predicts Unprecedented Reactivity and Intermediates. J. Am. Chem. Soc. 2005, 127, 210–216. [Google Scholar] [CrossRef]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Haldón, E.; Nicasio, M.C.; Pérez, P.J. Copper-catalysed azide–alkyne cycloadditions (CuAAC): An update. Org. Biomol. Chem. 2015, 13, 9528–9550. [Google Scholar] [CrossRef]
- Hein, J.E.; Fokin, V.V. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) and beyond: New reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010, 39, 1302–1315. [Google Scholar] [CrossRef]
- De Nino, A.; Maiuolo, L.; Costanzo, P.; Algieri, V.; Jiritano, A.; Olivito, F.; Tallarida, M.A. Recent Progress in Catalytic Synthesis of 1,2,3-Triazoles. Catalysts 2021, 11, 1120. [Google Scholar] [CrossRef]
- Saini, P.; Sonika; Singh, G.; Kaur, G.; Singh, J.; Singh, H. Robust and Versatile Cu(I) metal frameworks as potential catalysts for azide-alkyne cycloaddition reactions. Mol. Catal. 2021, 504, 111432. [Google Scholar] [CrossRef]
- Kalra, P.; Kaur, R.; Singh, H.; Singh, G.; Pawan; Kaur, G.; Singh, J. Metals as “Click” catalysts for alkyne-azide cycloaddition reactions: An overview. J. Organomet. Chem. 2021, 944, 121846. [Google Scholar] [CrossRef]
- Deobald, A.M.; Camargo, L.R.S.; Hörner, M.; Rodrigues, O.E.D.; Alves, D.; Braga, A.L. Synthesis of arylseleno-1,2,3-triazoles via copper-catalyzed 1,3-dipolar cycloaddition of azido arylselenides with alkynes. Synthesis 2011, 43, 2397–2406. [Google Scholar]
- Seus, N.; Saraiva, M.T.; Alberto, E.E.; Savegnago, L.; Alves, D. Selenium compounds in click chemistry: Copper catalyzed 1,3-dipolar cycloaddition of azidomethyl arylselenides and alkynes. Tetrahedron 2012, 68, 10419–10425. [Google Scholar] [CrossRef]
- Bhabak, K.P.; Mugesh, G. Functional Mimics of Glutathione Peroxidase: Bioinspired Synthetic Antioxidants. Accounts Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Potapov, V.A.; Amosova, S.V.; Petrov, P.A.; Romanenko, L.S.; Keiko, V.V. Exchange reactions of dialkyl dichalcogenides. Sulfur Lett. 1992, 15, 121–126. [Google Scholar]

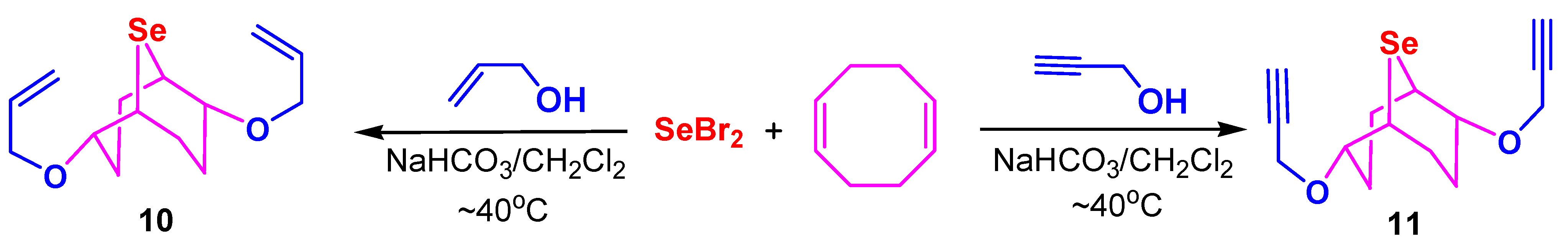

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musalov, M.V.; Potapov, V.A. Click Chemistry of Selenium Dihalides: Novel Bicyclic Organoselenium Compounds Based on Selenenylation/Bis-Functionalization Reactions and Evaluation of Glutathione Peroxidase-like Activity. Int. J. Mol. Sci. 2022, 23, 15629. https://doi.org/10.3390/ijms232415629
Musalov MV, Potapov VA. Click Chemistry of Selenium Dihalides: Novel Bicyclic Organoselenium Compounds Based on Selenenylation/Bis-Functionalization Reactions and Evaluation of Glutathione Peroxidase-like Activity. International Journal of Molecular Sciences. 2022; 23(24):15629. https://doi.org/10.3390/ijms232415629
Chicago/Turabian StyleMusalov, Maxim V., and Vladimir A. Potapov. 2022. "Click Chemistry of Selenium Dihalides: Novel Bicyclic Organoselenium Compounds Based on Selenenylation/Bis-Functionalization Reactions and Evaluation of Glutathione Peroxidase-like Activity" International Journal of Molecular Sciences 23, no. 24: 15629. https://doi.org/10.3390/ijms232415629
APA StyleMusalov, M. V., & Potapov, V. A. (2022). Click Chemistry of Selenium Dihalides: Novel Bicyclic Organoselenium Compounds Based on Selenenylation/Bis-Functionalization Reactions and Evaluation of Glutathione Peroxidase-like Activity. International Journal of Molecular Sciences, 23(24), 15629. https://doi.org/10.3390/ijms232415629






