Terpenoids from the Seeds of Toona sinensis and Their Ability to Attenuate High Glucose-Induced Oxidative Stress and Inflammation in Rat Glomerular Mesangial Cells
Abstract
:1. Introduction
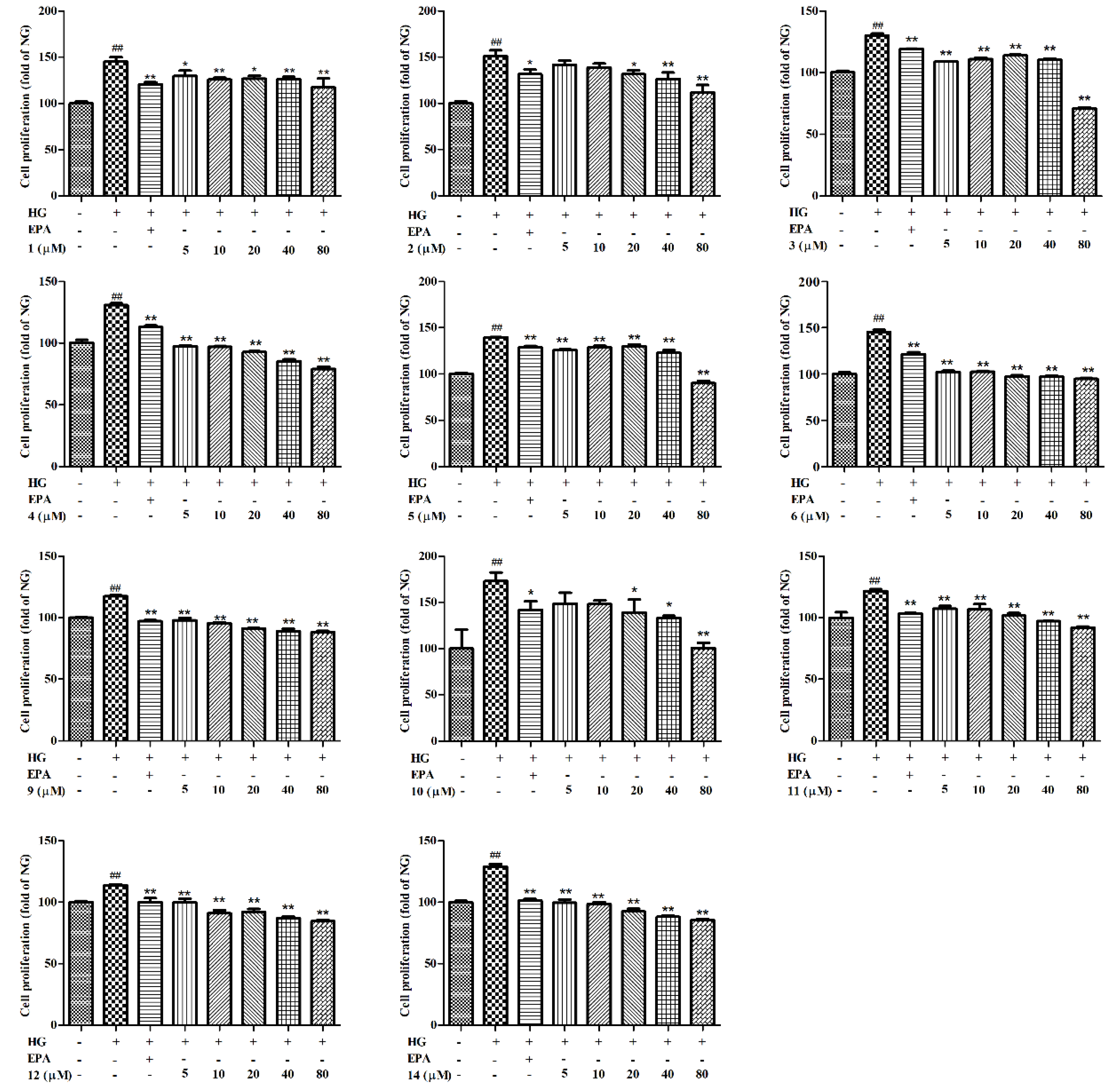
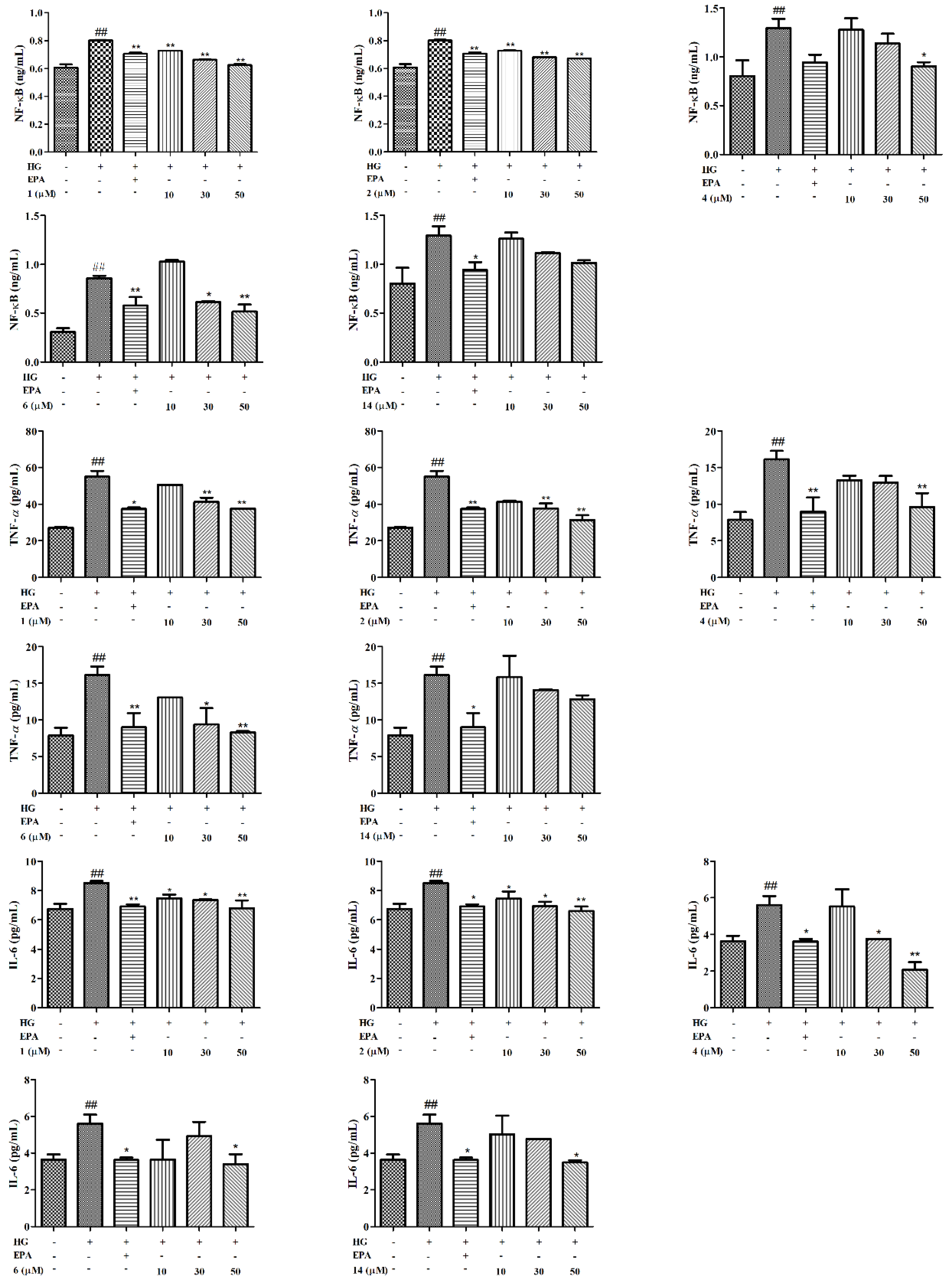
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. General Experimental Procedures
3.3. Extraction, Isolation and Purification
3.4. Cytotoxicity Assay
3.5. Cell Proliferation Assay
3.6. Elisa Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jiang, W.X.; Wang, R.S.; Liu, D.; Zuo, M.; Zhao, C.Z.; Zhang, T.L.; Li, W.Z. Protective effects of kaempferitrin on advanced glycation end products induce mesangial cell apoptosis and oxidative stress. Int. J. Mol. Sci. 2018, 19, 3334. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Chen, Z.; Hu, G.Y.; Li, Q. Therapeutic strategies of diabetic nephropathy: Recent progress and future perspectives. Drug Discov. Today 2015, 20, 332–346. [Google Scholar] [CrossRef]
- Umanath, K.; Lewis, J.B. Update on diabetic nephropathy: Core curriculum 2018. Am. J. Kidney Dis. 2018, 71, 884–895. [Google Scholar] [CrossRef]
- Shelton, L.M.; Park, B.K.; Copple, I.M. Role of Nrf2 in protection against acute kidney injury. Kidney Int. 2013, 84, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Wang, S.S.; Chiufai, K.; Wang, Q.; Cheng, X.L. Umbelliferone ameliorates renal function in diabetic nephropathy rats through regulating inflammation and TLR/NF-κB pathway. Chin. J. Nat. Med. 2019, 17, 346–354. [Google Scholar] [CrossRef]
- Avci, E.; Cakir, E.; Cevher, S.C.; Yaman, H.; Agilli, M.; Bilgi, C. Determination of oxidative stress and cellular inflammation in patients with diabetic nephropathy and non-diabetic nephropathy being administered hemodialysis treatment due to chronic renal failure. Ren. Fail. 2014, 36, 767–773. [Google Scholar] [CrossRef]
- Bao, L.P.; Li, J.S.; Zha, D.Q.; Zhang, L.; Gao, P.; Yao, T.; Wu, X.Y. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-ĸB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Liu, Z.; Ying, K.; Wang, H.M.; Liu, P.; Ji, X.F.; Chi, T.Y.; Zou, L.B.; Wang, S.J.; He, Z.G. WJ-39, an aldose reductase inhibitor, ameliorates renal lesions in diabetic nephropathy by activating Nrf2 signaling. Oxid. Med. Cell. Longev. 2020, 2020, 7950457. [Google Scholar] [CrossRef]
- Xiang, L.; Zhang, L.S.; Hu, J.Y. The complete chloroplast genome of Toona sinensis, an important economic andmedicinal plant endemic in China. Mitochondrial DNA B 2021, 6, 1025–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.S.; Zuo, M.; Ding, S.H.; Liu, B.; Meng, C.; Song, B.; Li, W.Z. Recovery of immune activity by administration of polysaccharides of Toona sinensis and its characterization of major component. Nat. Prod. Res. 2021, 35, 5513–5517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, C.; You, L.J.; Fu, X.; Chen, Y.S.; Luo, Y.Q. Structural identification of compounds from Toona sinensis leaves with antioxidant and anticancer activities. J. Funct. Foods 2014, 10, 427–435. [Google Scholar] [CrossRef]
- Peng, W.; Liu, Y.J.; Hu, M.B.; Zhang, M.M.; Yang, J.; Liang, F.; Huang, Q.W.; Wu, J.C. Toona sinensis: A comprehensive review on its traditional usages, phytochemisty, pharmacology and toxicology. Rev. Bras. Farmacogn. 2014, 29, 111–124. [Google Scholar] [CrossRef]
- Liu, D.; Wang, R.S.; Xuan, L.L.; Wang, X.H.; Li, W.Z. Two new apotirucallane-type triterpenoids from the pericarp of Toona sinensis and their ability to reduce oxidative stress in rat glomerular mesangial cells cultured under high-glucose conditions. Molecules 2020, 25, 801. [Google Scholar] [CrossRef]
- Wang, R.S.; Liu, D.; Liu, X.X.; Liu, F.; Xuan, L.L.; Tang, Y.; Li, W.Z. Cytotoxicity and polyol pathway inhibitory activities of chemical constituents isolated from the pericarp of Toona sinensis. Nat. Prod. Res. 2022, 36, 1593–1598. [Google Scholar] [CrossRef]
- Hou, L.; Fu, Y.H.; Tang, G.H.; Hao, X.J.; Zhao, Q.; He, H.P. Studies on chemical constituents of the fruits of Toona sinensis var. schensiana. J. Yunnan Univ. TCM 2011, 34, 21–27. [Google Scholar]
- Zhao, Q.; Sun, Q.Y.; Yang, Q.X. Effects of an anticomplementary polyphenol from the seed of Toona sinensis on complement-injured SH-SY5Y cells. Chin. Pharm. Bull. 2011, 27, 1086–1090. [Google Scholar]
- Yu, X.Q.; Zhang, Q.Q.; Yan, W.H.; Wang, L.; Guo, Z.X.; Yang, Q.Q.; Liu, C.X.; He, H.B.; Zou, K. Three new terpenoids from the Eupatorium chinense. Phytochem. Lett. 2017, 20, 224–227. [Google Scholar] [CrossRef]
- Tanaka, Y.J.; Sakamoto, A.; Inoue, T.; Yamada, T.; Kikuchi, T.; Kajimoto, T.; Muraoka, O.; Sato, A.; Wataya, Y.; Kim, H.S.; et al. Andirolides H-P from the flower of andiroba (Carapa guianensis, Meliaceae). Tetrahedron 2012, 68, 3669–3677. [Google Scholar] [CrossRef]
- Sakamoto, A.; Tanaka, Y.; Yamada, T.; Kikuchi, T.; Muraoka, O.; Ninomiya, K.; Morikawa, T.; Tanaka, R. Andirolides W-Y from the flower oil of andiroba (Carapa guianensis, Meliaceae). Fitoterapia 2015, 100, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, K.; Saito, H.; Yamamura, R.; Fukaya, H.; Hitotsuyanagi, Y.; Takeya, K. Hydroxylated gedunin derivatives from Cedrela sinensis. J. Nat. Prod. 2006, 69, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.D.; Wu, S.H.; Ma, Y.B.; Wu, D.G. Tirucallane triterpenoids from Dysoxylum hainanense. Phytochemistry 2000, 54, 801–805. [Google Scholar] [CrossRef]
- Pudhom, K.; Sommit, D.; Nuclear, P.; Ngamrojanavanich, N.; Petsom, A. Protoxylocarpin F-H, protolimonoids from seed kernels of Xylocarpus granatum. J. Nat. Prod. 2009, 72, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Kim, J.A.; Kim, S.Y.; Ryu, J.C.; Kim, Y.S.; Jung, S.H.; Kim, M.K.; Lee, S.H. Terpenoids and coumarins isolated from the fruits of Poncirus trifoliata. Chem. Pharm. Bull. 2008, 56, 839–842. [Google Scholar] [CrossRef]
- Biavatti, M.W.; Vieira, P.C.; Da Silva, M.F.; Fernandes, J.B.; Albuquerque, S. Triterpenoid constituents of Raulinoa echinata. J. Nat. Prod. 2002, 65, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cen, J.; Li, Q.; Wang, H.Y. Chemical constituents from Toona ciliata var. henryi and their anti-inflammatory activities. Chin. Tradit. Herbal Drugs 2014, 45, 755–759. [Google Scholar]
- Dong, X.J.; Zhu, Y.F.; Bao, G.H.; Hu, F.L.; Qin, G.W. New limonoids and a dihydrobenzofuran norlignan from the roots of Toona sinensis. Molecules 2013, 18, 2840–2850. [Google Scholar] [CrossRef]
- Zhao, W.Y.; Chen, J.J.; Zou, C.X.; Zhang, Y.Y.; Yao, G.D.; Wang, X.B.; Huang, X.X.; Lin, B.; Song, S.J. New tirucallane triterpenoids from Picrasma quassioides with their potential antiproliferative activities on hepatoma cells. Bioorg. Chem. 2019, 84, 309–318. [Google Scholar] [CrossRef]
- Yang, H.; Gu, Q.; Ying, X.; Cheng, B.; Shen, Y. Comparative study on two antioxidant flavanol-3-O-α-L-rhamnosides in the leaves of Toona sinensis (Chinese toon) from different production sites by HPLC-UV. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 687–691. [Google Scholar] [CrossRef]
- Xu, W.J.; Li, J.H.; Zhou, M.M.; Luo, J.; Jian, K.L.; Tian, X.M.; Xia, Y.Z.; Yang, L.; Luo, J.; Kong, L.Y. Toonasindiynes A-F, new polyacetylenes from Toona sinensis with cytotoxic and anti-inflammatory activities. Fitoterapia 2020, 146, 104667. [Google Scholar] [CrossRef]
- Lodewyk, M.W.; Siebert, M.R.; Tantillo, D.J. Computational prediction of 1H and 13C chemical shifts: A useful tool for natural product, mechanistic, and synthetic organic chemistry. Chem. Rev. 2012, 112, 1839–1862. [Google Scholar] [CrossRef] [PubMed]
- Grimblat, N.; Zanardi, M.M.; Sarotti, A.M. Beyond DP4: An improved probability for the stereochemical assignment of isomeric compounds using quantum chemical calculations of NMR shifts. J. Org. Chem. 2015, 80, 12526–12534. [Google Scholar] [CrossRef] [PubMed]
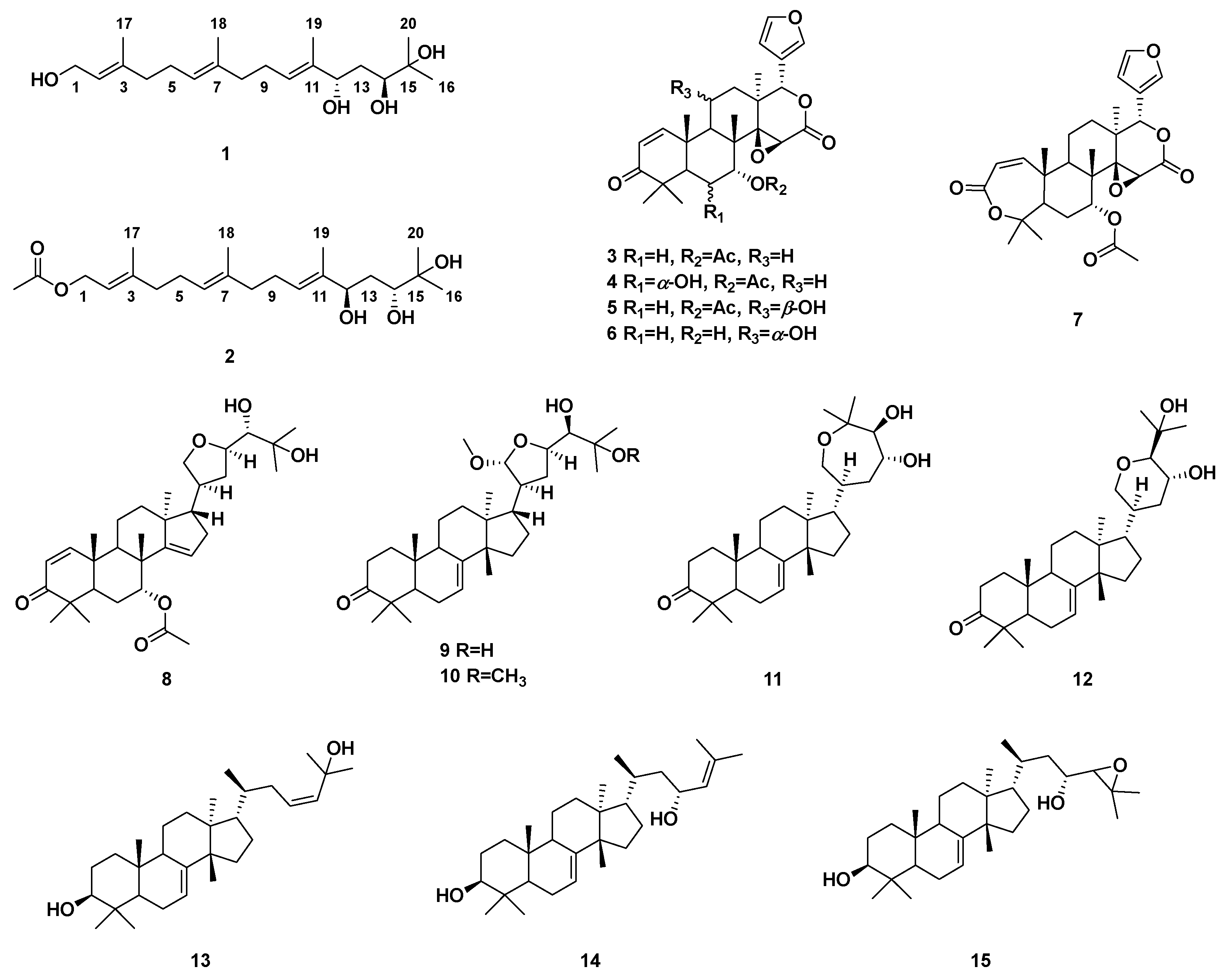
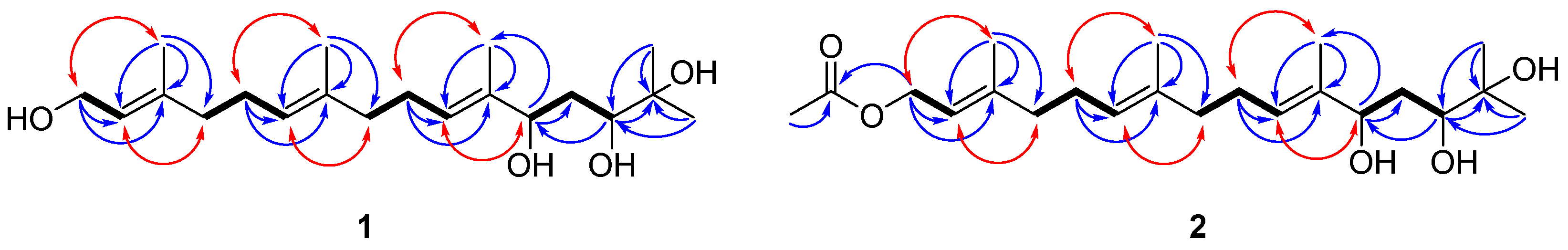


| NO. | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 4.14 (2H, d, 6.8) | 59.5 | 4.57 (2H, d, 7.1) | 61.5 |
| 2 | 5.39 (1H, t, 6.8) | 123.8 | 5.32 (1H, t, 7.1) | 118.4 |
| 3 | - | 139.4 | - | 142.3 |
| 4 | 2.05 (2H, m) | 39.6 | 2.02 (2H, m) | 39.5 |
| 5 | 2.11 (2H, m) | 26.2 | 2.15 (2H, m) | 26.4 |
| 6 | 5.09 (1H, t, 6.1) | 124.3 | 5.07 (1H, t, 6.3) | 124.4 |
| 7 | - | 135.1 | - | 135.1 |
| 8 | 2.03 (2H, m) | 39.3 | 2.02 (2H, m) | 39.5 |
| 9 | 2.11 (2H, m) | 26.1 | 2.08 (2H, m) | 26.2 |
| 10 | 5.45 (1H, t, 6.7) | 126.8 | 5.21 (1H, t, 6.8) | 129.5 |
| 11 | - | 134.6 | - | 131.8 |
| 12 | 4.52 (1H, m) | 81.9 | 3.68 (1H, t, 7.9) | 69.4 |
| 13 | 2.01 (1H, m); 1.91 (1H, m) | 39.1 | 2.61 (1H, m); 2.05 (1H, m) | 45.1 |
| 14 | 3.98 (1H, m) | 78.6 | 3.29 (1H, m) | 79.0 |
| 15 | - | 83.0 | - | 73.1 |
| 16 | 1.23 (3H, s) | 28.0 | 1.25 (3H, s) | 26.3 |
| 17 | 1.66 (3H, s) | 16.4 | 1.68 (3H, s) | 16.6 |
| 18 | 1.58 (3H, s) | 16.1 | 1.58 (3H, s) | 15.9 |
| 19 | 1.58 (3H, s) | 11.5 | 1.65 (3H, s) | 16.1 |
| 20 | 1.26 (3H, s) | 21.7 | 1.29 (3H, s) | 25.5 |
| 1-OCOCH3 | - | - | - | 171.4 |
| 1-OCOCH3 | - | - | 2.04 (3H, s) | 21.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Gao, H.; Liu, X.; Zhou, J.; Jiang, Y.; Wang, F.; Wang, R.; Li, W. Terpenoids from the Seeds of Toona sinensis and Their Ability to Attenuate High Glucose-Induced Oxidative Stress and Inflammation in Rat Glomerular Mesangial Cells. Molecules 2022, 27, 5784. https://doi.org/10.3390/molecules27185784
Chen Y, Gao H, Liu X, Zhou J, Jiang Y, Wang F, Wang R, Li W. Terpenoids from the Seeds of Toona sinensis and Their Ability to Attenuate High Glucose-Induced Oxidative Stress and Inflammation in Rat Glomerular Mesangial Cells. Molecules. 2022; 27(18):5784. https://doi.org/10.3390/molecules27185784
Chicago/Turabian StyleChen, Ying, Hong Gao, Xiaoxiao Liu, Jinyi Zhou, Yijin Jiang, Feng Wang, Rongshen Wang, and Wanzhong Li. 2022. "Terpenoids from the Seeds of Toona sinensis and Their Ability to Attenuate High Glucose-Induced Oxidative Stress and Inflammation in Rat Glomerular Mesangial Cells" Molecules 27, no. 18: 5784. https://doi.org/10.3390/molecules27185784
APA StyleChen, Y., Gao, H., Liu, X., Zhou, J., Jiang, Y., Wang, F., Wang, R., & Li, W. (2022). Terpenoids from the Seeds of Toona sinensis and Their Ability to Attenuate High Glucose-Induced Oxidative Stress and Inflammation in Rat Glomerular Mesangial Cells. Molecules, 27(18), 5784. https://doi.org/10.3390/molecules27185784








