Comparative Study on the Corro-Erosive Properties of Base Cemented Transition Metals TaC and HfC and TaX-HfX-C Coatings
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Materials
2.2. Deposition Parameter
2.3. Coating Characterization
3. Analysis and Results
3.1. Structural Analysis by X-ray Diffraction
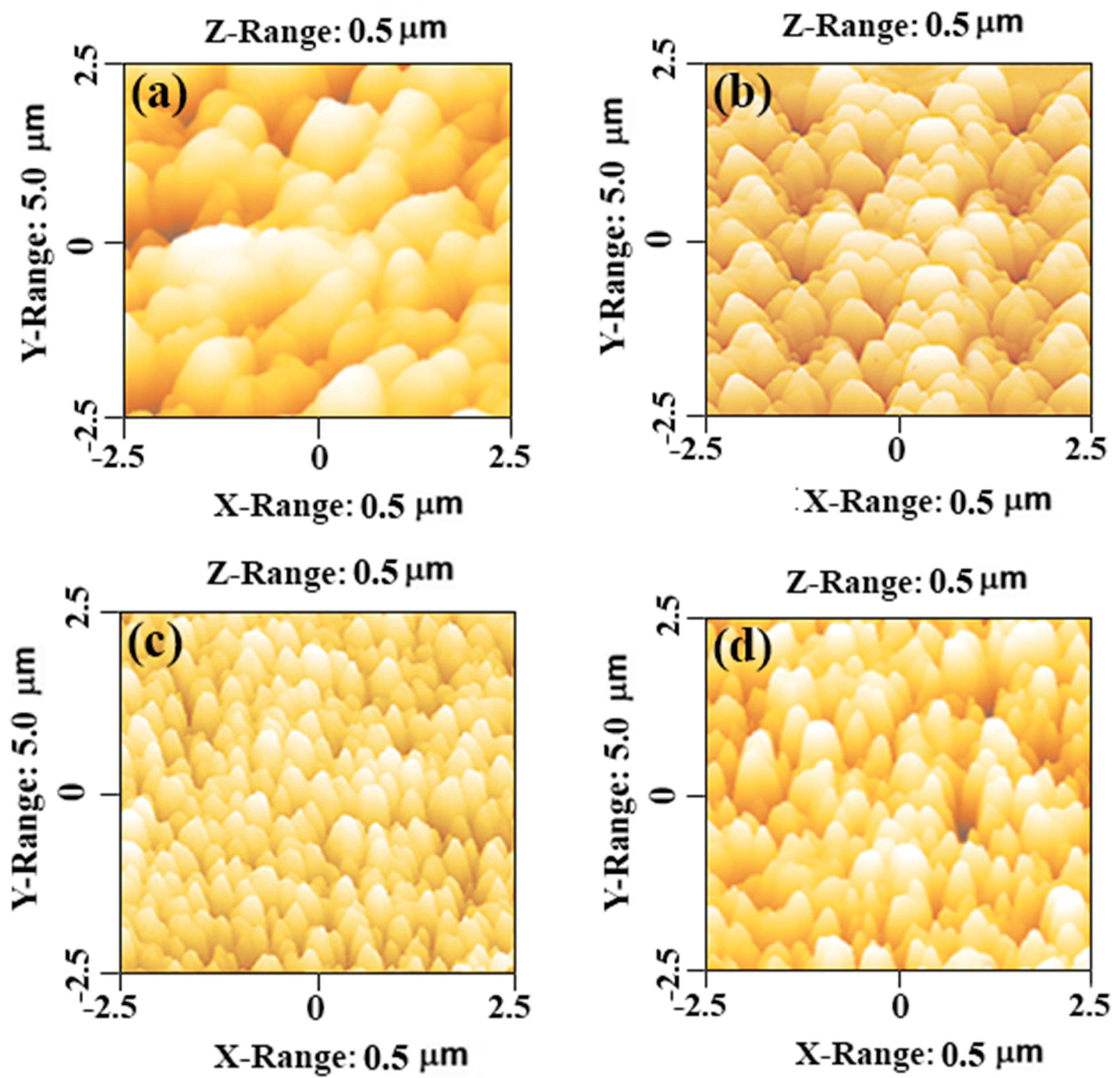
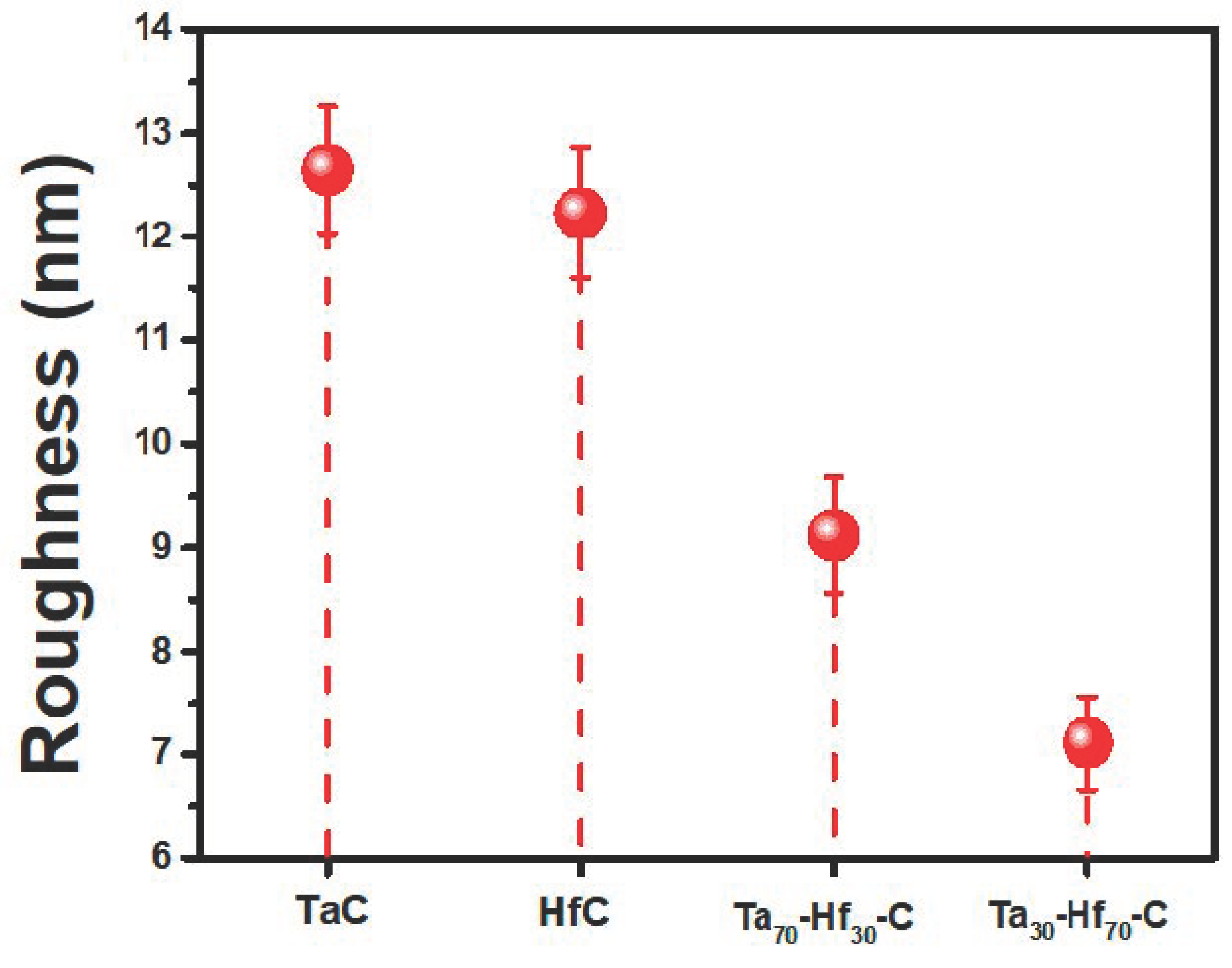
3.2. Morphological Analysis
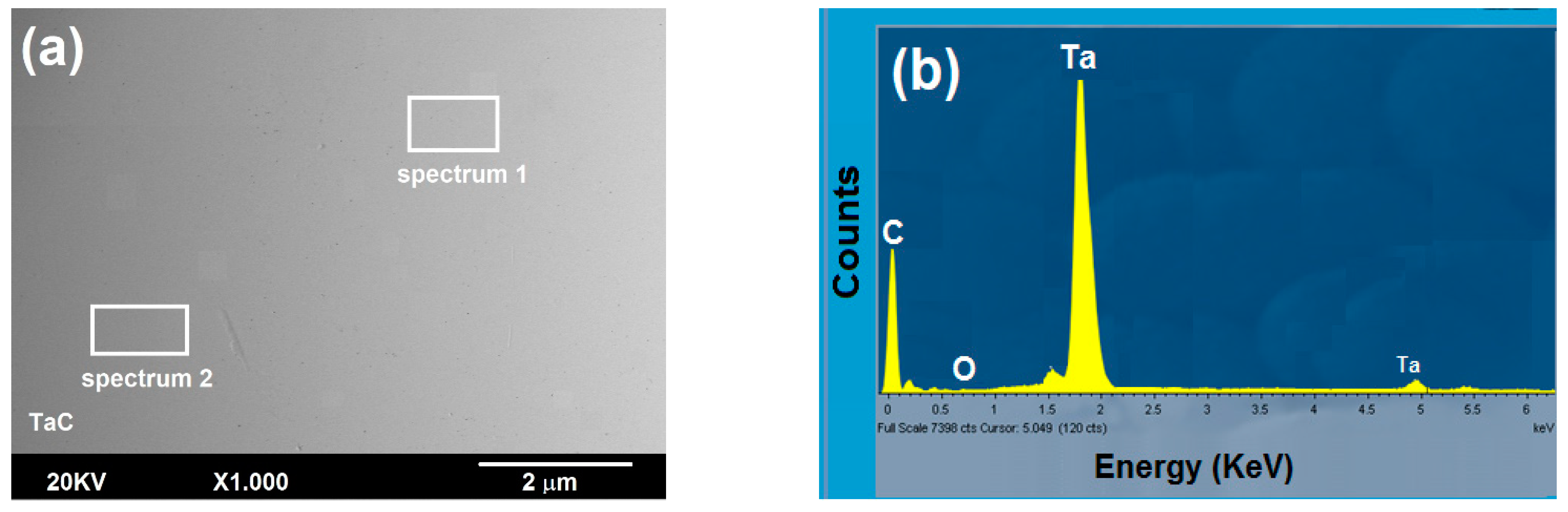
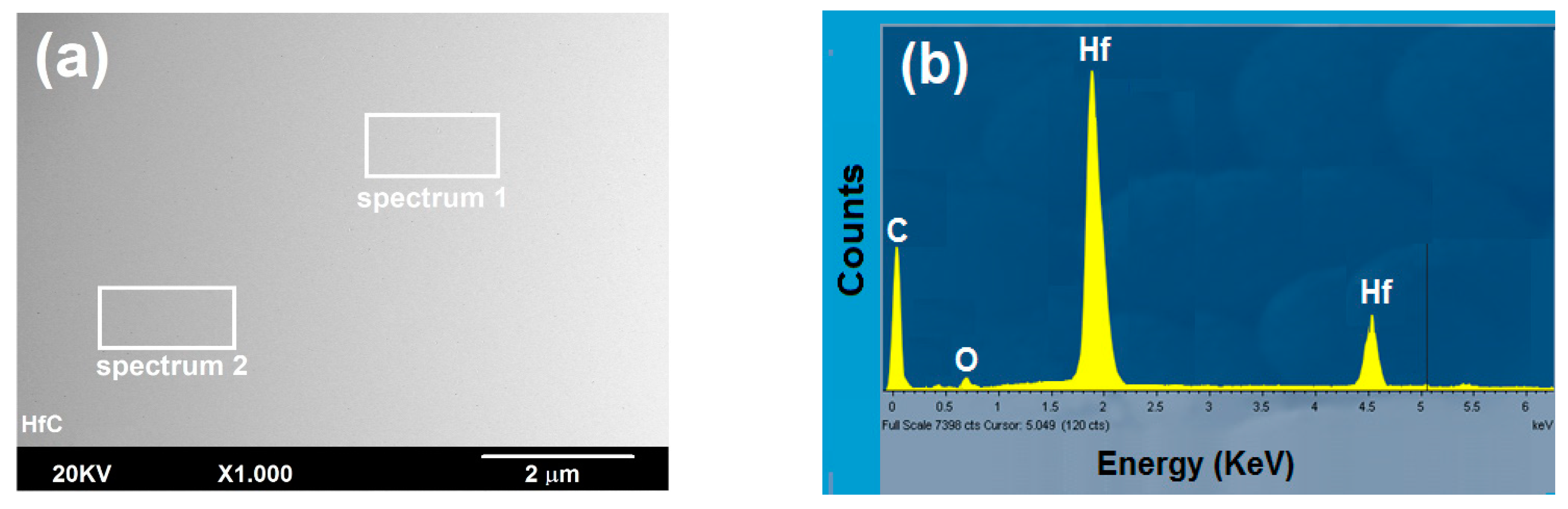
3.2.1. Surface Study by SEM-EDS
3.2.2. Cross-Section Analysis SEM
3.3. Corrosion Study
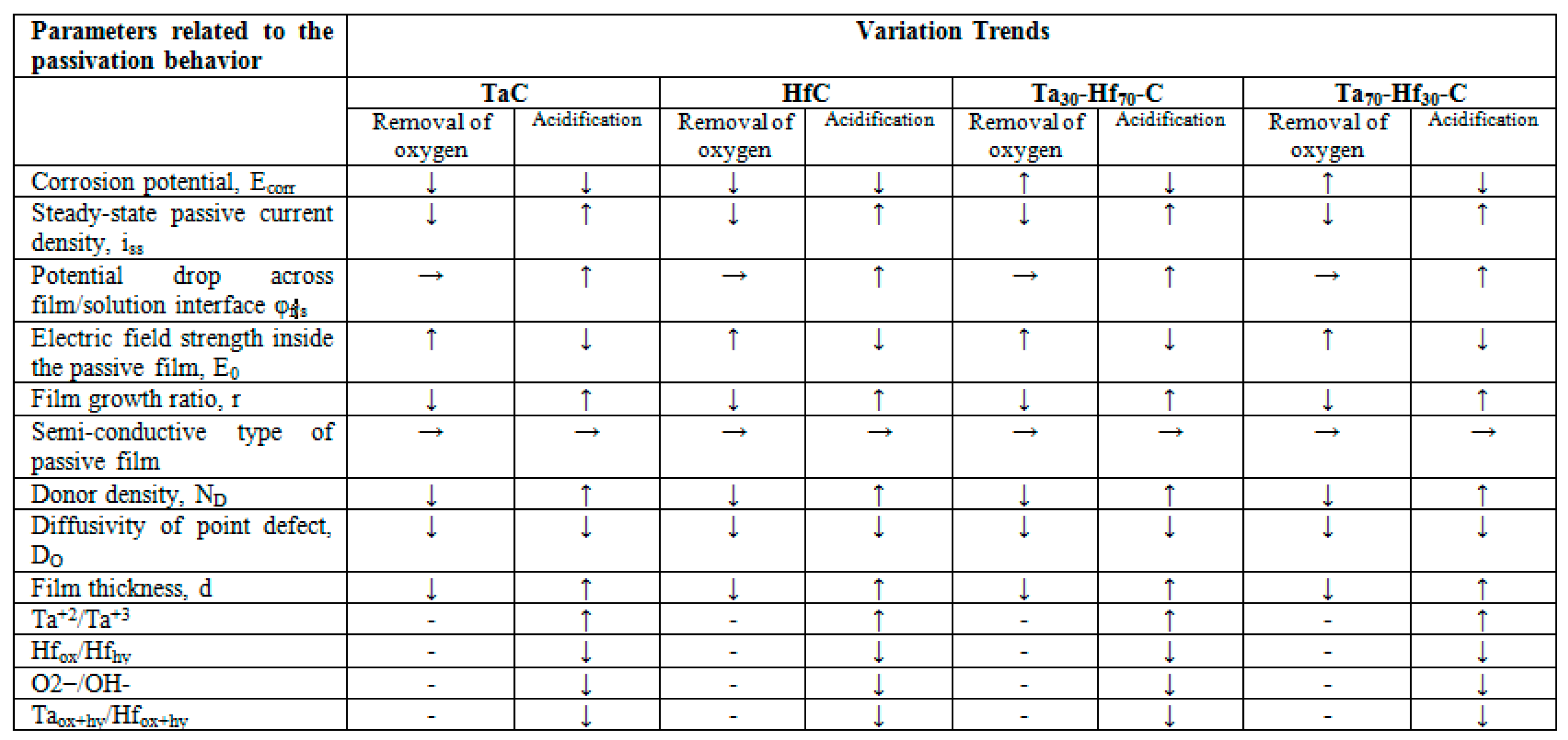
3.3.1. Electrochemical Impedance Spectroscopy (EIS)
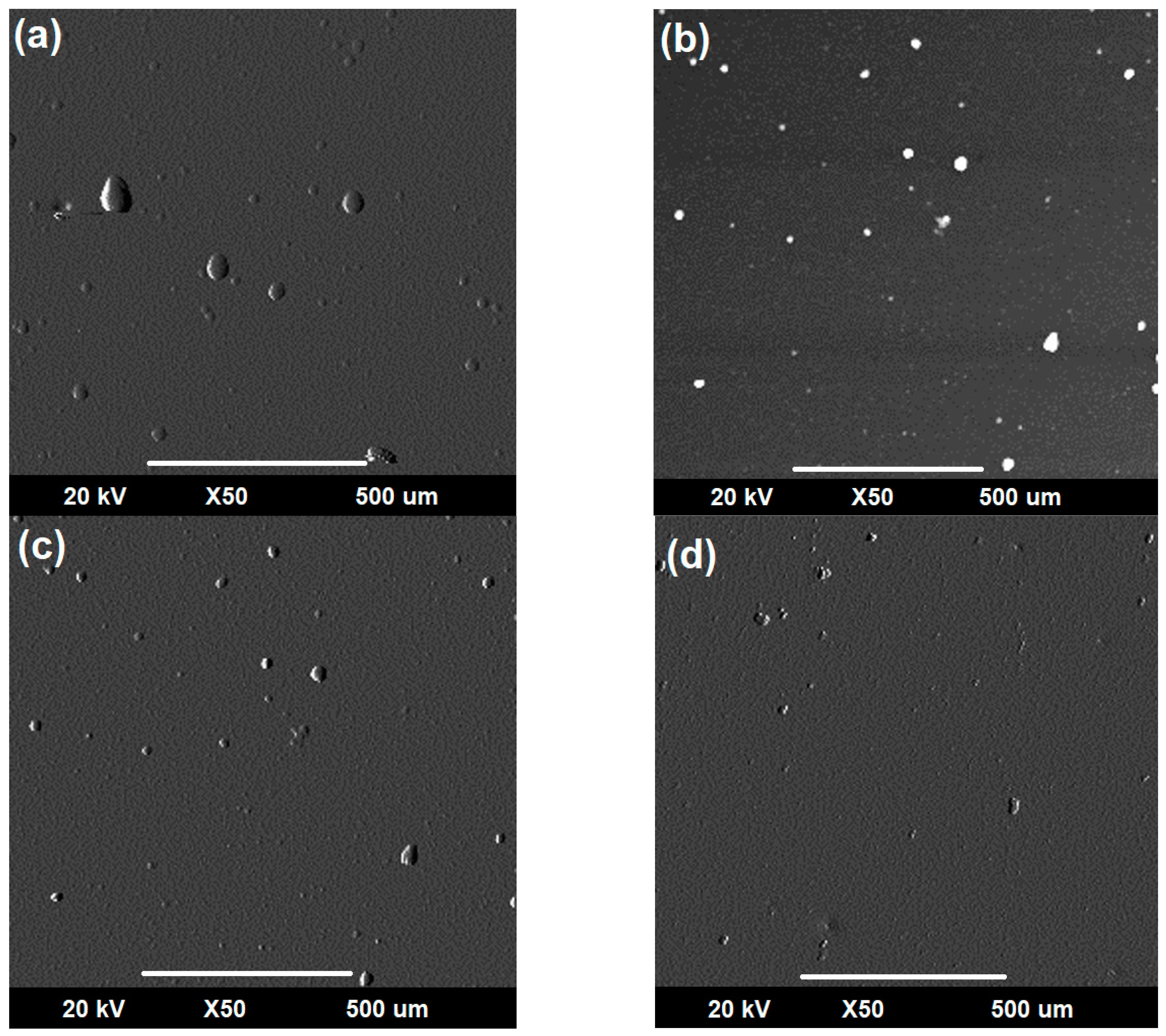
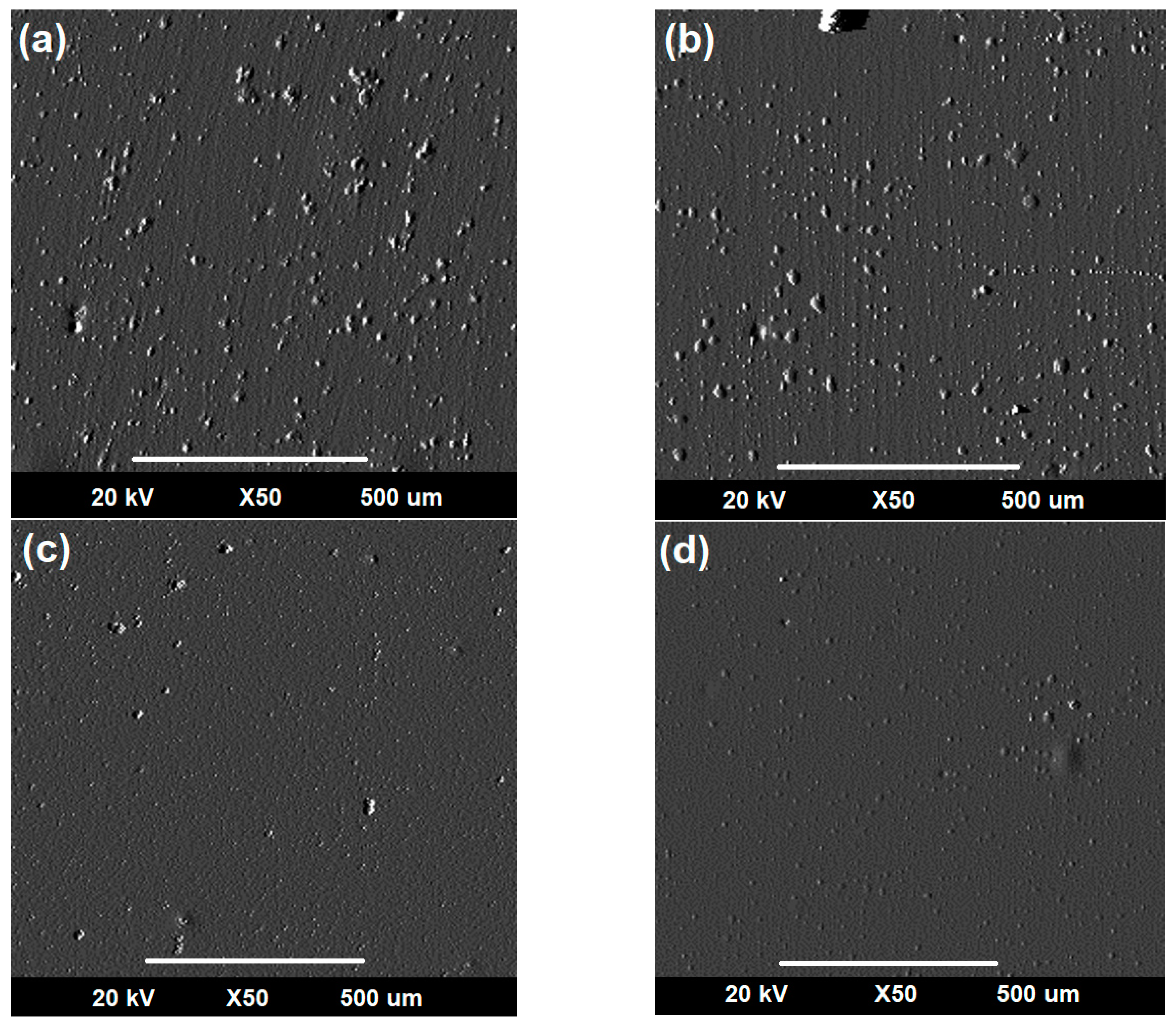
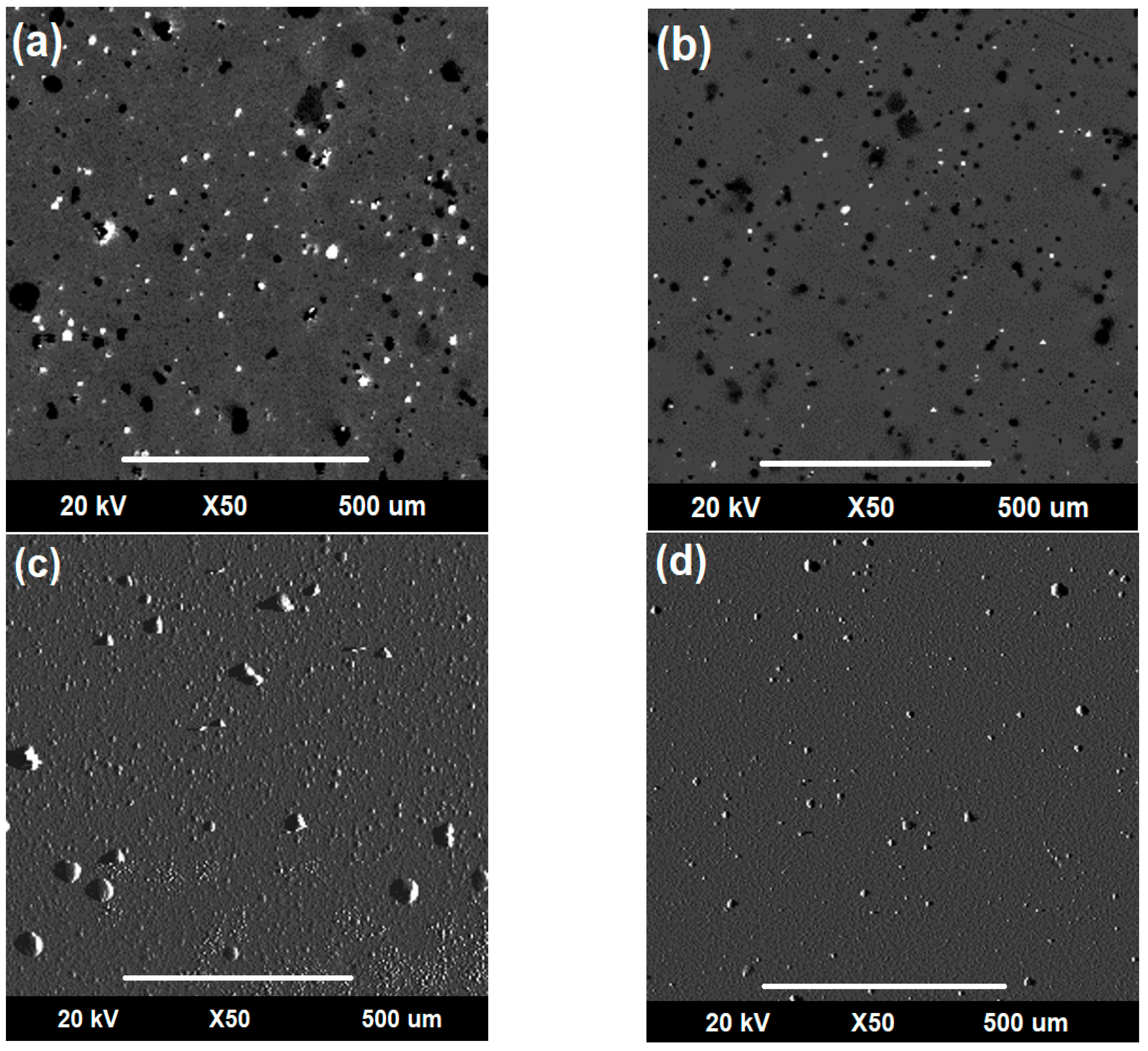
3.3.2. Evaluation of Corro-Erosive Damage by SEM as a Function of the Impact Angle
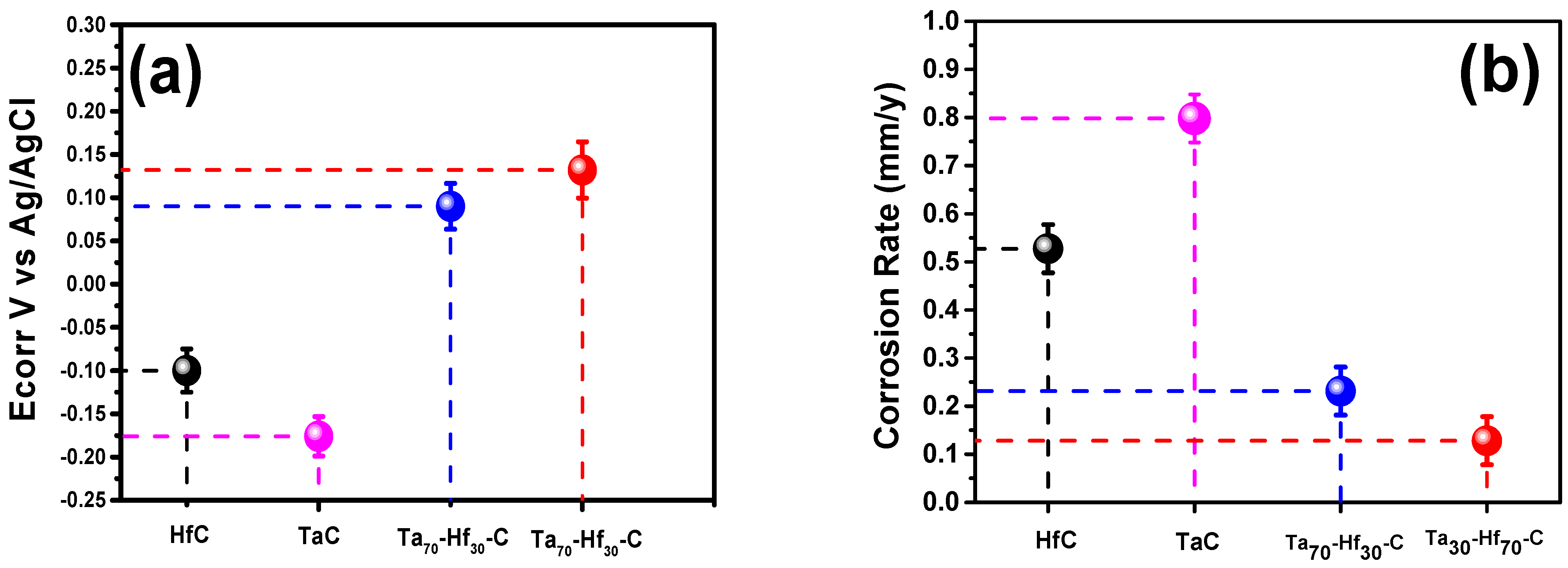
3.3.3. Tafel Polarization Curves
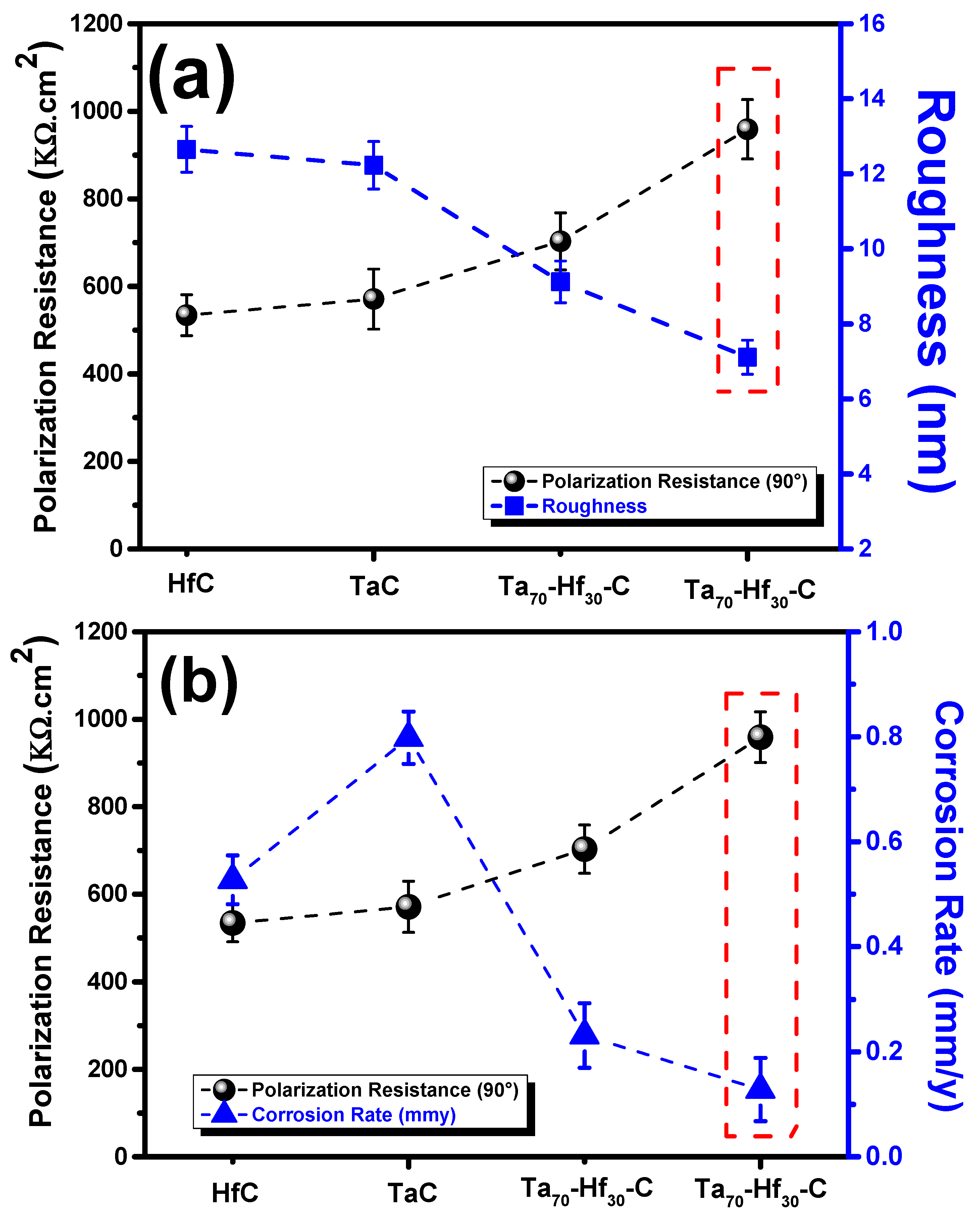
3.4. Merit Index
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wood, R. Erosion–corrosion interactions and their effect on marine and offshore materials. Wear 2006, 261, 1012–1023. [Google Scholar] [CrossRef]
- Okada, T.; Awazu, K.; Kawasaki, H. Influence of cavitation erosion on corrosion fatigue and the effect of surface coatings on resistance. Wear 1980, 63, 51–70. [Google Scholar] [CrossRef]
- Ou, X.-M.; Sun, Z.; Sun, M.; Zou, D.-L. Hot-corrosion mechanism of Ni-Cr coatings at 650 °C under different simulated corrosion conditions. J. China Univ. Min. Technol. 2008, 18, 444–448. [Google Scholar] [CrossRef]
- Feng, G.; Li, H.; Yao, X.; Zhou, H.; Yu, Y.; Lu, J. Ablation resistance of HfC-TaC/HfC-SiC alternate coating for SiC-coated carbon/carbon composites under cyclic ablation. J. Eur. Ceram. Soc. 2021, 41, 3207–3218. [Google Scholar] [CrossRef]
- Nyberg, H.; Tokoroyama, T.; Wiklund, U.; Jacobson, S. Design of low-friction PVD coating systems with enhanced running-in performance—carbon overcoats on TaC/aC coatings. Surf. Coat. Technol. 2013, 222, 48–54. [Google Scholar] [CrossRef]
- Guzmán, P.; Aperador, W.; Yate, L. Enhancement of the Pitting Corrosion Resistance of AISI 316LVM Steel with Ta-Hf-C/Au Bilayers for Biomedical Applications. J. Nanomater. 2017, 2017, 6825250. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Zhu, X.; Fu, Y.; Chen, R.; Gai, W. Design and preparation of multilayer TaC/HfC coating: Ablation behavior under oxyacetylene flame with different heat flux. Corros. Sci. 2022, 204, 110385. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J.; Peng, S. Synthesis and evaluation of TaC nanocrystalline coating with excellent wear resistance, corrosion resistance, and biocompatibility. Ceram. Int. 2021, 47, 20032–20044. [Google Scholar] [CrossRef]
- Ortiz, C.; Aperador, W.; Caicedo, J. Physical properties evolution of β-tricalcium phosphate/hydroxyapatite heterostructures in relation to the bilayer number. Thin Solid Film. 2022, 752, 139256. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Chen, R.; Zhu, X.; Fu, Y. Effect of microstructure on the ablation behavior and mechanical properties of CVD-HfC coating. Corros. Sci. 2021, 192, 109815. [Google Scholar] [CrossRef]
- Hernandez-Rengifo, E.H.-R.; Ortiz, C.; Hidalgo, C.; Ballesteros, J.; Caicedo, J. Comparative Study of Tribological and Mechanical Properties Between Single Layers of Al2O3 and Si3N4 Deposited on AISI 316 Stainless Steel. Tribol. Ind. 2021, 43, 259–273. [Google Scholar] [CrossRef]
- Chaparro, W.A.; López, E.V.; Uscategui, A.V. Electrochemical corrosion resistance study of electroplated nickel/copper coatings obtained by pulsed current. Ing. Y Desarro. 2010, 27, 14. [Google Scholar]
- Ortiz, C.H.; Caicedo, J.C.; Aperador, W. Corrosion Properties of Heterostructured [8YSZ/Al2O3] N Coatings as a Function of the Bilayer Number. J. Mater. Eng. Perform. 2021, 31, 1679–1692. [Google Scholar] [CrossRef]
- Tan, K.; Wharton, J.; Wood, R. Solid particle erosion–corrosion behaviour of a novel HVOF nickel aluminium bronze coating for marine applications—correlation between mass loss and electrochemical measurements. Wear 2005, 258, 629–640. [Google Scholar] [CrossRef]
- Ortiz, C.H.; Aperador, W.; Caicedo, J.C. Electrochemical Study of Anodized AZ31 Magnesium Alloy (Mg/MgO) Immersed under Watered Cementice Paste. J. Mater. Eng. Perform. 2022, 1, 1–10. [Google Scholar] [CrossRef]
- Caicedo, J.; Cabrera, G.; Caicedo, H.; Amaya, C.; Aperador, W. Nature in corrosion–erosion surface for [TiN/TiAlN] n nanometric multilayers growth on AISI 1045 steel. Thin Solid Film. 2012, 520, 4350–4361. [Google Scholar] [CrossRef]
- Ortiz, C.; Aperador, W.; Caicedo, J. Electrochemical response of (β-TCP and HA) individual coatings and [β-TCP/HA] multilayers coatings exposed to biocompatible environments. Surf. Coat. Technol. 2022, 435, 128266. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Qin, T.-W.; Wang, H.-M.; Zhang, Z. Comparison of corrosion behaviors of AZ31, AZ91, AM60 and ZK60 magnesium alloys. Trans. Nonferrous Met. Soc. China 2009, 19, 517–524. [Google Scholar] [CrossRef]
- Morks, M.F.; Cole, I.; Corrigan, P.; Kobayashi, A. Electrochemical Characterization of Plasma Sprayed Alumina Coatings. J. Surf. Eng. Mater. Adv. Technol. 2011, 1, 107–111. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, S.; Dou, Y.; Han, S.; Wang, L.; Man, C.; Wang, X.; Chen, S.; Cheng, Y.F.; Li, X. Passivation behavior and surface chemistry of 2507 super duplex stainless steel in artificial seawater: Influence of dissolved oxygen and pH. Corros. Sci. 2019, 150, 218–234. [Google Scholar] [CrossRef]
- Zhao, K.; Li, X.-Q.; Wang, L.-W.; Yang, Q.-R.; Cheng, L.-J.; Cui, Z.-Y. Passivation Behavior of 2507 Super Duplex Stainless Steel in Hot Concentrated Seawater: Influence of Temperature and Seawater Concentration. Acta Met. Sin. 2021, 35, 326–340. [Google Scholar] [CrossRef]


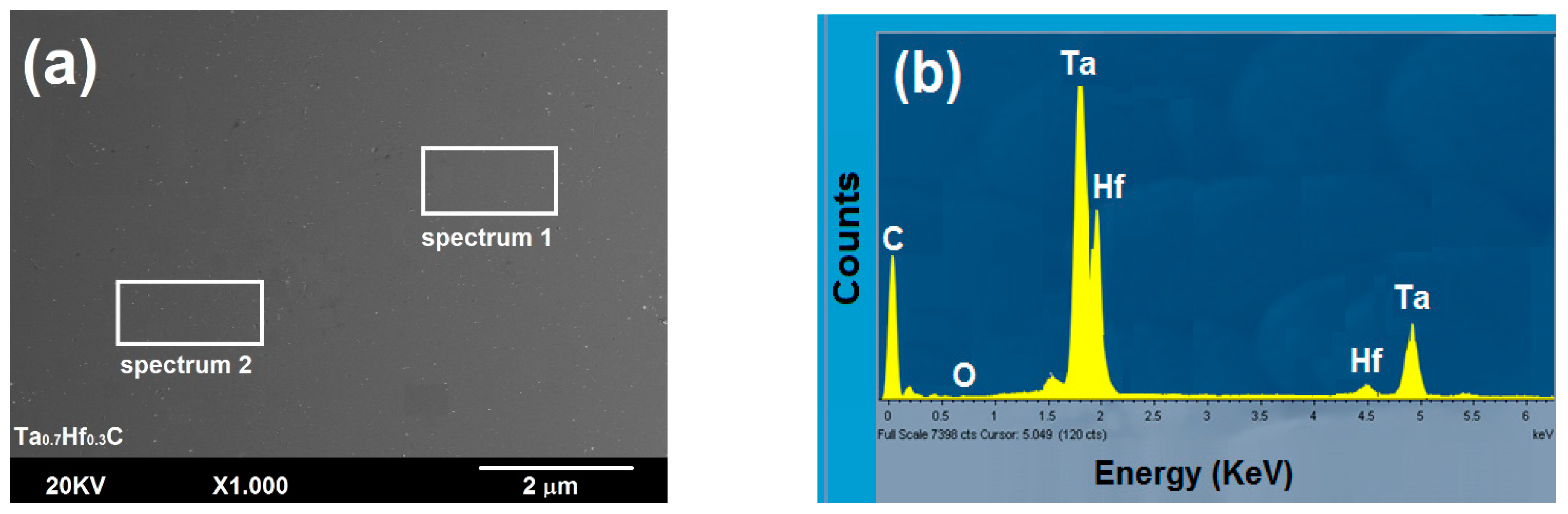
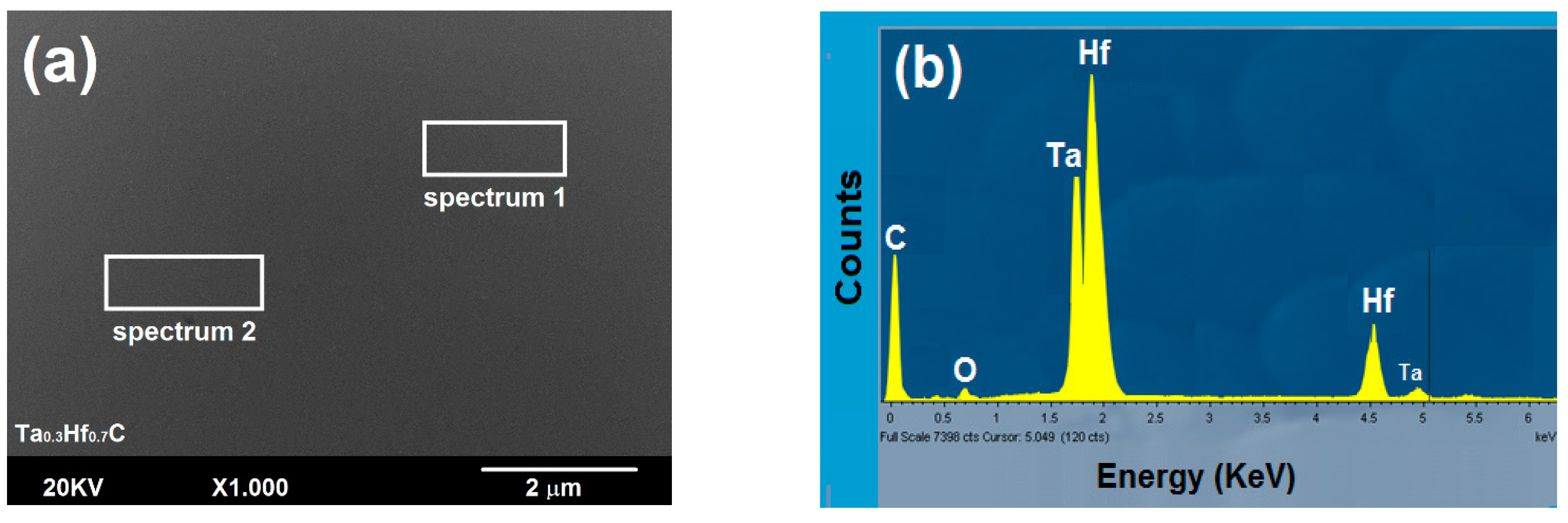

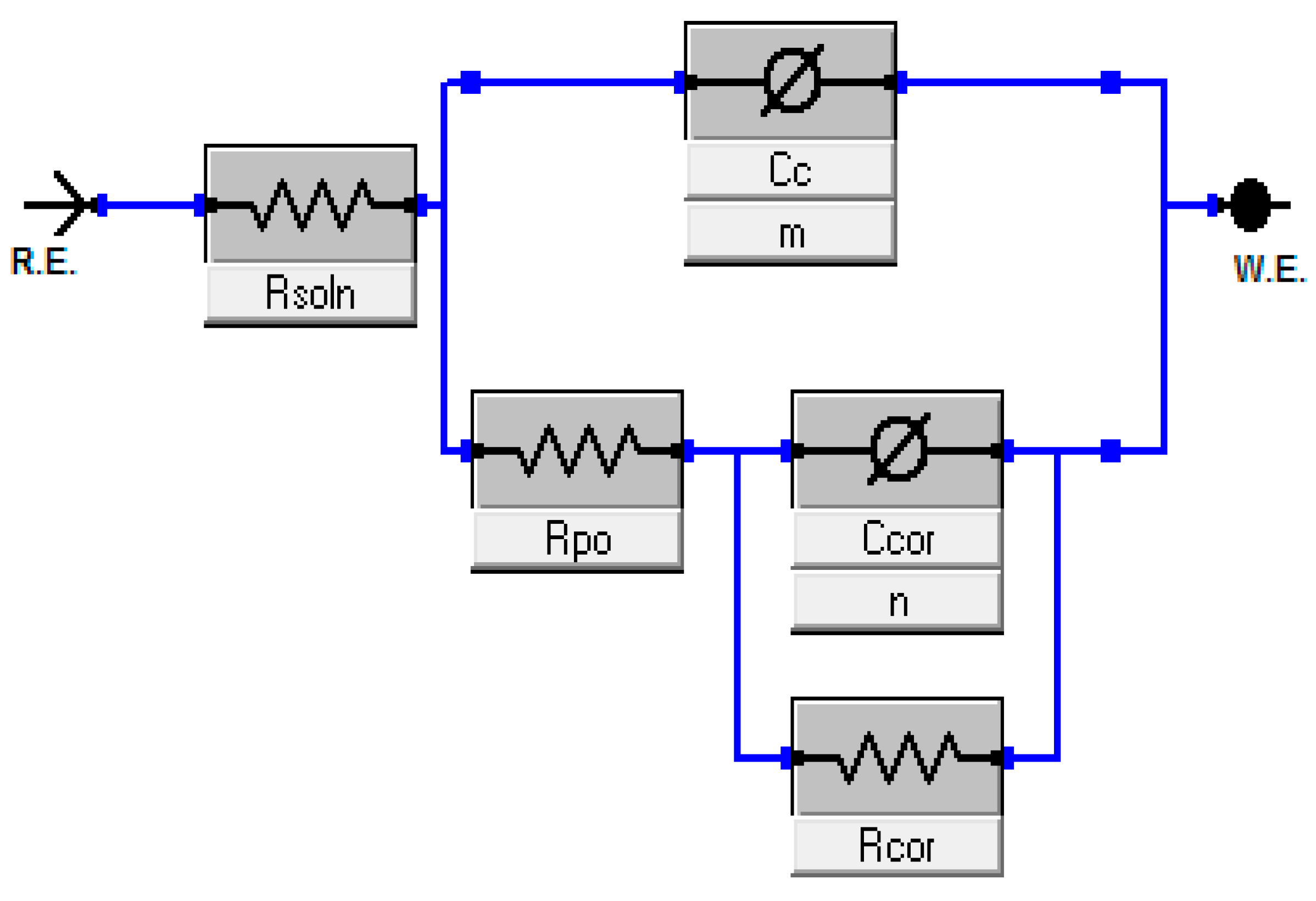

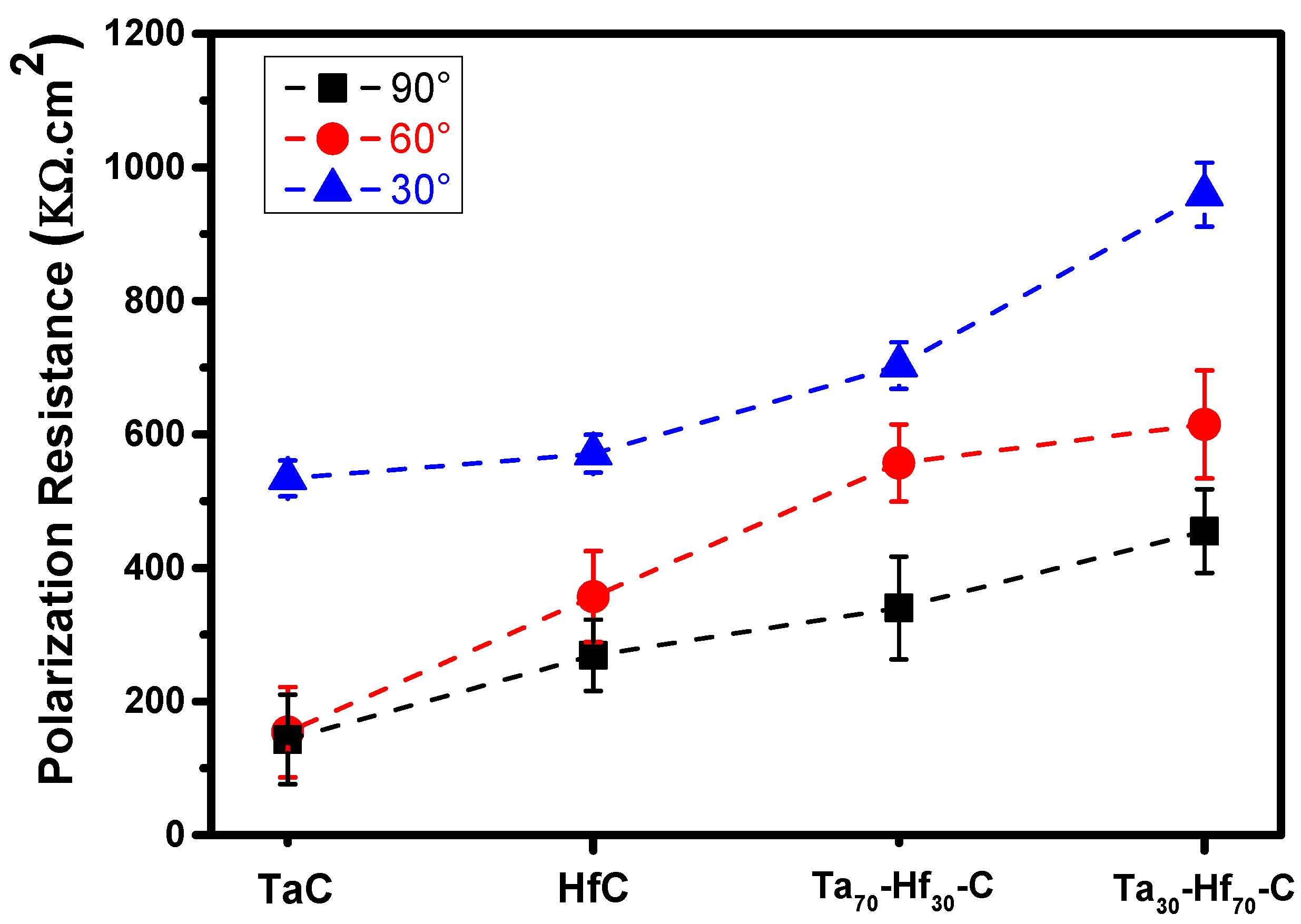

| Elements | Fe | Cr | Ni | Mo | Mn | Si | C | P | S |
|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 63.73 | 16.86 | 14.59 | 2.59 | 1.81 | 0.37 | 0.0024 | 0.017 | 0.009 |
| Coatings | Target Ta | Target Hf | Target C |
|---|---|---|---|
| TaC | 100 W | - | 380 W |
| [Ta30-Hf70-C] | 30 W | 70 W | 380 W |
| [Ta70-Hf30-C] | 70 W | 30 W | 380 W |
| HfC | - | 100 | 380 W |
| Coatings | Anodic Slope (A. cm2) | Cathodic Slope (A. cm2) | Corrosion Rate (mm/y) | Ecorr (V) |
|---|---|---|---|---|
| TaC | 1.97 × 10−6 | 3.47 × 10−6 | 0.7981 | −0.176 |
| HfC | 3.36 × 10−6 | 5.51 × 10−6 | 0.52732 | −0.1 |
| [Ta70-Hf30-C] | 1.62 × 10−6 | 1.56 × 10−6 | 0.2314 | 0.09 |
| [Ta30-Hf70-C] | 6.24 × 10−7 | 6.87 × 10−7 | 0.1282 | 0.132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz, C.H.; Sanchez, S.S.; Caicedo, J.C.; Aperador, W.; Sánchez-Molina, J.; Ruiz, J.B. Comparative Study on the Corro-Erosive Properties of Base Cemented Transition Metals TaC and HfC and TaX-HfX-C Coatings. Metals 2022, 12, 1516. https://doi.org/10.3390/met12091516
Ortiz CH, Sanchez SS, Caicedo JC, Aperador W, Sánchez-Molina J, Ruiz JB. Comparative Study on the Corro-Erosive Properties of Base Cemented Transition Metals TaC and HfC and TaX-HfX-C Coatings. Metals. 2022; 12(9):1516. https://doi.org/10.3390/met12091516
Chicago/Turabian StyleOrtiz, C. H., S. S. Sanchez, J. C. Caicedo, W. Aperador, Jorge Sánchez-Molina, and J. Bautista Ruiz. 2022. "Comparative Study on the Corro-Erosive Properties of Base Cemented Transition Metals TaC and HfC and TaX-HfX-C Coatings" Metals 12, no. 9: 1516. https://doi.org/10.3390/met12091516
APA StyleOrtiz, C. H., Sanchez, S. S., Caicedo, J. C., Aperador, W., Sánchez-Molina, J., & Ruiz, J. B. (2022). Comparative Study on the Corro-Erosive Properties of Base Cemented Transition Metals TaC and HfC and TaX-HfX-C Coatings. Metals, 12(9), 1516. https://doi.org/10.3390/met12091516








