Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Anodizing Process
2.3. Microstructural Characterization
2.4. Vickers Microhardness Measurements
2.5. Electrochemical Techniques
3. Results
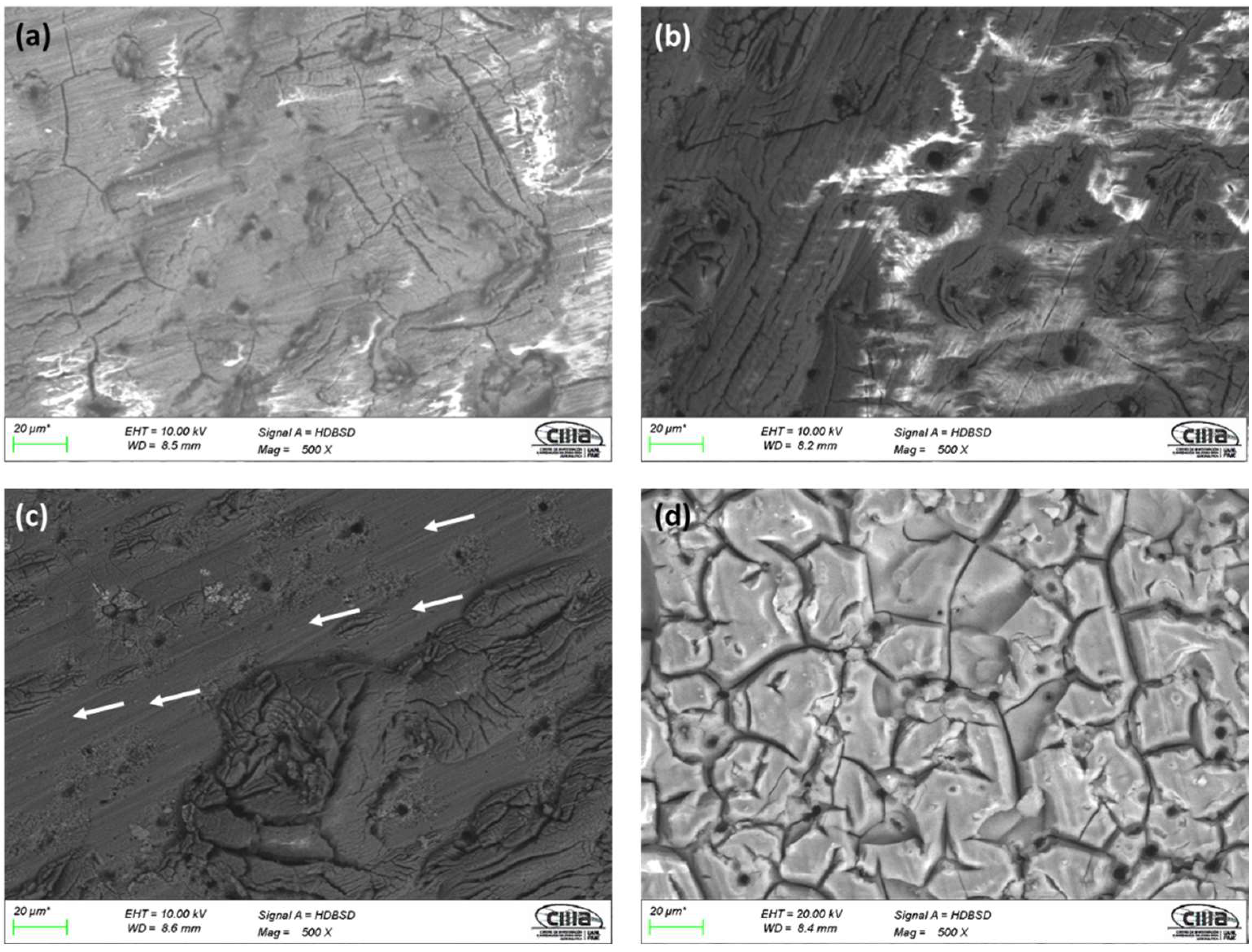
3.1. Microstructural Characterization by SEM
3.1.1. Surface Morphology
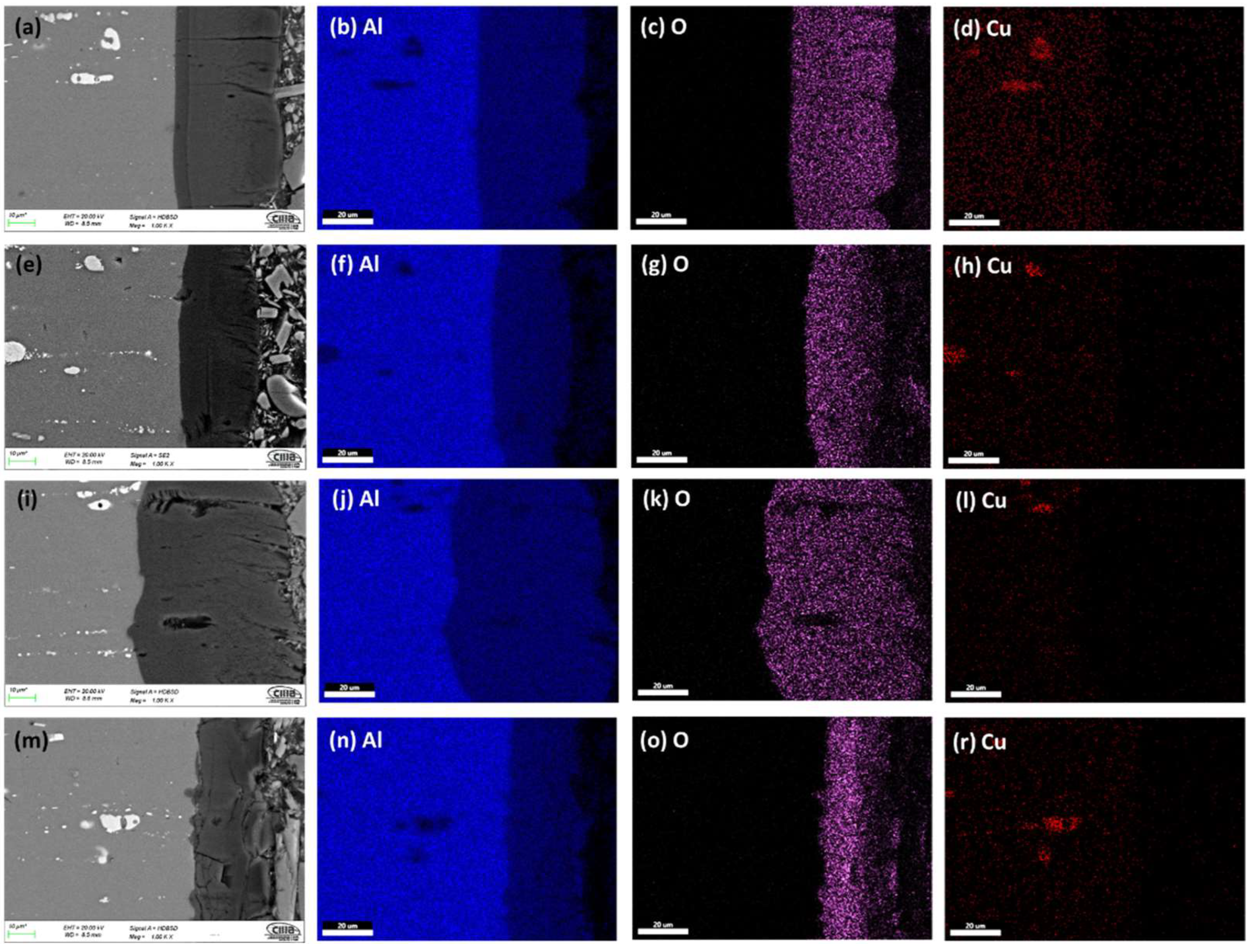
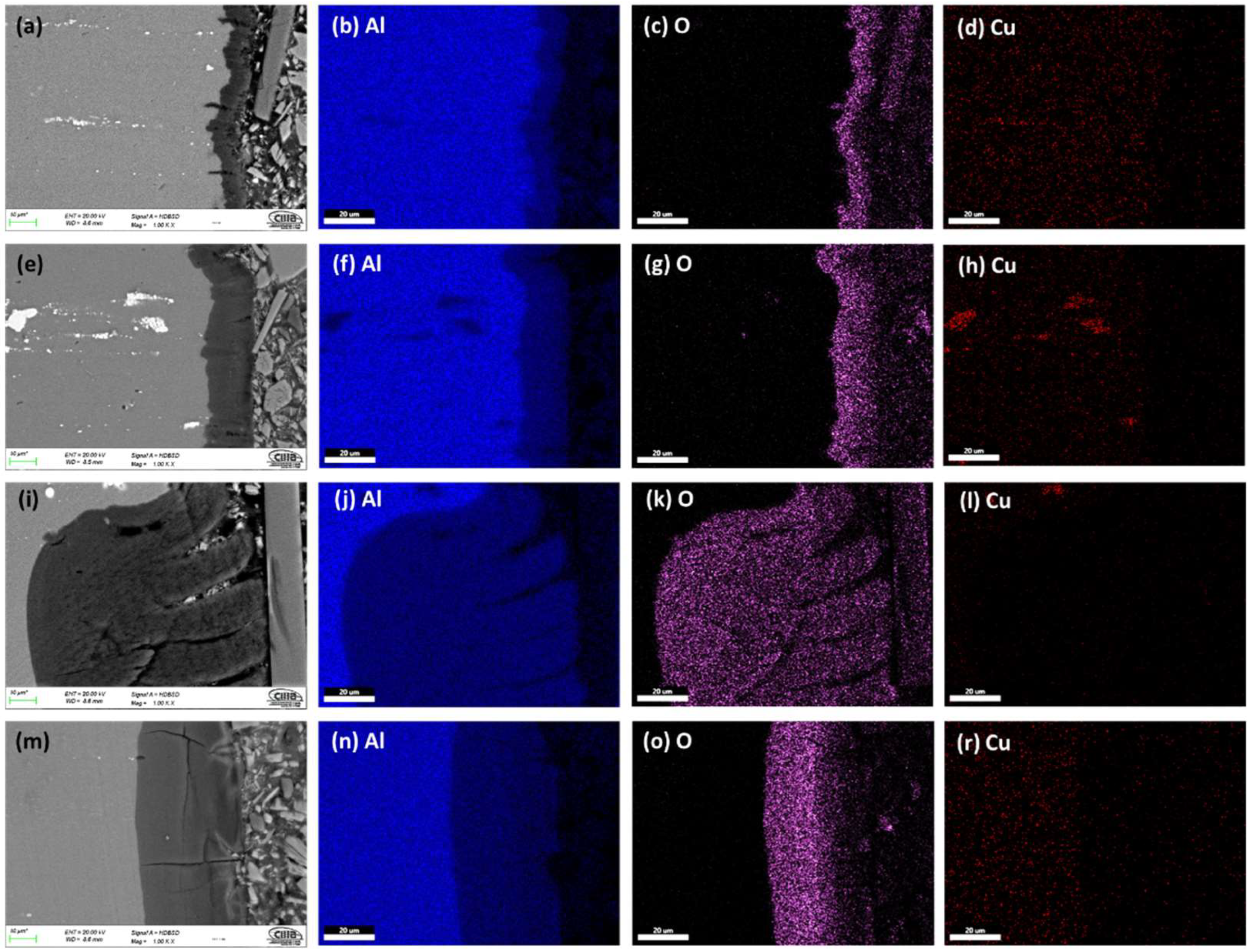
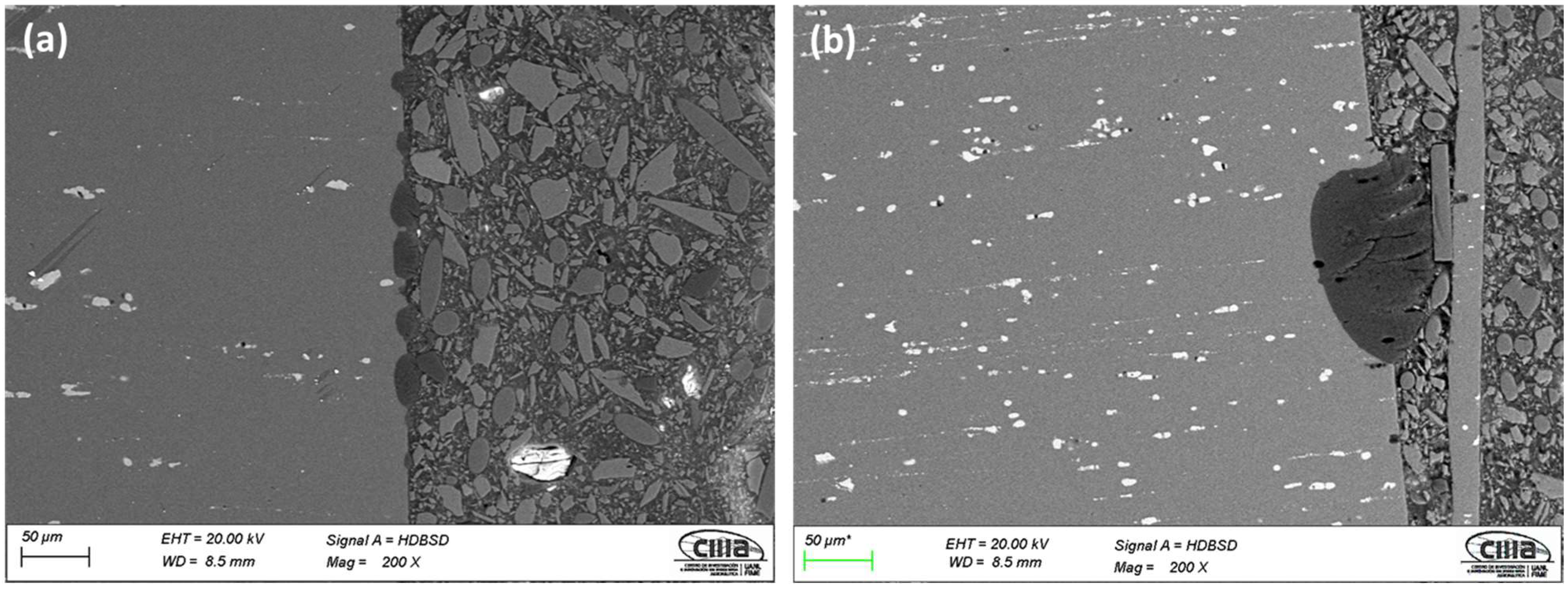
3.1.2. Morphology of Cross-Sections
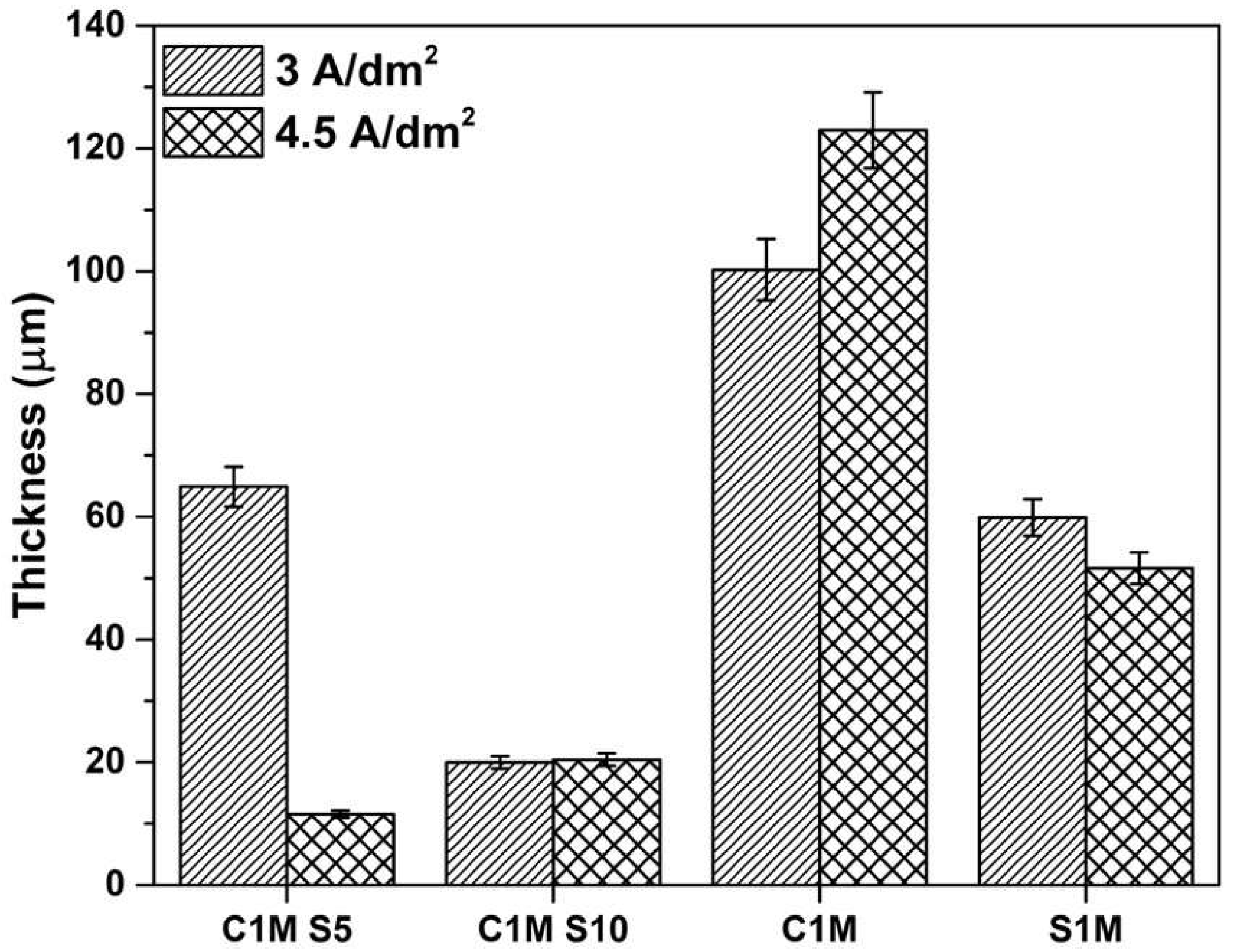
3.1.3. The Thickness of Anodized Samples of AA2024
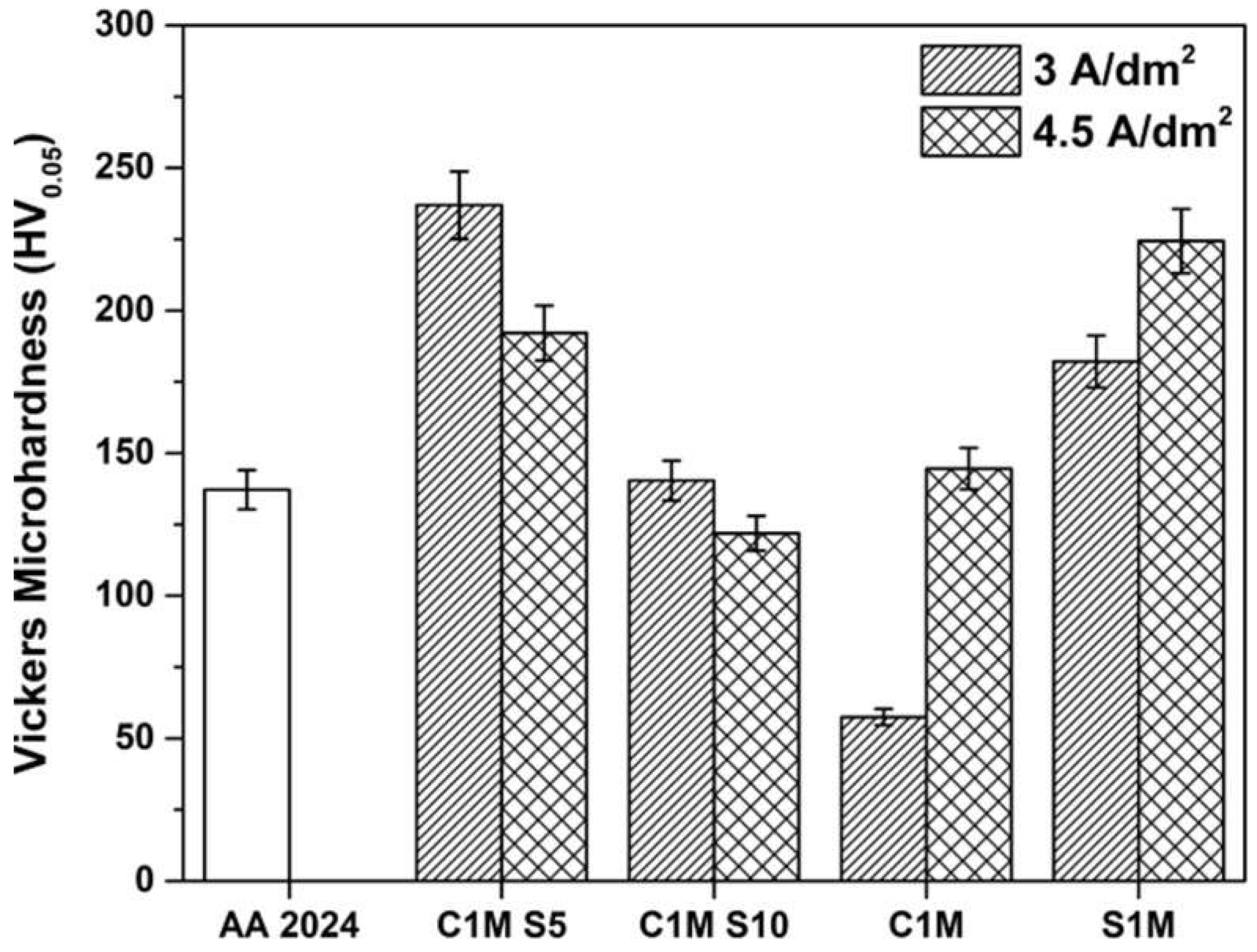
3.2. Vickers Microhardness Measurements
3.3. Electrochemical Techniques
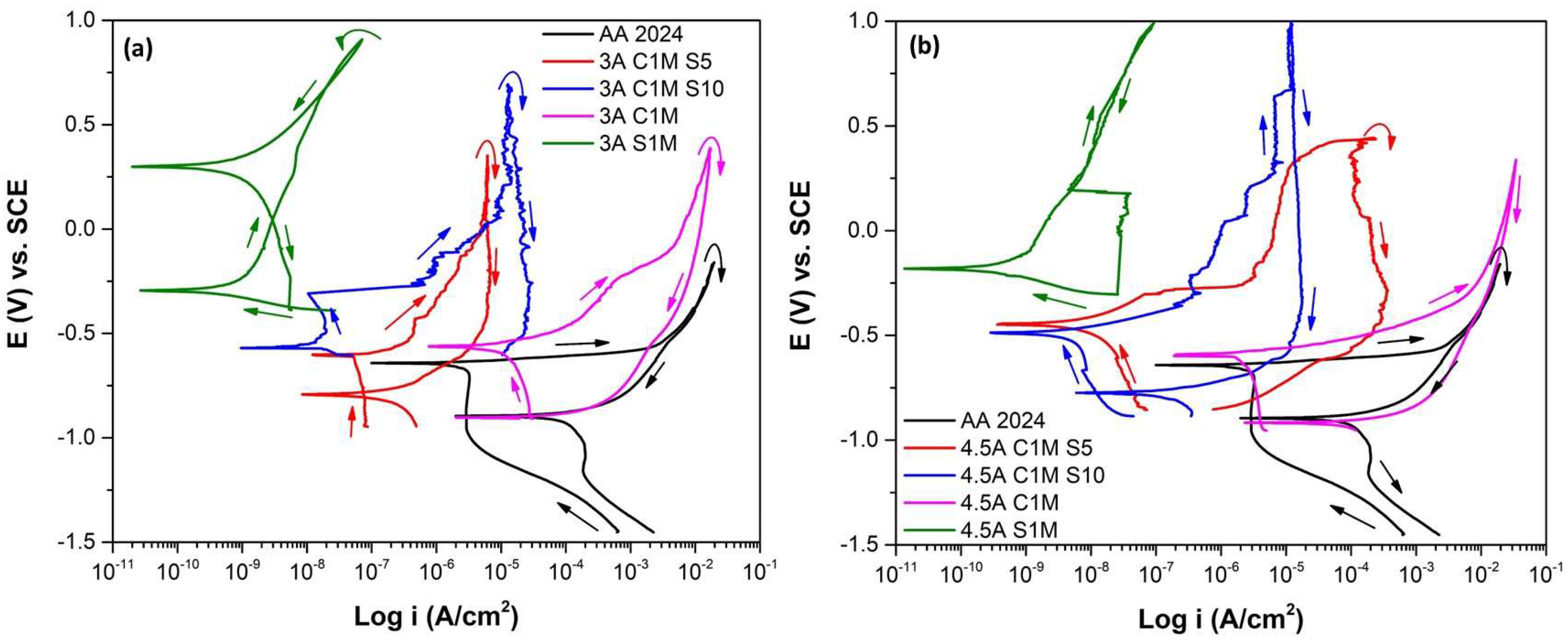
3.3.1. Cyclic Potentiodynamic Polarization
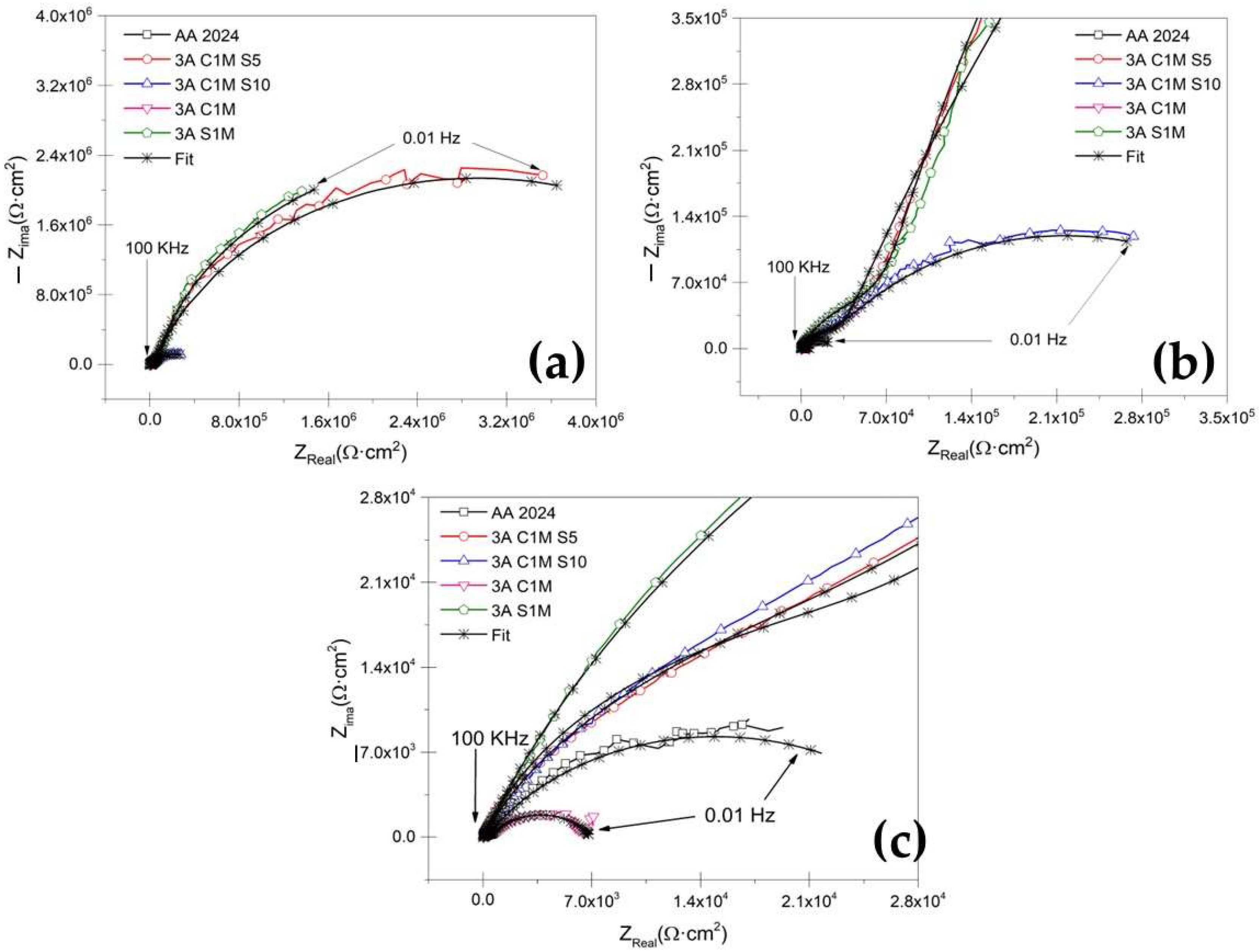
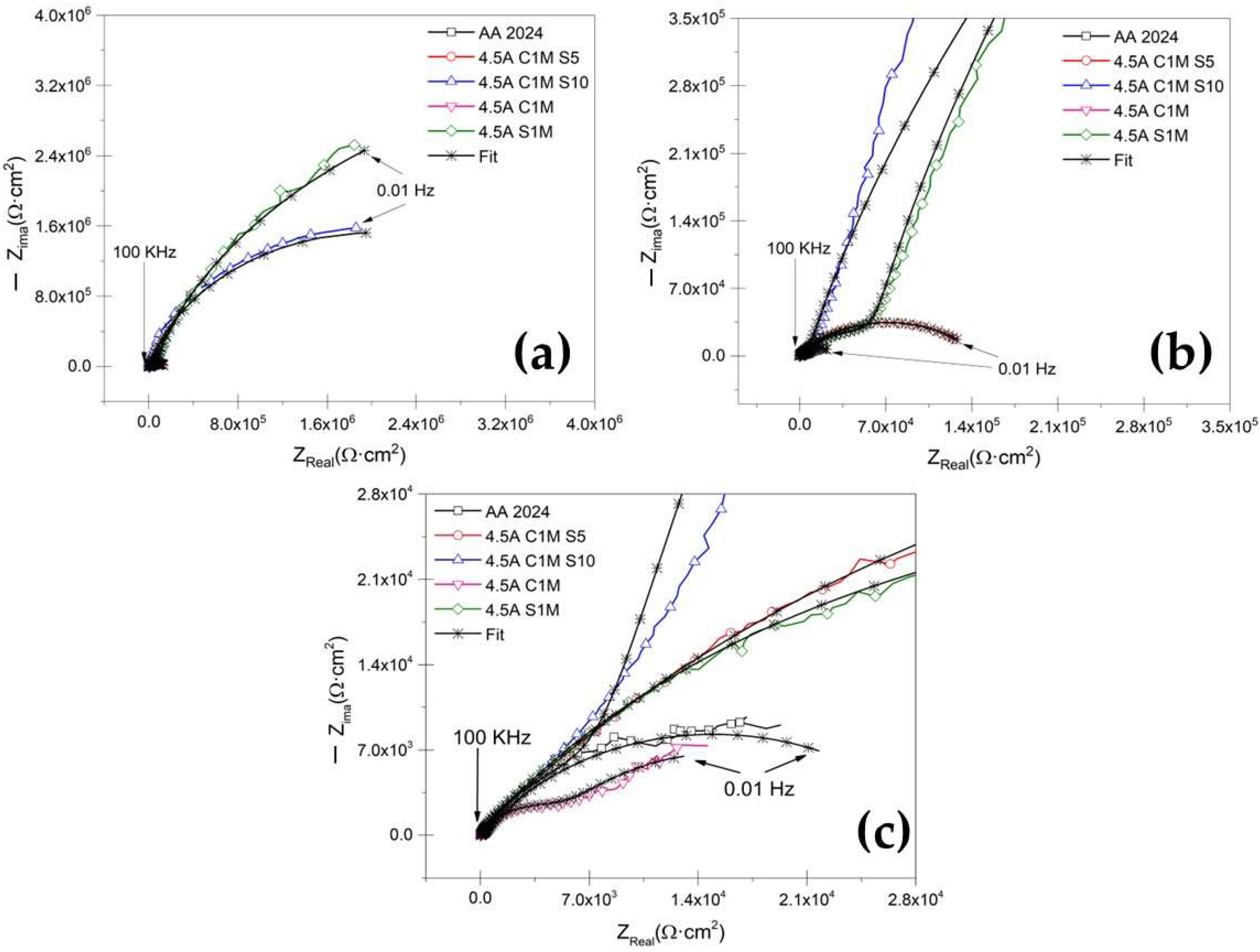
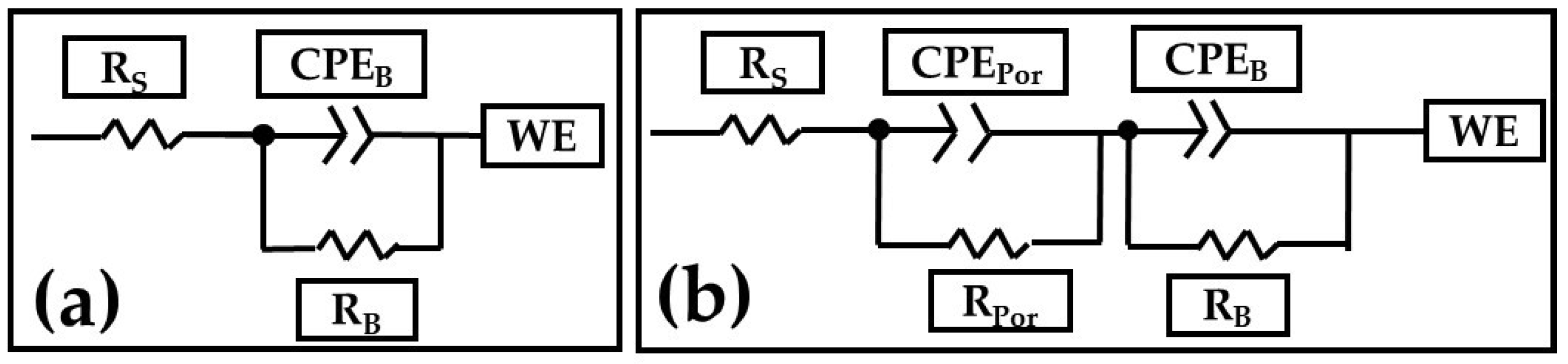
3.3.2. Electrochemical Impedance Spectroscopy Measurements
4. Discussion
5. Conclusions
- Porous anodic alumina films were successfully produced under hard anodizing conditions on AA2024 alloy. Intermetallic phases in aluminum alloys influence the anodic layer growth rate and morphology. Oxide layers formed on AA2024 alloys with coarse intermetallic phases contained large cavities and surface defects.
- Anodic films with porosity, cracks, and lateral porosity, commonly encountered during anodizing of AA2024 alloy, were obtained. In addition, oxidation of the second phase particles is achieved, and, as a result, its consumption generates cavities on the surface of the films and in the cross-section.
- The thickness and Vickers microhardness obtained in the hard anodic coatings were low due to secondary phases rich in copper that prevent the film’s homogeneous growth. These secondary phases are associated with the generation of lateral porosity that also decreases properties. Lower thickness and microhardness are presented because of the formation of large cavities and defects induced by the activity of the Cu and Fe coarse intermetallic phases.
- The cyclic potentiodynamic polarization technique indicated that higher Ecorr, and lower corrosion current densities (icorr), were presented in samples 3A CIM S5 and 4.5A C1M, representing that this sample provides more corrosion resistance than conventional sulfuric acid anodizing or the un-anodized alloy.
- The EIS results indicate that hard-anodized coatings with citric–sulfuric acid showed resistance when exposed to a 3.5 wt. % NaCl solution for the samples 3A CIM S5 and 4.5A C1M S10.
- Type III hard anodizing is possible with mixtures of citric–sulfuric acid solutions, which will present good mechanical properties and greater corrosion resistance than the material without anodizing.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kwolek, P. Hard Anodic Coatings on Aluminum Alloys. Adv. Manuf. Sci. Technol. 2017, 41, 35–46. [Google Scholar]
- The history of aluminium industry. Available online: https://www.aluminiumleader.com/history/industry_history/ (accessed on 11 July 2022).
- Abdel-Salam, O.E.; Shoeib, M.A.; Elkilany, H.A. Characterization of the hard anodizing layers formed on 2014-T3 Al alloy, in sulphuric acid electrolyte containing sodium lignin sulphonate. Egypt. J. Petrol. 2017, 27, 497–504. [Google Scholar] [CrossRef]
- Mouritz, P.A. Aluminium alloys for aircraft structures. In Introduction to Aerospace Materials, 1st ed.; Mouritz, P.A., Ed.; Woodhead Publishing: Boston, MA, USA, 2012; Volume 1, pp. 173–201. [Google Scholar]
- Gloria, A.; Nontanari, R.; Richetta, M.; Varone, A. Alloys for Aeronautic Applications: State of the Art and Perspectives. Metals 2019, 9, 662. [Google Scholar] [CrossRef]
- Applications of 2024 Aluminum Alloy. Available online: https://www.howardprecision.com/applications-of-2024-aluminum-alloy/ (accessed on 11 July 2022).
- Huda, Z.; Taib, N.I.; Zaharinie, T. Characterization of 2024-T3: An aerospace aluminum alloy. Mater. Chem. Phys. 2009, 113, 515–517. [Google Scholar] [CrossRef]
- Technical Report TR-17. In Hard Anodizing of Aluminum Alloys and Its Effect on Bal Seal Performance; Bal Seal Engineering, Inc.: Lake Forest, CA, USA, 2016; Available online: https://www.balseal.com/wp-content/uploads/2019/06/Effects_Of_Hard_Anodizing_Aluminum_Alloys_On_Bal_SealTR_17.pdf (accessed on 10 September 2022).
- Renaud, A.; Paint, Y.; Lanzutti, A.; Bonnaud, L.; Fedrizzi, L.; Dubois, P.; Poorteman, M.; Oliver, M.G. Sealing porous anodic layers on AA2024-T3 with a low viscosity benzoxazine resin for corrosion protection in aeronautical applications. RSC Adv. 2019, 9, 16819–16830. [Google Scholar] [CrossRef]
- Safyari, M.; Hojo, T.; Moshtaghi, M. Effect of environmental relative humidity on hydrogen-induced mechanical degradation in an Al-Zn-Mg-Cu alloy. Vacuum 2021, 192, 110489. [Google Scholar] [CrossRef]
- Aerospace aluminum alloy 2024 for industrial application. Available online: http://www.aluminium-alloys.com/aerospace-aluminum-alloy-2024-for-industrial-application/ (accessed on 11 July 2022).
- 2024 Aluminum Alloy: Properties. Available online: https://www.gabrian.com/wp-content/uploads/2018/10/2024-Aluminum-Alloy-Properties.pdf (accessed on 12 July 2022).
- Cotell, C.M. Surface Engineering of Aluminum and Aluminum Alloys. In ASM Handbook; ASM International: Novelty, OH, USA, 1994; Volume 5, pp. 4–7. [Google Scholar]
- Montoya-Rangel, M.; de Garza-Montes, O.N.; Gaona-Tiburcio, C.; Colás, R.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Electrochemical noise measurements of advanced high-strength steels in different solutions. Metals 2020, 10, 1232. [Google Scholar] [CrossRef]
- Martínez Viademonte, M.; Abrahami, S.T.; Hack, T.; Burchardt, M.; Terryn, H. A Review on Anodizing of Aerospace Aluminum Alloys for Corrosion Protection. Coatings 2020, 10, 1106. [Google Scholar] [CrossRef]
- Jalal, H.; Saound, Y.; Karabet, F. Effect of Organic Additives on AA6066 Anodization. J. Chem. Technol. Metall. 2019, 54, 447–453. [Google Scholar]
- Hitzig, J.; Jüttner, K.; Lorenz, W.J.; Paatsch, W. AC-Impedance Measurements on Porous Aluminium Oxide Films. Corros. Sci. 1984, 24, 945–952. [Google Scholar] [CrossRef]
- Gabe, D.R. Hard anodizing—What do we mean by hard? Met. Finish. 2002, 100, 52–58. [Google Scholar] [CrossRef]
- Memerifard, S.; Rahimipour, M.; Mobashrpour, I. Investigation of organic additives on voltage rate in Aluminum hard-anodizing process. Int. J. Bio-Inorg. Hybr. Nanomater 2017, 6, 59–63. [Google Scholar]
- Curioni, M.; Saenz de Miera, M.; Skeldon, P.; Thompson, G.E.; Ferguson, J. Macroscopic and Local Filming Behavior of AA2024 T3 Aluminum Alloy during Anodizing in Sulfuric Acid Electrolyte. J. Electrochem. Soc. 2008, 155, C387. [Google Scholar] [CrossRef]
- Boisier, G.; Pébère, N.; Druez, C.; Villarte, M.; Suel, S. FESEM and EIS Study of Sealed AA2024 T3 Anodized in Sulfuric Acid Electrolytes: Influence of Tartaric Acid. J. Electrochem. Soc. 2008, 155, C521–C529. [Google Scholar] [CrossRef]
- Torrescano Alvarez, J.M. Hard Anodic Films for Aluminum Alloys. Ph.D. Thesis, University of Manchester, Manchester, UK, 2018. [Google Scholar]
- Udochukwu Udochukwa, S.; Fernandes, F.A.O.; Pereira, A.B. The Sealing Step in Aluminum Anodizing: A Focus on Sustainable Strategies for Enhancing Both Energy Efficiency and Corrosion Resistance. Coatings 2020, 10, 226–282. [Google Scholar]
- Norek, M.; Wlodarski, M. Morphological and chemical characterization of highly ordered conical-pore anodic alumina prepared by multistep citric acid anodizing and chemical etching process. J. Porous Mater. 2018, 25, 45–53. [Google Scholar] [CrossRef]
- Shoshan, T.A.; De Kok, J.M.M.; Terryn, H.; Mol, J.M.C. Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: A review. Front. Chem. Sci. Eng. 2017, 11, 465–482. [Google Scholar]
- Abd El-Hameed, A.M.; Abdel-Aziz, Y.A.; El-Tokhy, F.S. Anodic Coating Characteristics of Different Aluminum Alloys for Spacecraft Materials Applications. Mat. Sci. Appl. 2017, 8, 197–208. [Google Scholar] [CrossRef]
- Nakajima, D.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Growth behavior of anodic oxide formed by aluminum anodizing in glutaric and its derivative acid electrolytes. Appl. Surf. Sci. 2014, 321, 364–370. [Google Scholar] [CrossRef]
- Solmaz, R.; Kardas, G.; Yazici, B.; Erbil, M. Citric acid as natural corrosion inhibitor for aluminum protection. Corros. Eng. Sci. Technol. 2008, 43, 186–191. [Google Scholar] [CrossRef]
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 1995.
- ASTM E407-07; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2011.
- Cabral-Miramontes, J.; Almeraya-Calderón, F.; López, F.E.; Lara Banda, M.; Olguín-Coca, J.; López-León, L.D.; Castañeda-Robles, I.; Alcalá, M.Á.E.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Citric Acid as an Alternative to Sulfuric Acid for the Hard-Anodizing of AA6061. Metals 2021, 11, 1838. [Google Scholar] [CrossRef]
- Franco, M.; Krishna, T.H.; Pillai, A.M.; Rajendra, A.; Sharma, A.K. A comparative study on the corrosion behavior of hard anodic coatings on aa 6061 obtained using dc and pulsed dc power sources. Acta Metall. Sin. Engl. Lett. 2013, 26, 647–656. [Google Scholar] [CrossRef]
- Cabral-Miramontes, J.A.; Barceinas-Sánchez, J.D.O.; Poblano-Salas, C.A.; Pedraza-Basulto, G.K.; Nieves-Mendoza, D.; Zambrano-Robledo, P.C.; Almeraya-Calderón, F.; Chacón-Nava, J.G. Corrosion Behavior of AISI 409Nb Stainless Steel Manufactured by Powder Metallurgy Exposed in H2SO4 and NaCl Solutions. Int. J. Electrochem. Sci. 2013, 8, 564–577. [Google Scholar]
- Cabral Miramontes, J.A.; Barceinas Sánchez, J.D.O.; Almeraya Calderón, F.; Martínez Villafañe, A.; Chacón Nava, J.G. Effect of Boron Additions on Sintering and Densification of a Ferritic Stainless Steel. J. Mater. Eng. Perform. 2010, 19, 880–884. [Google Scholar] [CrossRef]
- ASTM G61-86; Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements for Localized Corrosion Susceptibility of Iron-, Nickel-, or Cobalt-Based Alloys. ASTM International: West Conshohocken, PA, USA, 2018.
- Ramirez-Arteaga, A.M.; Gonzalez-Rodriguez, J.G.; Campillo, B.; Gaona-Tiburcio, C.; Dominguez-Patiño, G.; Leduc Lezama, L.; Chacon-Nava, J.G.; Neri-Flores, M.A.; Martinez-Villafañe, A. An Electrochemical Study of the Corrosion Behavior of a Dual Phase Steel in 0.5m H2SO4. Int. J. Electrochem. Sci. 2010, 5, 1786. [Google Scholar]
- Martínez-Villafañe, A.; Almeraya-Calderón, F.; Gaona-Tiburcio, C.; Gonzalez-Rodriguez, J.; Porcayo-Calderón, J. High-Temperature Degradation and Protection of Ferritic and Austenitic Steels in Steam Generators. J. Mater. Eng. Perform. 1998, 7, 108–113. [Google Scholar] [CrossRef]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado, E.M.; Cabral-Miramontes, J.A.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-López, F.; Chacón-Nava, J.G.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef]
- Almeraya- Calderon, F.; Montoya-R., M.; Garza-Montes-de-Oca, N.; Castorena, J.; Estupiñan-Lopez, F.; Miramontes, J.; Gaona-Tiburcio, C. Corrosion behavior of multilayer coatings deposited by PVD on Inconel 718 in Chloride and Sulphuric Acid solutions. Int. J. Electrochem. Sci. 2019, 14, 9596–9609. [Google Scholar] [CrossRef]
- Jáquez-Muñoz, J.M.; Gaona-Tiburcio, C.; Chacón-Nava, J.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Delgado, A.D.; Flores-De los Rios, J.P.; Bocchetta, P.; Almeraya-Calderón, F. Electrochemical corrosion of titanium and titanium alloys anodized in H2SO4 and H3PO4 solutions. Coatings 2022, 12, 325. [Google Scholar] [CrossRef]
- Huang, Y.L.; Shih, H.; Huang, H.; Daugherty, J.; Wu, S.; Ramanathan, S.; Chang, C.; Mansfeld, F. Evaluation of the corrosion resistance of anodized aluminum 6061 using electrochemical impedance spectroscopy (EIS). Corros. Sci. 2008, 50, 3569–3575. [Google Scholar] [CrossRef]
- Zuo, Y.; Zhao, P.H.; Zhao, J.M. The influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solutions. Surf. Coat. Technol. 2003, 166, 237–242. [Google Scholar] [CrossRef]
- Suay, J.J.; Gimenez, E.; Rodriguez, T.; Habbib, K.; Saura, J.J. Characterization of anodized and sealed aluminium by EIS. Corros. Sci. 2003, 45, 611–624. [Google Scholar] [CrossRef]
- ASTM G106-15; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Cabral-Miramontes, J.A.; Bastidas, D.M.; Baltazar, M.A.; Zambrano-Robledo, P.; Bastidas, J.M.; Almeraya-Calderón, F.M.; Gaona-Tiburcio, C. Corrosion behavior of Zn-TiO2, and Zn-ZnO Electrodeposited Coating in 3.5% NaCl Solution. Int. J. Electrochem. Sci. 2019, 14, 4226–4239. [Google Scholar] [CrossRef]
- Maldonado-Bandala, E.; Jiménez-Quero, V.; Olguin-Coca, J.; Lizarraga, L.G.; Baltazar-Zamora, M.A.; Ortiz, C.A.; Almeraya, C.F.; Zambrano, R.P.; Gaona-Tiburcio, C. Electrochemical Characterization of Modified Concretes with Sugar Cane Bagasse Ash. Int. J. Electrochem. Sci. 2011, 6, 4915–4926. [Google Scholar]
- Cabral-Miramontes, J.; Gaona-Tiburcio, C.; Estupinán-López, F.; Lara-Banda, M.; Zambrano-Robledo, P.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Chacón-Nava, J.; Almeraya-Calderón, F. Corrosion Resistance of Hard Coat Anodized AA 6061 in Citric–Sulfuric Solutions. Coatings 2020, 10, 601. [Google Scholar] [CrossRef]
- Samaniego-Gámez, P.; Almeraya-Calderón, F.; Martin, U.; Ress, J.; Gaona-Tiburcio, C.; Silva-Vidaurri, L.; Cabral-Miramontes, J.; Bastidas, J.M.; Chacón-Nava, J.G.; Bastidas, D.M. Efecto del tratamiento de sellado en el comportamiento frente a corrosión de la aleación anodizada de aluminio-litio AA2099. Rev. Met. 2020, 56, e180. [Google Scholar] [CrossRef]
- Iglesias-Rubianes, L.; Garcia-Vergara, S.J.; Skeldon, P.; Thompson, G.E.; Ferguson, J.; Beneke, M. Cyclic oxidation processes during anodizing of Al-Cu alloys. Electrochim. Acta 2007, 52, 7148–7157. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, Y.; Li, J.; Li, Y.; Zhang, Z.; Feng, C.; Sun, R. Fabrication of self-ordered alumina films with large interpore distance by Janus anodization in citric acid. Sci. Rep. 2016, 6, 39165. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, Z.; Li, Y.; Ma, Y.; Chen, L.; Zhang, Z.; Sun, R. Self-Organization process of aluminum oxide during hard anodization. Electrochim. Acta 2016, 213, 14–20. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, Y.; Li, J.; Lu, J.; Li, Y.; Yang, Y.; Feng, C.; Hao, C.; Zhang, Z.; Hu, J.; et al. Pore nucleation mechanism of self-ordered alumina with large period in stable anodization in citric acid. J. Electrochem. Soc. 2018, 165, E311–E317. [Google Scholar] [CrossRef]
- Tabesh, S.; Davar, F.; Loghman-Estarki, M.R. The effects of chelating agent type on the morphology and phase evolutions of alumina nanostructures. Ceram. Int. 2017, 43, 10247. [Google Scholar] [CrossRef]
- MIL-A-8625F; Anodic Coatings for Aluminum and Aluminum Alloys. Departments and Agencies of the Department of Defense: Washington, VA, USA, 1993; pp. 1–19.
- Thomas, R.W. Hard Anodizing of Aluminum; Brace, A.W., Ed.; Technicopy Ltd.: Stonehouse, UK, 1987; pp. 1–7. [Google Scholar]
- Lerner, L.M. Hard anodizing of aerospace aluminium alloys. Trans. Inst. Met. Finish. 2010, 88, 21–24. [Google Scholar] [CrossRef]
- Wang, L.W.; Tian, H.Y.; Gao, H.; Xie, F.Z.; Zhao, K.; Cui, Z.Y. Electrochemical and XPS analytical investigation of the accelerative effect of bicarbonate/carbonate ions on AISI 304 in alkaline environment. Appl. Surf. Sci. 2019, 492, 792–807. [Google Scholar] [CrossRef]
- Yang, Y.J.; Zhang, Z.P.; Jin, Z.S.; Sun, W.C.; Xia, C.Q.; Ma, M.Z.; Zhang, X.Y.; Li, G.; Liu, R.P. A study on the corrosion behavior of the in-situ Ti-based bulk metallic glass matrix composites in acid solutions. J. Alloy. Compd. 2019, 782, 927–935. [Google Scholar] [CrossRef]
- Silverman, D.C. Practical Corrosion prediction using electrochemical techniques. In Uhlig’s Corrosion Handbook, 3rd ed.; Winston Revie, R., Ed.; John Wiley & Son, Inc.: Hoboken, NJ, USA, 2011; pp. 1129–1166. [Google Scholar]
- Silverman, D.C. Tutorial on Cyclic Potentiodynamic Polarization Technique. CORROSION 98, San Diego, CA, USA, 22–27 March 1998; 1998. [Google Scholar]
- Gaona-Tiburcio, C.; Almeraya-Calderón, F.; Chacon-Nava, J.; Matutes-Aquino, A.M.; Martinez-Villafañe, A. Electrochemical response of permanent magnets in different solutions. J. Alloys Compd. 2004, 369, 78–80. [Google Scholar] [CrossRef]
- Huang, Y.; Esra, K.; Mansfeld, F. Evaluation of the corrosion resistance of anodizing aluminum samples using electrochemical impedance spectroscopy. In Proceedings of the Annual Graduate Student Research Symposium, Los Angeles, CA, USA, 13 February 2007. [Google Scholar]
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2021, 11, 4. [Google Scholar] [CrossRef]
- Feliu, S.J. Electrochemical impedance spectroscopy for the measurement of the corrosion rate of magnesium alloys: Brief review and challenges. Metals 2020, 10, 775. [Google Scholar] [CrossRef]
- Hoar, T.P.; Wood, G.C. The sealing of porous anodic oxide films on aluminium. Electrochim. Acta 1962, 7, 333–353. [Google Scholar] [CrossRef]
- Samaniego-Gámez, P.O.; Almeraya-Calderon, F.; Maldonado-Bandala, E.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Lopez-Leon, L.D.; Silva Vidaurri, L.G.; Zambrano-Robledo, P.; Gaona-Tiburcio, C. Corrosion Behavior of AA2055 Aluminum-Lithium Alloys Anodized in the Presence of Sulfuric Acid Solution. Coatings 2021, 11, 1278. [Google Scholar] [CrossRef]
- Kwolek, P.; Krupa, K.; Obłój, A.; Kocurek, P.; Wierzbinka, M.; Sieniawski, J. Tribological Properties of the Oxide Coatings Produced onto 6061-T6 Aluminum Alloy in the Hard Anodizing Process. J. Mater. Eng. Perform. 2018, 27, 3268–3275. [Google Scholar] [CrossRef]
- Samaniego-Gámez, O.; Almeraya-Calderón, F.; Chacón-Nava, J.; Maldonado-Bandala, E.; Nieves-Mendoza, D.; Flores-De los Rios, J.P.; Jáquez-Muñoz, J.M.; Delgado, A.D.; Gaona-Tiburcio, C. Corrosion Behavior of Passivated CUSTOM450 and AM350 Stainless Steels For Aeronautical Applications. Metals 2022, 12, 666. [Google Scholar] [CrossRef]
- Cesiulis, H.; Tsyntsaru, N.; Ramanavicius, A.; Ragoisha, G. The Study of Thin Films by Electrochemical I. In Nanostructures and Thin Films for Multifunctional Applications; Tiginyanu, I., Topala, P., Ursaki, V., Eds.; Nanoscience and Technology; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kramer, G.R.; Mendez, C.M.; Ares, A.E. Evaluation of corrosion resistance of aluminum-based alloys in bioethanol produced in Misiones. Proc. Mat. Sci. 2015, 9, 341–349. [Google Scholar] [CrossRef]
- Elkilany, H.A.; Shoeib, M.A.; Abdel-Salam, O.E. Influence of hard anodizing on the mechanical and corrosion properties of different aluminum alloys. Metallogr. Microstruct. Anal. 2019, 8, 861–879. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Paez, M.A.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Xhou, X. Oxidation of copper and mobility of copper ions during anodizing of an Al–1.5 wt.% Cu alloy. Surf. Interface Anal. 1995, 23, 892–898. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Dimitrov, N.; Sieradzki, K. Dealloying and corrosion of Al alloy 2024 T3. J. Electrochem. Soc. 2002, 149, 428–439. [Google Scholar] [CrossRef]
- Buchheit, R.G.; Martinez, M.A.; Montes, L.P. Evidence for Cu ions formation by dissolution and dealloying of Al2CuMg intermetallic compound in rotating disk. J. Electrochem. Soc. 2000, 147, 119–124. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.P.; Pagettia, J.; Normand, B. An electrochemical approach to the anodic oxidation of Al 2024 alloy in sulfuric acid containing inhibitors. Surf. Coat. Technol. 2002, 161, 267–274. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhou, X.; Skeldon, P.; Thompson, G.E. Structure of the Copper–enriched layer introduced by anodic oxidation of copper-containing aluminum Alloy. Electrochim. Acta 2015, 179, 394–401. [Google Scholar] [CrossRef]
- Hashimoto, T.; Zhang, X.; Zhou, X.; Skeldon, P.; Haigh, S.J.; Thompson, G.E. Investigation of dealloying of S phase (Al2CuMg) in AA 2024-T3 aluminum alloy using high-resolution 2D and 3D electron imaging. Corros. Sci. 2016, 103, 157–164. [Google Scholar] [CrossRef]
- Snogan, F.; Blanc, C.; Mankowski, G.; Pebere, N. Characterisation of sealed anodic films on 7050 T74 and 2214 T6 aluminum alloys. Surf. Coat. Technol. 2002, 154, 94–103. [Google Scholar] [CrossRef]
- Fratila-Apachitei, L.E.; Tichelaar, F.D.; Thompson, G.E.; Terryn, H.; Skeldon, P.; Duszczyk, J.; Katgerman, L. A transmission electron microscopy study of hard anodic oxide layers on AlSi(Cu) alloys. Electrochim. Acta. 2004, 49, 3169–3177. [Google Scholar] [CrossRef]
- Cheng, T.C.; Chou, C.C. The electrical and mechanical properties of porous anodic 6061-T6 aluminum alloy oxide film. J. Nanomat. 2015, 2015, 141. [Google Scholar] [CrossRef]
- Koczera, A.E. The Effects of Carboxylic Acids in Aluminum Anodizing. Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 2017. [Google Scholar]
- Peng, L.; Li, M. Improved technology for hard anodizing dissolution of aluminum alloy part. Open Mat. Sci. J. 2015, 9, 82–85. [Google Scholar] [CrossRef]
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Yasumori, A. Fabrication of ideally ordered nanoporous alumina films and integrated alumina nanotubule arrays by high-field anodization. Adv. Mater. 2005, 17, 2115–2119. [Google Scholar] [CrossRef]
- Schwirn, K.; Lee, W.; Hillebrand, R.; Steinhart, M.; Nielsch, K.; Gösele, U. Self-ordered anodic aluminum oxide formed by H2SO4 hard anodization. ACS Nano 2008, 2, 302–310. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, G.; Ye, J.; Hua, R.; Sun, Z.; Cui, J. Fast growth of self-aligned titania nanotube arrays with excellent transient photoelectric response. Electrochim. Acta. 2016, 211, 552–560. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Norek, M.; Michalska-Domańska, M.; Bojar, Z. Ultra-small nanopores obtained by self-organized anodization of aluminum in oxalic acid at low voltages. Mater. Lett. 2013, 111, 20–23. [Google Scholar] [CrossRef]
- Noh, K.; Brammer, K.; Kim, H.; Jung, S.; Seong, T.; Jin, S. Highly self-assembled nanotubular aluminum oxide by hard anodization. J. Mater. Res. 2011, 26, 186–193. [Google Scholar] [CrossRef]
- Dimogerontakis, T.; Kaplanoglou, L.; Kaplanoglou, I. Oxygen evolution during the formation of barrier type anodic film on 2024-T3 aluminium alloy. Corros. Sci. 1998, 40, 1939–1951. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. the composition of the alloy/film interface during anodic oxidation of Al-W alloys. J. Electrochem. Soc. 1996, 143, 2465–2470. [Google Scholar] [CrossRef]
- Habazaki, H.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C.; Zhou, X. Effects of alloying elements in anodizing of aluminium. Trans IMF 1997, 75, 18–23. [Google Scholar] [CrossRef]
- Habazaki, H.; Zhou, X.; Shimizu, K.; Skeldon, P.; Thompson, G.E.; Wood, G.C. Mobility of copper ions in anodic alumina films. Electrochim. Acta. 1997, 42, 2627–2635. [Google Scholar] [CrossRef]
- Torrescano-Alvarez, J.M.; Curioni, M.; Habazaki, H.; Hashimoto, T.; Skeldon, P.; Zhou, X. Incorporation of alloying elements into porous anodic films on aluminium alloys: The role of cell diameter. Electrochim. Acta. 2019, 296, 783–789. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Norek, M.; Stępniowski, W.J.; Budner, B. Fabrication of high quality anodic aluminum oxide (AAO) on low purity aluminum-A comparative study with the AAO produced on high purity aluminum. Electrochim. Acta. 2013, 105, 424–432. [Google Scholar] [CrossRef]
- Zajączkowska, L.; Siemiaszko, D.; Norek, M. Towards self-organized anodization of aluminum in malic acid solutions-new aspects of anodization in the organic acid. Materials 2020, 13, 3899. [Google Scholar] [CrossRef] [PubMed]
- Jalal, H.; Hussein, K.; Karabet, F.; Saound, Y. Study of the effect mechanism of organic additives on the aluminum alloy 6066 anodization. J. Miner. Met. Mater. Eng. 2019, 5, 73–81. [Google Scholar]
- Sposito, G. The Environmental Chemistry of Aluminum, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 173–174, 303–305. [Google Scholar]
- Hidber, P.C.; Graule, T.J.; Gauckler, L.J. Citric Acid—A Dispersant for Aqueous Alumina Suspensions. J. Am. Ceram. Soc. 1996, 79, 1857–1867. [Google Scholar] [CrossRef]
- Ban, C.L.; He, Y.D.; Xin, S. Effect of citric acid on microstructure and electrochemical characteristics of high voltage anodized alumina film formed on etched Al Foils. Trans. Nonferrous Met. Soc. China. 2011, 21, 133–138. [Google Scholar] [CrossRef]
- Xiangfeng, M.; Guoying, W.; Hongliang, G.; Yundan, Y.; Ying, C.; Dettinger, H. Anodization for 2024 Al Alloy from Sulfuric-Citric Acid and Anticorrosion Performance of Anodization Films. Int. J. Electrochem. Sci. 2013, 8, 10660–10671. [Google Scholar]
- Tang, C.W. The Study of Anodic Treatment of Aluminum in Tertiary Mixed Acid after High Temperature Pre-Immersing. Ph.D. Thesis, Tatung University, Taipei City, Taiwan, 2005. [Google Scholar]
- Torato, P.; Krusid, B. Effect of current density ramping on the growth rate and structure of AA2024-T3. Materials 2022, 15, 3258. [Google Scholar]
- Liu, Y.; Skeldon, P.; Thompson, G.E.; Hashimoto, T.; Smith, C.J.E. Influence of copper on chromate conversion coating of aluminium alloys. Trans. Inst. Met. Finish. 2005, 83, 125–129. [Google Scholar] [CrossRef]
- Thompson, G.E. Porous anodic alumina: Fabrication, characterization, and applications. Thin Solid Film. 1997, 297, 192–201. [Google Scholar] [CrossRef]
- López, V.; Otero, E.; Bautista, A.; González, J.A. Sealing of anodic films obtained in oxalic acid baths. Surf. Coat. Technol. 2000, 124, 76–84. [Google Scholar] [CrossRef]
- Bautista, A.; González, J.A.; López, V. Influence of triethanolamine additions on the sealing mechanism of anodised aluminium. Surf. Coat. Technol. 2002, 154, 49–54. [Google Scholar] [CrossRef]
- Santiago, G.; Baltazar, M.A.; Galván, R.; López, L.; Zapata, F.; Zambrano, P.; Gaona, C.; Almeraya, F. Electrochemical Evaluation of Reinforcement Concrete Exposed to Soil Type SP Contaminated with Sulphates. Int. J. Electrochem. Sci. 2016, 11, 4850–4864. [Google Scholar] [CrossRef]
- Bocchetta, P.; Chen, L.-Y.; Tardelli, J.D.C.; Reis, A.C.D.; Almeraya-Calderón, F.; Leo, P. Passive layers and corrosion resistance of biomedical Ti-6Al-4V and β-Ti alloys. Coatings 2021, 11, 487. [Google Scholar] [CrossRef]
- Dasquet, J.P.; Caillard, U.D.; Conforto, E.; Bonino, J.P.; Bes, R. Investigation of the anodic oxide layer on 1050 and 2024T3 aluminium alloys by electron microscopy and electrochemical impedance spectroscopy. Thin Solid Film. 2000, 371, 183–190. [Google Scholar] [CrossRef]
- MacDonald, J.R.; Johnson, W.B. Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987; pp. 154–156. [Google Scholar]
- Fonseca, C.; Barbosa, M.A. Corrosion behavior of titanium in biofluids containing H2O2 studied by electrochemical impedance spectroscopy. Corros. Sci. 2001, 43, 547–559. [Google Scholar] [CrossRef]
- Macdonald, D. Reflections on the history of electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1376–1388. [Google Scholar] [CrossRef]

| Material | Anodizing | Sealing Process | Samples | |||
|---|---|---|---|---|---|---|
| Current Density (A/dm2) | Time (min) | Bath for Anodizing | ||||
| Sulfuric Acid | Citric Acid | |||||
| AA2024 | 3 | 60 | 5 mL/L | 1 M | Deionized water Temperature at 95 °C Time 60 min | 3A C1M S5 |
| 10 mL/L | 1 M | 3A C1M S10 | ||||
| - | 1 M | 3A C1M | ||||
| 1 M | - | 3A S1M | ||||
| 4.5 | 60 | 5 mL/L | 1 M | Deionized water Temperature at 95 °C Time 60 min | 4.5A C1M S5 | |
| 10 mL/L | 1 M | 4.5A C1M S10 | ||||
| - | 1 M | 4.5A C1M | ||||
| 1 M | - | 4.5A S1M | ||||
| Sample | Ecorr (V) | Epit (V) | EA–C (V) | Ipass (A/cm2) | icorr (A/cm2) | Hysteresis |
|---|---|---|---|---|---|---|
| AA2024 | −0.656 | −0.656 | −0.895 | - | 3.43 × 10−7 | Positive |
| 3A C1M S5 | −0.600 | −0.430 | −0.791 | - | 1.57 × 10−7 | Positive |
| 3A C1M S10 | −0.598 | −0.308 | - | 1.38 × 10−8 | 1.14 × 10−8 | Negative |
| 3A C1M | −0.563 | −0.242 | −0.903 | - | 2.31 × 10−5 | Positive |
| 3A S1M | −0.293 | −0.293 | 0.298 | 6.77 × 10−9 | 1.36 × 10−10 | Negative |
| 4.5A C1M S5 | −0.446 | 0.334 | - | 7.52 × 10−6 | 2.35 × 10−8 | Positive |
| 4.5A C1M S10 | −0.488 | 0.034 | −0.774 | 2.44 × 10−6 | 1.26 × 10−9 | Positive |
| 4.5A C1M | −0.593 | −0.593 | −0.917 | - | 2.55 × 10−6 | Positive |
| 4.5A S1M | −0.180 | −0.180 | - | 1.87 × 10−8 | 8.30 × 10−11 | Negative |
| Samples | RSol (Ω·cm2) | CPEPor (F/cm2) | RPor (Ω·cm2) | nPor | CPEB (F/cm2) | RB (Ω·cm2) | nB | Error | χ2 |
|---|---|---|---|---|---|---|---|---|---|
| AA2024 | 28.5 | - | - | - | 1.27 × 10−4 | 13,920 | 0.80 | ˂1.42 | 1 × 10−2 |
| 3A C1M S5 | 28.78 | 2.28 × 10−7 | 46,014 | 0.78 | 9.30 × 10−7 | 5.81 × 106 | 0.81 | ˂1.96 | 1 × 10−2 |
| 3A C1M S10 | 14.26 | 8.80 × 10−7 | 47,545 | 0.74 | 3.12 × 10−6 | 0.36 × 106 | 0.69 | ˂1.14 | 1 × 10−2 |
| 3A C1M | 22.77 | 1.41 × 10−6 | 560 | 0.81 | 4.02 × 10−5 | 6381 | 0.64 | ˂1.95 | 3 × 10−3 |
| 3A S1M | 24.40 | 6.68 × 10−7 | 152,820 | 0.77 | 8.41 × 10−7 | 6.20 × 106 | 0.94 | ˂1.79 | 1 × 10−2 |
| 4.5A C1M S5 | 63.58 | 1.13 × 10−7 | 2677 | 0.85 | 6.11 × 10−6 | 0.14 × 106 | 0.54 | ˂2.84 | 1 × 10−2 |
| 4.5A C1M S10 | 16.88 | 2.27 × 10−6 | 15,651 | 0.75 | 1.26 × 10−6 | 4.11 × 106 | 0.90 | ˂2.04 | 3 × 10−2 |
| 4.5A C1M | 25.58 | 5.43 × 10−5 | 6555 | 0.74 | 4.81 × 10−4 | 19,478 | 0.71 | ˂2.25 | 1 × 10−3 |
| 4.5A S1M | 25.35 | 6.55 × 10−7 | 89,049 | 0.61 | 1.26 × 10−6 | 10.2 × 106 | 0.91 | ˂2.17 | 7 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miramontes, J.C.; Gaona Tiburcio, C.; García Mata, E.; Esneider Alcála, M.Á.; Maldonado-Bandala, E.; Lara-Banda, M.; Nieves-Mendoza, D.; Olguín-Coca, J.; Zambrano-Robledo, P.; López-León, L.D.; et al. Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution. Materials 2022, 15, 6401. https://doi.org/10.3390/ma15186401
Miramontes JC, Gaona Tiburcio C, García Mata E, Esneider Alcála MÁ, Maldonado-Bandala E, Lara-Banda M, Nieves-Mendoza D, Olguín-Coca J, Zambrano-Robledo P, López-León LD, et al. Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution. Materials. 2022; 15(18):6401. https://doi.org/10.3390/ma15186401
Chicago/Turabian StyleMiramontes, José Cabral, Citlalli Gaona Tiburcio, Estefanía García Mata, Miguel Ángel Esneider Alcála, Erick Maldonado-Bandala, Maria Lara-Banda, Demetrio Nieves-Mendoza, Javier Olguín-Coca, Patricia Zambrano-Robledo, Luis Daimir López-León, and et al. 2022. "Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution" Materials 15, no. 18: 6401. https://doi.org/10.3390/ma15186401
APA StyleMiramontes, J. C., Gaona Tiburcio, C., García Mata, E., Esneider Alcála, M. Á., Maldonado-Bandala, E., Lara-Banda, M., Nieves-Mendoza, D., Olguín-Coca, J., Zambrano-Robledo, P., López-León, L. D., & Almeraya Calderón, F. (2022). Corrosion Resistance of Aluminum Alloy AA2024 with Hard Anodizing in Sulfuric Acid-Free Solution. Materials, 15(18), 6401. https://doi.org/10.3390/ma15186401








